A Longitudinal Study of Episodic and Semantic Autobiographical Memory in aMCI and Alzheimer’s Disease Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Materials
2.3.1. General Cognitive Screening
2.3.2. Assessment of Autobiographical Memory
2.3.3. Follow-Up Assessment
2.4. Data Analysis
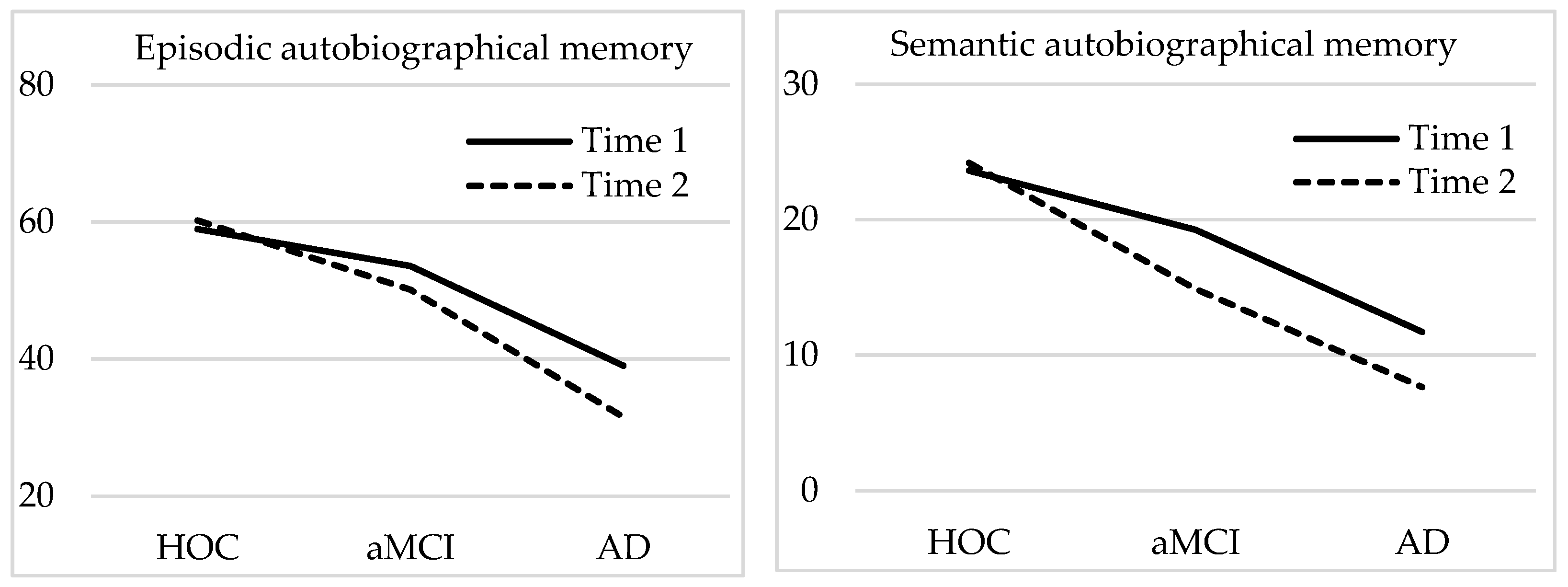
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pitarque, A.; Meléndez, J.C.; Sales, A.; Mayordomo, T.; Satorres, E.; Escudero, J.; Algarabel, S. The effects of healthy aging, amnestic mild cognitive impairment, and Alzheimer’s disease on recollection, familiarity and false recognition, estimated by an associative process-dissociation recognition procedure. Neuropsychologia 2016, 91, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Irish, M.; Landin-Romero, R.; Mothakunnel, A.; Ramanan, S.; Hsieh, S.; Hodges, J.R.; Piguet, O. Evolution of autobiographical memory impairments in Alzheimer’s disease and frontotemporal dementia–A longitudinal neuroimaging study. Neuropsychologia 2018, 110, 14–25. [Google Scholar] [CrossRef]
- Fivush, R.; Habermas, T.; Waters, T.E.; Zaman, W. The making of autobiographical memory: Intersections of culture, narratives and identity. Int. J. Psychol. 2011, 46, 321–345. [Google Scholar] [CrossRef]
- Piolino, P.; Desgranges, B.; Benali, K.; Eustache, F. Episodic and semantic remote autobiographical memory in ageing. Memory 2002, 10, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Tulving, E. Episodic memory and autonoesis: Uniquely human. In The Missing Link in Cognition: Origins of Self-Reflective Consciousness; Herbert, S., Metcalfe, J., Eds.; Oxford University Press: New York, NY, USA, 2005; pp. 3–56. [Google Scholar]
- Baddeley, A. The concept of episodic memory. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2001, 356, 1345–1350. [Google Scholar] [CrossRef]
- Maguire, E.A. Neuroimaging studies of autobiographical event memory. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2001, 356, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, E.; McKinnon, M.C.; Levine, B. The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia 2006, 44, 2189–2208. [Google Scholar] [CrossRef] [Green Version]
- Piolino, P.; Desgranges, B.; Eustache, F. Episodic autobiographical memory over the course of time: Cognitive, neuropsychological and neuroimaging findings. Neuropsychologia 2009, 47, 2314–2329. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.H.; Rubin, D.C.; Giovanello, K.S. Effects of task instruction on autobiographical memory specificity in young and older adults. Memory 2014, 22, 722–736. [Google Scholar] [CrossRef]
- Masdeu, J.C.; Zubieta, J.L.; Arbizu, J. Neuroimaging as a marker of the onset and progression of Alzheimer’s disease. J. Neurol. Sci. 2005, 236, 55–64. [Google Scholar] [CrossRef]
- Murphy, K.J.; Troyer, A.K.; Levine, B.; Moscovitch, M. Episodic, but not semantic, autobiographical memory is reduced in amnestic mild cognitive impairment. Neuropsychologia 2008, 46, 3116–3123. [Google Scholar] [CrossRef] [Green Version]
- Meléndez, J.C.; Escudero, J.; Satorres, E.; Pitarque, A. Type of memory and emotional valence in healthy aging, mild cognitive impairment, and Alzheimer’s disease. Psicothema 2019, 31, 60–65. [Google Scholar] [CrossRef]
- Berna, F.; Schönknecht, P.; Seidl, U.; Toro, P.; Schröder, J. Episodic autobiographical memory in normal aging and mild cognitive impairment: A population-based study. Psychiatry Res. 2012, 200, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Leyhe, T.; Müller, S.; Milian, M.; Eschweiler, G.W.; Saur, R. Impairment of episodic and semantic autobiographical memory in patients with mild cognitive impairment and early Alzheimer’s disease. Neuropsychologia 2009, 47, 2464–2469. [Google Scholar] [CrossRef] [PubMed]
- Irish, M.; Lawlor, B.A.; O’Mara, S.M.; Coen, R.F. Exploring the recollective experience during autobiographical memory retrieval in amnestic mild cognitive impairment. J. Int. Neuropsychol. Soc. 2010, 16, 546–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnabe, A.; Whitehead, V.; Pilon, R.; Arsenault-Lapierre, G.; Chertkow, H. Autobiographical memory in mild cognitive impairment and Alzheimer’s disease: A comparison between the Levine and Kopelman interview methodologies. Hippocampus 2012, 22, 1809–1825. [Google Scholar] [CrossRef]
- Irish, M.; Hornberger, M.; Lah, S.; Miller, L.; Pengas, G.; Nestor, P.J.; Hodges, J.R.; Piguet, O. Profiles of recent autobiographical memory retrieval in semantic dementia, behavioural-variant frontotemporal dementia, and Alzheimer’s disease. Neuropsychologia 2011, 49, 2694–2702. [Google Scholar] [CrossRef]
- El Haj, M.; Antoine, P.; Nandrino, J.L.; Kapogiannis, D. Autobiographical memory decline in Alzheimer’s disease, a theoretical and clinical overview. Ageing Res. Rev. 2015, 23, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Müller, S.; Mychajliw, C.; Reichert, C.; Melcher, T.; Leyhe, T. Autobiographical memory performance in Alzheimer’s disease depends on retrieval frequency. J. Alzheimers Dis. 2016, 52, 1215–1225. [Google Scholar] [CrossRef]
- Maguire, E.A.; Kumaran, D.; Hassabis, D.; Kopelman, M.D. Autobiographical memory in semantic dementia: A longitudinal fMRI study. Neuropsychologia 2010, 48, 123–136. [Google Scholar] [CrossRef] [Green Version]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Reisberg, B.; Ferri, S.H.; de Leon, M.J.; Crook, T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am. J. Psychiatry 1982, 139, 1136–1139. [Google Scholar] [CrossRef] [Green Version]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Radloff, L.S. The CES-D scale a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Folstein, M.; Folstein, S.; Mc Hugh, P. Mini Mental State. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Peña-Casanova, J. Test Barcelona Revisado. Normalidad, Semiología y Patologías Neuropsicológicas. [Barcelona Test Revised. Normality, Semiology and Neuropsychological Pathologies]; Masson: Barcelona, Spain, 2005. [Google Scholar]
- Benedet, M.J.; Alejandre, M.A. Test de Aprendizaje Verbal España Complutense [Spanish Verbal Learning Test]; TEA Ediciones: Madrid, Spain, 1998. [Google Scholar]
- Wechsler, D. WAIS-III.; Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Rey, A. Test de Copia y Reproducción de Memoria de Figuras Geométricas Complejas [Copy and Reproduction of Complex Geometric Figures from Memory Test]; TEA Ediciones: Madrid, Spain, 1999. [Google Scholar]
- Kopelman, M.D.; Wilson, B.A.; Baddeley, A.D. The Autobiographical Memory Interview; Thames Valley Test Company: Bury St. Edmunds, UK, 1990. [Google Scholar]
- Piolino, P.; Coste, C.; Martinelli, P.; Macé, A.L.; Quinette, P.; Guillery-Girard, B.; Belleville, S. Reduced specificity of autobiographical memory and aging: Do the executive and feature binding functions of working memory have a role? Neuropsychologia 2010, 48, 429–440. [Google Scholar] [CrossRef]
- St Jacques, P.L.; Levine, B. Ageing and autobiographical memory for emotional and neutral events. Memory 2007, 15, 129–144. [Google Scholar] [CrossRef] [Green Version]
- Spaan, P.E.; Raaijmakers, J.G.; Jonker, C. Alzheimer’s disease versus normal ageing: A review of the efficiency of clinical and experimental memory measures. J. Clin. Exp. Neuropsychol. 2003, 25, 216–233. [Google Scholar] [CrossRef]
- Donix, M.; Brons, C.; Jurjanz, L.; Poettrich, K.; Winiecki, P.; Holthoff, V.A. Overgenerality of autobiographical memory in people with amnestic mild cognitive impairment and early Alzheimer’s disease. Arch. Clin. Neuropsychol. 2009, 25, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Devanand, D.P.; Pradhaban, G.; Liu, X.; Khandji, A.; De Santi, S.; Segal, S.; Rusinek, H.; Pelton, G.H.; Honig, L.S.; Mayeux, R.; et al. Hippocampal and entorhinal atrophy in mild cognitive impairment prediction of Alzheimer disease. Neurology 2007, 68, 828–836. [Google Scholar] [CrossRef]
- Meléndez, J.C.; Agusti, A.I.; Satorres, E.; Pitarque, A. Are semantic and episodic autobiographical memories influenced by the life period remembered? Comparison of young and older adults. Eur. J. Ageing 2018, 15, 417–424. [Google Scholar] [CrossRef]
- Hirjak, D.; Wolf, R.C.; Remmele, B.; Seidl, U.; Thomann, A.K.; Kubera, K.M.; Schroder, J.; Maier-Hein, K.H.; Thomann, P.A. Hippocampal formation alterations differently contribute to autobiographic memory deficits in mild cognitive impairment and Alzheimer’s disease. Hippocampus 2017, 27, 702–715. [Google Scholar] [CrossRef] [PubMed]
| Variables | a. HOC (n = 26) | b. aMCI (n = 17) | c. AD (n = 16) | Significant Differences (p < 0.05) | ||||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | T1 | T2 | T1 | T2 | |
| Age | 74.53 (4.90) | 77.35 (4.76) | 77.07 (4.54) | a = b = c | ||||
| Gender | 10/16 | 6/11 | 5/11 | |||||
| Education | 2.78 | 2.47 | 2.50 | a = b = c | ||||
| GDS | 1.21 | 1.1 | 2.29 | 2.52 | 3.35 | 3.71 | a < b < c | a < b < c |
| CES-D | 5.28 | 5.9 | 9.01 | 8.71 | 9.21 | 9.14 | a = b = c | a = b = c |
| MMSE | 29.04 | 28.46 | 21.64 | 21.35 | 17.57 | 17.49 | a > b > c | a > b > c |
| VFTC | 23.75 | 22.64 | 13.01 | 11.47 | 7.64 | 5.76 | a > b > c | a > b > c |
| VFTP | 37.78 | 38.71 | 23.41 | 22.29 | 14.64 | 12.35 | a > b > c | a > b > c |
| TAVEC-I | 53.28 | 56.53 | 27.78 | 25.35 | 15.85 | 13.71 | a > b > c | a > b > c |
| TAVEC-D | 12.57 | 12.67 | 3.41 | 3.01 | 1.07 | 0.93 | a > (b = c) | a > (b = c) |
| DSF | 8.18 | 8.07 | 7.46 | 7.21 | 5.64 | 4.98 | (a = b) > c | (a = b) > c |
| DSB | 4.96 | 5.02 | 3.46 | 3.25 | 2.21 | 1.97 | a > (b = c) | a > (b = c) |
| Rey-I | 34.39 | 34.42 | 24.37 | 24.37 | 16.71 | 10.35 | a > b > c | a > b > c |
| Rey-D | 18.35 | 19.67 | 4.53 | 3.81 | 0.71 | 0.42 | a > (b = c) | a > (b = c) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meléndez, J.C.; Pitarque, A.; Delhom, I.; Real, E.; Abella, M.; Satorres, E. A Longitudinal Study of Episodic and Semantic Autobiographical Memory in aMCI and Alzheimer’s Disease Patients. Int. J. Environ. Res. Public Health 2021, 18, 6849. https://doi.org/10.3390/ijerph18136849
Meléndez JC, Pitarque A, Delhom I, Real E, Abella M, Satorres E. A Longitudinal Study of Episodic and Semantic Autobiographical Memory in aMCI and Alzheimer’s Disease Patients. International Journal of Environmental Research and Public Health. 2021; 18(13):6849. https://doi.org/10.3390/ijerph18136849
Chicago/Turabian StyleMeléndez, Juan C., Alfonso Pitarque, Iraida Delhom, Elena Real, Mireia Abella, and Encarnación Satorres. 2021. "A Longitudinal Study of Episodic and Semantic Autobiographical Memory in aMCI and Alzheimer’s Disease Patients" International Journal of Environmental Research and Public Health 18, no. 13: 6849. https://doi.org/10.3390/ijerph18136849
APA StyleMeléndez, J. C., Pitarque, A., Delhom, I., Real, E., Abella, M., & Satorres, E. (2021). A Longitudinal Study of Episodic and Semantic Autobiographical Memory in aMCI and Alzheimer’s Disease Patients. International Journal of Environmental Research and Public Health, 18(13), 6849. https://doi.org/10.3390/ijerph18136849








