Difference in Incontinence Pad Use between Patients after Radical Prostatectomy and Cancer-Free Population with Subgroup Analysis for Open vs. Minimally Invasive Radical Prostatectomy: A Descriptive Analysis of Insurance Claims-Based Data
Abstract
:1. Introduction
2. Methods
2.1. Data
2.2. Measures of Incontinence Pads
2.3. Selection of Patients
2.4. Analysis
3. Results
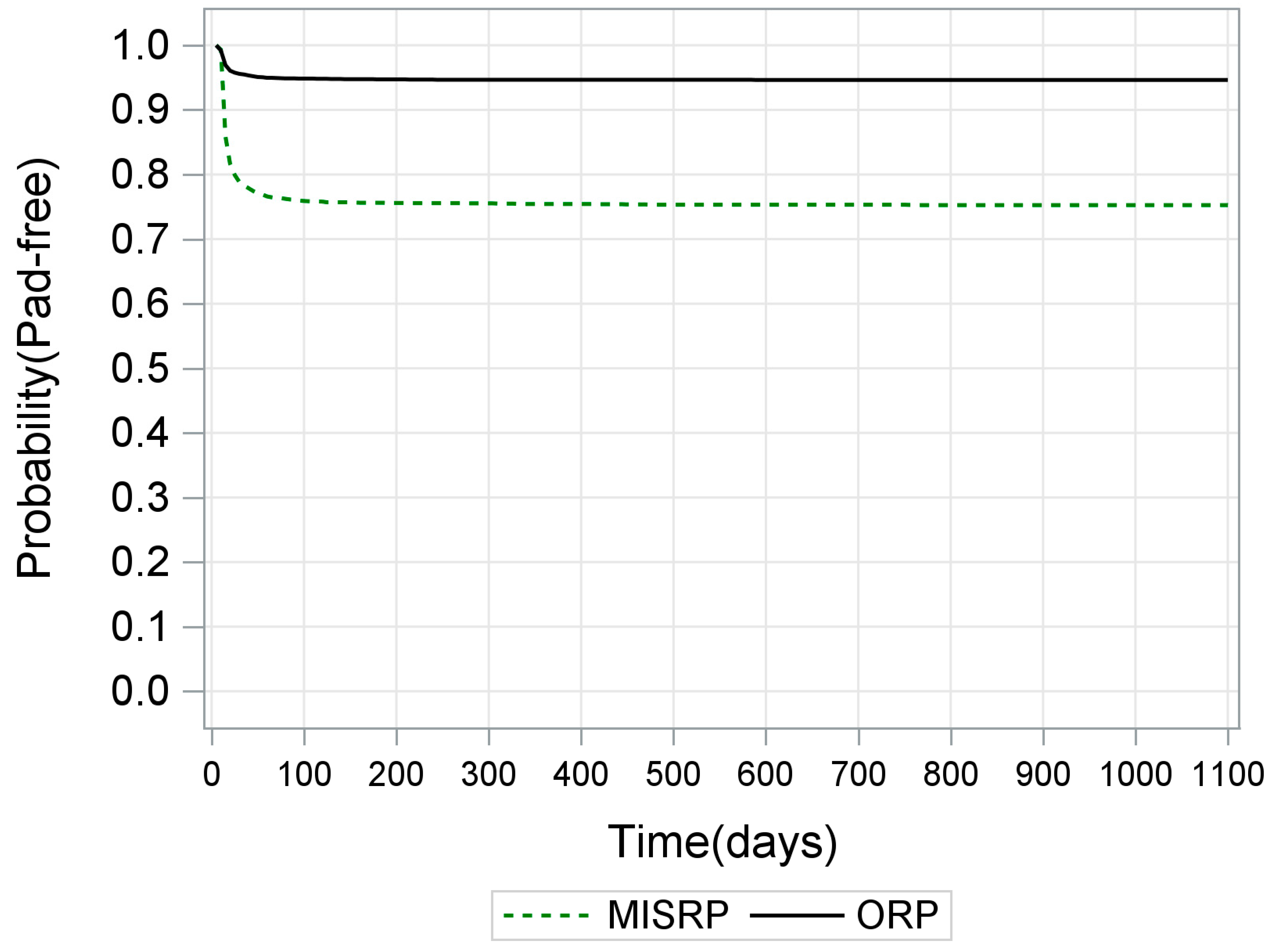
3.1. Effect of Type of RP
3.2. Effect of Age
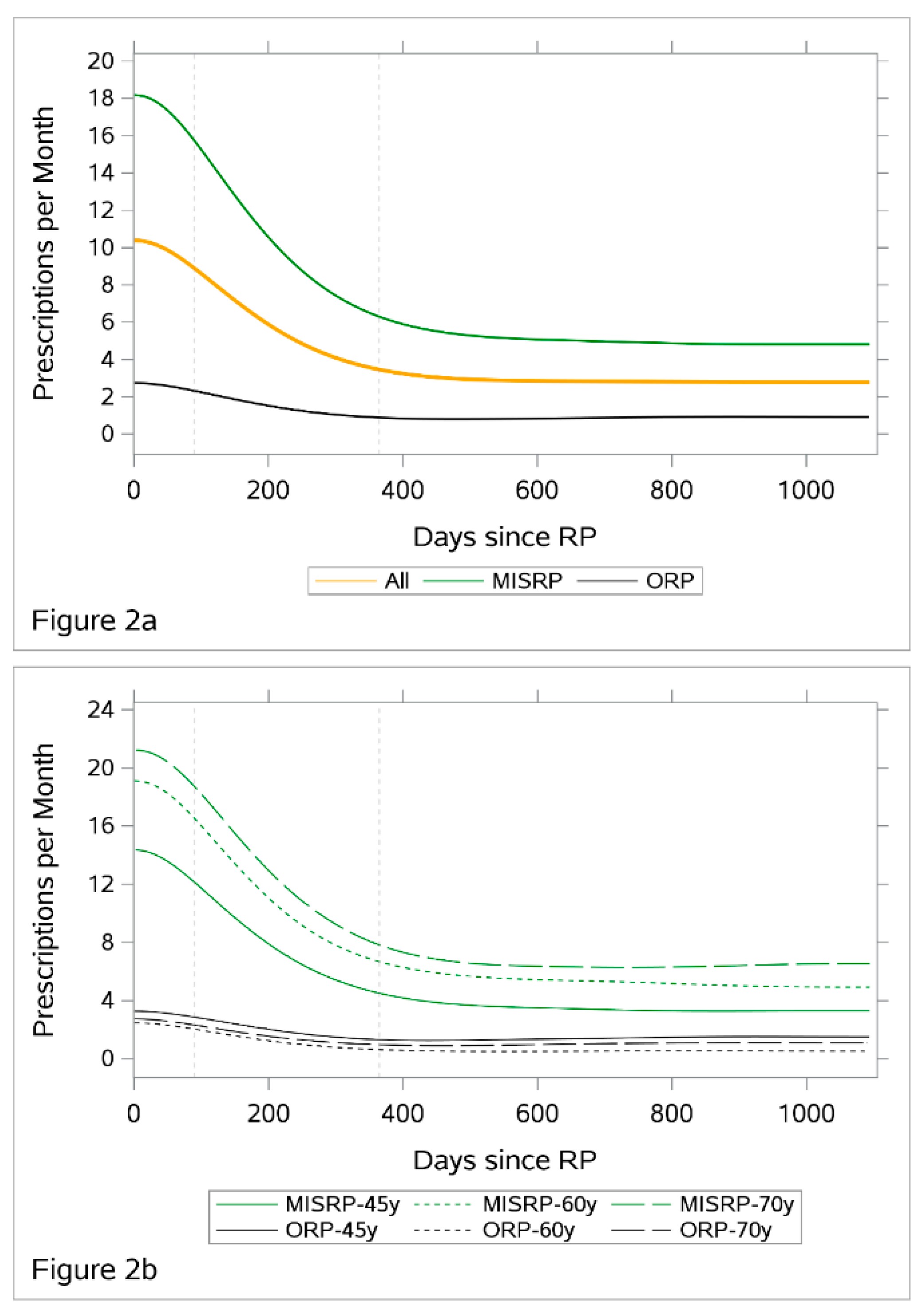
3.3. Number of Prescribed Pads
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Mason, M.; Metcalfe, C.; Holding, P.; Davis, M.; Peters, T.; Turner, E.L.; Martin, R.M.; et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N. Engl. J. Med. 2016, 375, 1415–1424. [Google Scholar] [CrossRef] [Green Version]
- Wilt, T.J.; Jones, K.M.; Barry, M.J.; Andriole, G.L.; Culkin, D.; Wheeler, T.; Aronson, W.J.; Brawer, M.K. Follow-Up of Prostatectomy Versus Observation for Early Prostate Cancer. N. Engl. J. Med. 2017, 377, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Wallerstedt, A.; Carlsson, S.; Nilsson, A.E.; Johansson, E.; Nyberg, T.; Steineck, G.; Wiklund, N.P. Pad Use and Patient Reported Bother From Urinary Leakage After Radical Prostatectomy. J. Urol. 2012, 187, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Resnick, M.J.; Koyama, T.; Fan, K.-H.; Albertsen, P.C.; Goodman, M.; Hamilton, A.S.; Hoffman, R.M.; Potosky, A.L.; Stanford, J.L.; Stroup, A.M.; et al. Long-Term Functional Outcomes after Treatment for Localized Prostate Cancer. N. Engl. J. Med. 2013, 368, 436–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epidemiology of Urinary (UI) and Faecal (FI) Incontinence and Pelvic Organ Prolapse (POP). Available online: https://www.ics.org/Publications/ICI_4/files-book/comite-1.pdf (accessed on 9 November 2020).
- De Nunzio, C.; Pastore, A.L.; Lombardo, R.; Cancrini, F.; Carbone, A.; Fuschi, A.; Dutto, L.; Tubaro, A.; Witt, J.H. The Eortc Quality of Life Questionnaire Predicts Early and Long-Term Incontinence in Patients Treated with Robotic Assisted Radical Prostatectomy: Analysis of a Large Single Center Cohort. Urol. Oncol. Semin. Orig. Investig. 2019, 37, 1006–1013. [Google Scholar] [CrossRef]
- Bourke, L.; Boorjian, S.A.; Briganti, A.; Klotz, L.; Mucci, L.; Resnick, M.J.; Rosario, D.J.; Skolarus, T.A.; Penson, D.F. Survivorship and Improving Quality of Life in Men with Prostate Cancer. Eur. Urol. 2015, 68, 374–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlsson, S.; Jäderling, F.; Wallerstedt, A.; Nyberg, T.; Stranne, J.; Thorsteinsdottir, T.; Carlsson, S.V.; Bjartell, A.; Hugosson, J.; Haglind, E.; et al. Oncological and Functional Outcomes 1 Year After Radical Prostatectomy for Very-Low-Risk Prostate Cancer: Results from the Prospective LAPPRO Trial. BJU Int. 2016, 118, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Yaxley, J.W.; Coughlin, G.D.; Chambers, S.K.; Occhipinti, S.; Samaratunga, H.; Zajdlewicz, L.; Dunglison, N.; Carter, R.; Williams, S.; Payton, D.J.; et al. Robot-Assisted Laparoscopic Prostatectomy Versus Open Radical Retropubic Prostatectomy: Early Outcomes from a Randomised Controlled Phase 3 Study. Lancet 2016, 388, 1057–1066. [Google Scholar] [CrossRef]
- Coughlin, G.D.; Yaxley, J.W.; Chambers, S.K.; Occhipinti, S.; Samaratunga, H.; Zajdlewicz, L.; Teloken, P.; Dunglison, N.; Williams, S.; Lavin, M.F.; et al. Robot-Assisted Laparoscopic Prostatectomy Versus Open Radical Retropubic Prostatectomy: 24-Month Outcomes from a Randomised Controlled Study. Lancet Oncol. 2018, 19, 1051–1060. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Cornford, P.; De Santis, M.; Fanti, S.; Gillessen, S.; Grummet, J.; Henry, A.M.; Lam, T.B.; et al. European Association of Urology Guidelines. 2020 Edition. EAU Annu. Congr. Amst. 2020. Available online: https://uroweb.org/eau-guidelines-2020-now-available-online/ (accessed on 5 November 2020).
- Olsson, L.; Salomon, L.; Nadu, A.; Hoznek, A.; Cicco, A.; Saint, F.; Chopin, D.; Abbou, C. Prospective Patient-Reported Continence after Laparoscopic Radical Prostatectomy. Urology 2001, 58, 570–572. [Google Scholar] [CrossRef]
- Donnellan, S.M.; Duncan, H.J.; MacGregor, R.J.; Russell, J.M. Prospective Assessment of Incontinence after Radical Retropubic Prostatectomy: Objective and Subjective Analysis. Urology 1997, 49, 225–230. [Google Scholar] [CrossRef]
- Bachner, F.; Bobek, J.; Habimana, K.; Ladurner, J.; Lepuschütz, L.; Ostermann, H.; Rainer, L.; Schmidt, A.E.; Zuba, M.; Quentin, W.; et al. Health Systems in Transition Austria Health System Review. 2018, Volume 20. Available online: https://jasmin.goeg.at/id/eprint/434 (accessed on 14 November 2020).
- Competence Center Integrierte Versorgung. Available online: https://www.cciv.at/cdscontent/?contentid=10007.678424&portal=ccivportal (accessed on 25 October 2020).
- Fuchs, M.; Hollan, K.; Schenk, M. Analyse der Nicht-Krankenversicherten Personen in Österreich (2015/17). Studie im Auftrag des Hauptverbands der österreichischen Sozialversicherungsträger 2018. Available online: https://www.researchgate.net/publication/327201409_Analyse_der_nicht-krankenversicherten_Personen_in_Osterreich_201517_Studie_im_Auftrag_des_Hauptverbands_der_osterreichischen_Sozialversicherungstrager (accessed on 15 November 2020).
- Sacco, E.; Prayer-Galetti, T.; Pinto, F.; Fracalanza, S.; Betto, G.; Pagano, F.; Artibani, W. Urinary Incontinence after Radical Prostatectomy: Incidence by Definition, Risk Factors and Temporal Trend in a Large Series with a Long-Term Follow-Up. BJU Int. 2006, 97, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Liss, M.A.; Osann, K.; Canvasser, N.; Chu, W.; Chang, A.; Gan, J.; Li, R.; Santos, R.; Skarecky, D.; Finley, D.S.; et al. Continence Definition After Radical Prostatectomy Using Urinary Quality of Life: Evaluation of Patient Reported Validated Questionnaires. J. Urol. 2010, 183, 1464–1468. [Google Scholar] [CrossRef]
- Haglind, E.; Carlsson, S.; Stranne, J.; Wallerstedt, A.; Wilderäng, U.; Thorsteinsdottir, T.; Lagerkvist, M.; Damber, J.-E.; Bjartell, A.; Hugosson, J.; et al. Urinary Incontinence and Erectile Dysfunction After Robotic Versus Open Radical Prostatectomy: A Prospective, Controlled, Nonrandomised Trial. Eur. Urol. 2015, 68, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.C. Comparative Effectiveness of Minimally Invasive vs Open Radical Prostatectomy. JAMA 2009, 302, 1557–1564. [Google Scholar] [CrossRef]
- Touijer, K.; Eastham, J.A.; Secin, F.P.; Romero-Otero, J.; Serio, A.; Stasi, J.; Sanchez-Salas, R.; Vickers, A.; Reuter, V.E.; Scardino, P.T.; et al. Comprehensive Prospective Comparative Analysis of Outcomes Between Open and Laparoscopic Radical Prostatectomy Conducted in 2003 to 2005. J. Urol. 2008, 179, 1811–1817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, F.F. Editorial: Risk Factors for Urinary Incontinence after Radical Prostatectomy. J. Urol. 1996, 156, 1724. [Google Scholar] [CrossRef]
- Begg, C.B.; Riedel, E.R.; Bach, P.B.; Kattan, M.; Schrag, D.; Warren, J.L.; Scardino, P.T. Variations in Morbidity after Radical Prostatectomy. N. Engl. J. Med. 2002, 346, 1138–1144. [Google Scholar] [CrossRef]
- Gershman, B.; Meier, S.K.; Jeffery, M.M.; Moreira, D.; Tollefson, M.K.; Kim, S.P.; Karnes, R.J.; Shah, N.D. Redefining and Contextualizing the Hospital Volume-Outcome Relationship for Robot-Assisted Radical Prostatectomy: Implications for Centralization of Care. J. Urol. 2017, 198, 92–99. [Google Scholar] [CrossRef]
- Gershman, B.; Psutka, S.P.; McGovern, F.J.; Dahl, D.M.; Tabatabaei, S.; Gettman, M.T.; Frank, I.; Carlson, R.E.; Rangel, L.J.; Barry, M.J.; et al. Patient-Reported Functional Outcomes Following Open, Laparoscopic, and Robotic Assisted Radical Prostatectomy Performed by High-volume Surgeons at High-volume Hospitals. Eur. Urol. Focus 2016, 2, 172–179. [Google Scholar] [CrossRef]
- Hoffman, K.E.; Penson, D.F.; Zhao, Z.; Huang, L.-C.; Conwill, R.; Laviana, A.A.; Joyce, D.D.; Luckenbaugh, A.N.; Goodman, M.; Hamilton, A.S.; et al. Patient-Reported Outcomes Through 5 Years for Active Surveillance, Surgery, Brachytherapy, or External Beam Radiation With or Without Androgen Deprivation Therapy for Localized Prostate Cancer. JAMA 2020, 323, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Booth, C.M.; Karim, S.; MacKillop, W.J. Real-World Data: Towards Achieving the Achievable in Cancer Care. Nat. Rev. Clin. Oncol. 2019, 16, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Sacco, E.; Bientinesi, R.; Gandi, C.; Di Gianfrancesco, L.; Pierconti, F.; Racioppi, M.; Bassi, P. Patient Pad Count is a Poor Measure of Urinary Incontinence Compared with 48-h Pad Test: Results of a Large-Scale Multicentre Study. BJU Int. 2019, 123, E69–E78. [Google Scholar] [CrossRef] [PubMed]
- Harlow, B.L.; Bavendam, T.G.; Palmer, M.H.; Brubaker, L.; Burgio, K.L.; Lukacz, E.S.; Miller, J.M.; Mueller, E.R.; Newman, D.K.; Rickey, L.M.; et al. The Prevention of Lower Urinary Tract Symptoms (PLUS) Research Consortium: A Transdisciplinary Approach Toward Promoting Bladder Health and Preventing Lower Urinary Tract Symptoms in Women Across the Life Course. J. Women Health 2018, 27, 283–289. [Google Scholar] [CrossRef] [PubMed]
| Prostate Cancer Patients Underwent RP 1 | Cancer Free Controls | ||||
|---|---|---|---|---|---|
| Open | Minimally Invasive | Total | With Non-Operated BPH 2 | Without BPH | |
| Number of Cases (n) | 3262 | 2986 | 6248 | 7158 | 50,257 |
| 2013 | 1227 | 932 | 2159 | ||
| 2014 | 1055 | 1008 | 2063 | ||
| 2015 | 980 | 1046 | 2026 | ||
| Age (years), median (Q1–Q3) | 64.6 (58.8–68.7) | 64.6 (58.8–69.6) | 64.6 (58.8–69.1) | 70.8 (62.5–77.9) | 64.3 (54.3–74.4) |
| Patients received incontinence pad prescription (n) | 54 | 504 | |||
| Preoperative | 15 | 36 | 51 | ||
| Postoperative | 175 | 737 | 912 | ||
| Total number of incontinence pad prescription (n) | |||||
| Postoperative | 13,280 | 4971 | 18,251 | ||
| Total number of prescribed incontinence pad (n) | 42,813 | 591,390 | |||
| Preoperative (from 1/1/2011) | 1613 | 6947 | 8560 | ||
| Postoperative | 135,106 | 809,899 | 945,005 | ||
| Monthly rate of prescribed incontinence pads [95% CI] | 0.06 (0.04–0.09) | 0.12 (0.10–0.14) | |||
| Preoperative | 0.01 (0.01–0.02) | 0.05 (0.03–0.08) | 0.03 (0.02–0.05) | ||
| 3 months postoperative | 3.61 (2.58–3.78) | 22.94 (20.97–24.97) | 12.61 (11.59–13.65) | ||
| 12 months postoperative | 1.70 (1.32–2.11) | 12.19 (11.01–13.42) | 6.71 (6.10–7.34) | ||
| 36 months postoperative | 1.15 (0.84–1.50) | 7.51 (6.69–8.35) | 4.91 (3.76–4.62) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mun, D.-H.; Yang, L.; Shariat, S.F.; Reitter-Pfoertner, S.; Gredinger, G.; Waldhoer, T. Difference in Incontinence Pad Use between Patients after Radical Prostatectomy and Cancer-Free Population with Subgroup Analysis for Open vs. Minimally Invasive Radical Prostatectomy: A Descriptive Analysis of Insurance Claims-Based Data. Int. J. Environ. Res. Public Health 2021, 18, 6891. https://doi.org/10.3390/ijerph18136891
Mun D-H, Yang L, Shariat SF, Reitter-Pfoertner S, Gredinger G, Waldhoer T. Difference in Incontinence Pad Use between Patients after Radical Prostatectomy and Cancer-Free Population with Subgroup Analysis for Open vs. Minimally Invasive Radical Prostatectomy: A Descriptive Analysis of Insurance Claims-Based Data. International Journal of Environmental Research and Public Health. 2021; 18(13):6891. https://doi.org/10.3390/ijerph18136891
Chicago/Turabian StyleMun, Dong-Ho, Lin Yang, Shahrokh F. Shariat, Sylvia Reitter-Pfoertner, Gerald Gredinger, and Thomas Waldhoer. 2021. "Difference in Incontinence Pad Use between Patients after Radical Prostatectomy and Cancer-Free Population with Subgroup Analysis for Open vs. Minimally Invasive Radical Prostatectomy: A Descriptive Analysis of Insurance Claims-Based Data" International Journal of Environmental Research and Public Health 18, no. 13: 6891. https://doi.org/10.3390/ijerph18136891
APA StyleMun, D.-H., Yang, L., Shariat, S. F., Reitter-Pfoertner, S., Gredinger, G., & Waldhoer, T. (2021). Difference in Incontinence Pad Use between Patients after Radical Prostatectomy and Cancer-Free Population with Subgroup Analysis for Open vs. Minimally Invasive Radical Prostatectomy: A Descriptive Analysis of Insurance Claims-Based Data. International Journal of Environmental Research and Public Health, 18(13), 6891. https://doi.org/10.3390/ijerph18136891







