Air Pollution and COVID-19: A Possible Dangerous Synergy for Male Fertility
Abstract
1. Introduction
- (A).
- The virus infects multiple tissues, including the testis [3,4] and a meta-analysis of over three million reported global cases showed that male patients with confirmed Coronavirus disease 2019 (COVID-19) have both a higher requirement for intensive treatment unit (ITU) admission and a higher mortality rate than females [5].
- (B).
- The spread of the Coronavirus (COVID-19) during the first pandemic wave primarily affected the elderly population. Epidemiological data from China, Italy, Japan, Singapore, Canada and South Korea showed that susceptibility to infections in individuals under 20 years of age was about half that of adults over 20 years of age and that clinical symptoms occurred in 21% (95% confidence interval: 12–31%) of infections in people aged 10–19 years, rising to 69% (57–82%) of infections in people older than 70 years [6]. Nevertheless, while the majority of cases in most territories around the world settled into an older age profile, positive cases in Hong Kong were concentrated among younger age groups, with the highest incidence of cases reported in the 15–24 age group [7]. During June−August, the COVID-19 pandemic in the United States affected more young people than during January−May 2020 [8]. The shift toward the younger age group happened in all four U.S. census regions, regardless of changes in incidence during this period, and was evidenced in visits for COVID-19-related diseases, positive SARS-CoV-2 RT-PCR test results and confirmed COVID-19 cases. A similar trend was observed in Europe, where the median age of COVID-19 cases decreased from 54 years in the January−May period to 39 years in the June−July period, during which time persons aged 20 to 29 years accounted for the highest proportion of cases (19.5%) [9]. This shift has also been boosted by the spread of the most significant variants of the coronavirus SARS-CoV-2 from September 2020 to March 2021: the UK, Brazilian, South African, Indian variants. These variants exhibit a greater contagiousness with significant prevalence in young people and children [10].
- (C).
- Several studies point out that chronic exposure to air pollutants in the most polluted areas of the world leads to more severe and lethal forms of COVID-19.
- (D).
- The concept of “mild COVID symptoms”, such as dyspnea, chest heaviness, palpitations and fatigue, muscle pains that reduce people’s ability to perform physical or caring activities, needs to be redefined. Indeed, clinicians do not yet know whether prolonged ill-health following asymptomatic or mild or severe symptomatic infection will generate, long-term, moderate, or heavy effects in the lungs [11] or potentially in other tissues, including the testis.
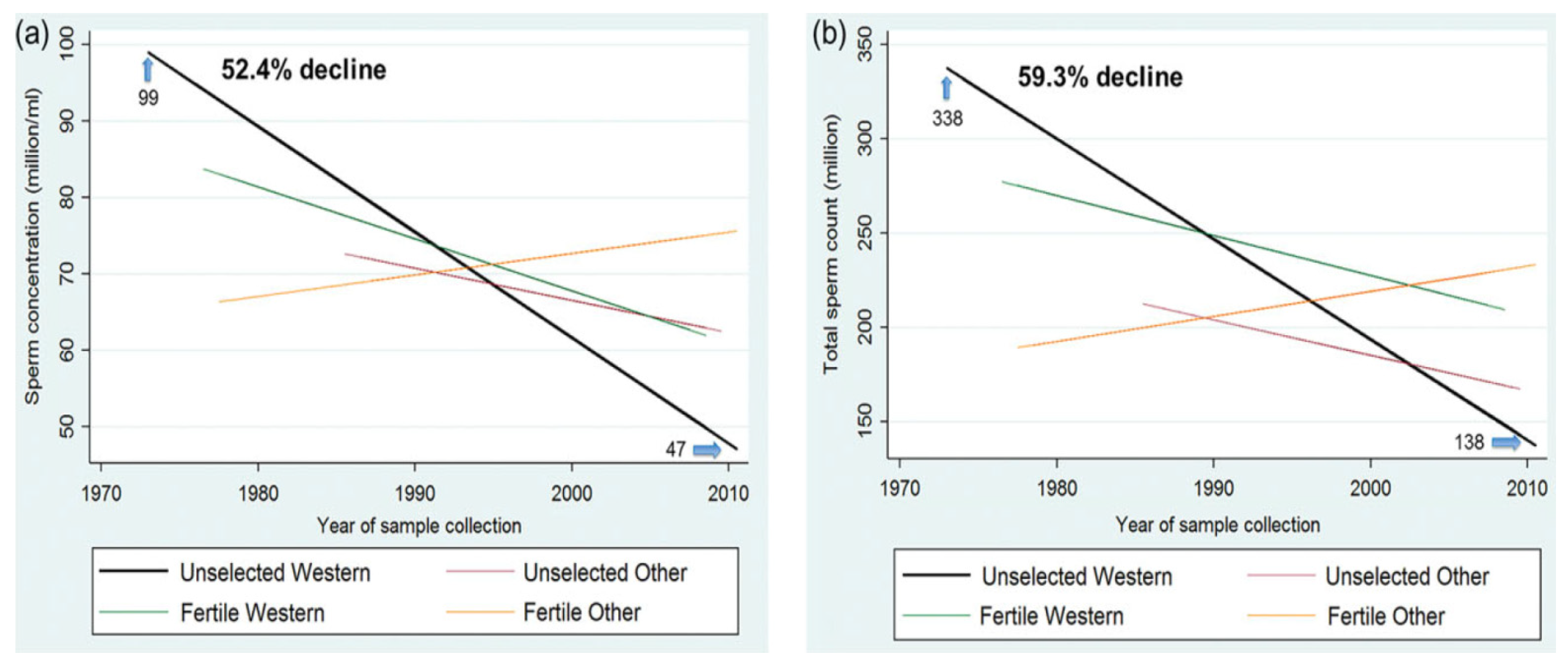
2. Sperm Decline in the World
3. Air Pollution and Impact on Male Fertility
4. SARS-CoV-2 Impact on the Male Reproductive System
5. SARS-CoV-2 and Pollution
6. Epidemiology and Mechanisms of Damage by Air Pollutants
7. Mechanism of Damage of SARS-CoV-2
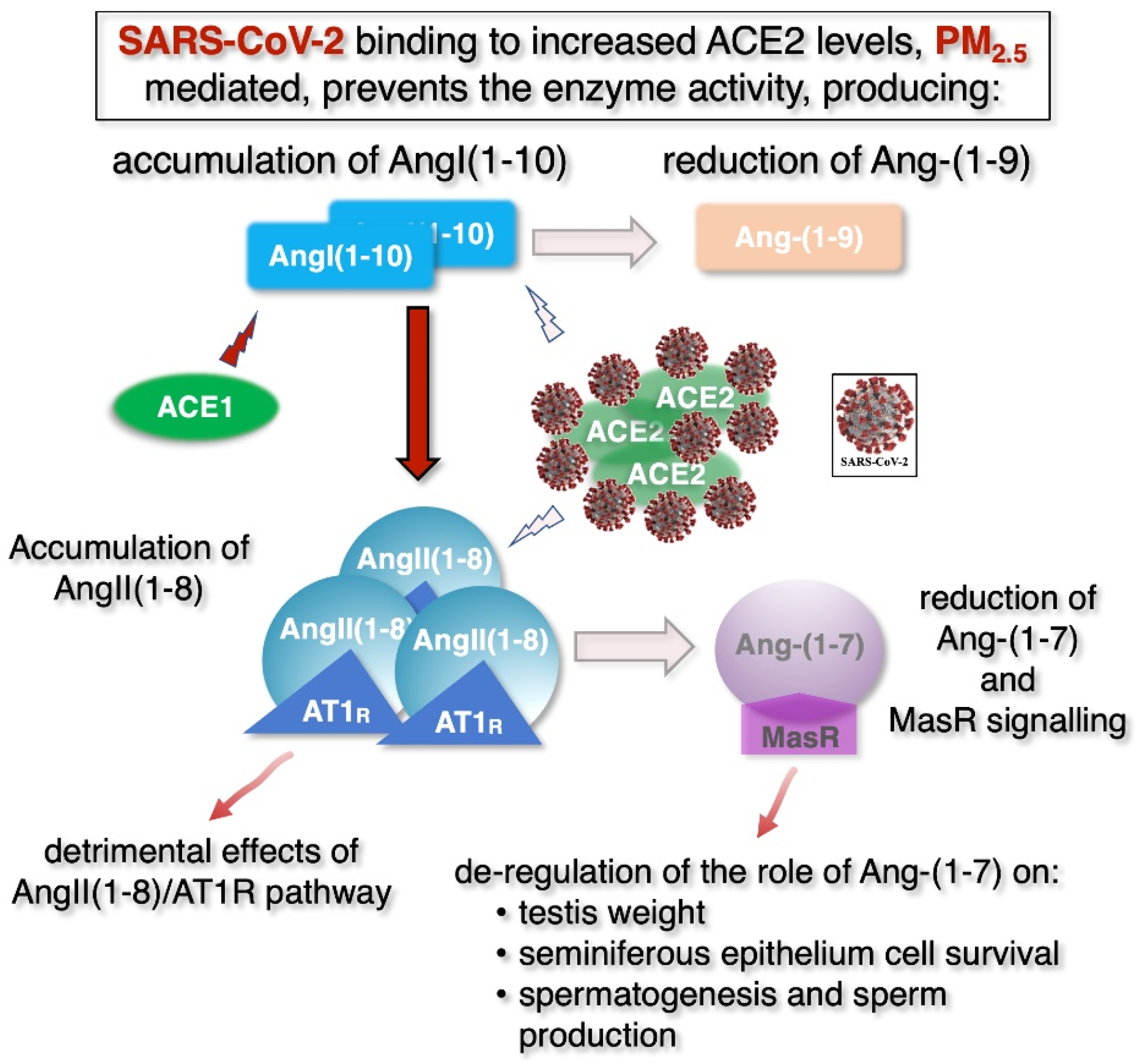
8. Male Fertility: The Possible Dangerous Synergy of Air Pollution and COVID-19
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hui, D.S.C.; Zumla, A. Severe Acute Respiratory Syndrome: Historical, Epidemiologic, and Clinical Features. Infect. Dis. Clin. N. Am. 2019, 33, 869–889. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, C.; Alsafi, Z.; O’Neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World Health Organization Declares Global Emergency: A Review of the 2019 Novel Coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76. [Google Scholar] [CrossRef]
- Batiha, O.; Al-Deeb, T.; Al-Zoubi, E.; Alsharu, E. Impact of COVID-19 and Other Viruses on Reproductive Health. Andrologia 2020, 52, e13791. [Google Scholar] [CrossRef] [PubMed]
- Baughn, L.B.; Sharma, N.; Elhaik, E.; Sekulic, A.; Bryce, A.H.; Fonseca, R. Targeting TMPRSS2 in SARS-CoV-2 Infection. Mayo Clin. Proc. 2020, 95, 1989–1999. [Google Scholar] [CrossRef]
- Peckham, H.; de Gruijter, N.M.; Raine, C.; Radziszewska, A.; Ciurtin, C.; Wedderburn, L.R.; Rosser, E.C.; Webb, K.; Deakin, C.T. Male Sex Identified by Global COVID-19 Meta-Analysis as a Risk Factor for Death and ITU Admission. Nat. Commun. 2020, 11, 6317. [Google Scholar] [CrossRef]
- Davies, N.G.; Klepac, P.; Liu, Y.; Prem, K.; Jit, M.; Eggo, R.M. Age-Dependent Effects in the Transmission and Control of COVID-19 Epidemics. Nat. Med. 2020, 26, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.J.P.; Ganly, R.; Li, Z.; Gietel-Basten, S. Exploring the Young Demographic Profile of COVID-19 Cases in Hong Kong: Evidence from Migration and Travel History Data. PLoS ONE 2020, 15, e0235306. [Google Scholar] [CrossRef] [PubMed]
- Stokes, E.K. Coronavirus Disease 2019 Case Surveillance—United States, January 22–30 May 2020. MMWR Morb. Mortal Wkly Rep. 2020, 69. [Google Scholar] [CrossRef]
- Coronavirus Disease 2019 (COVID-19) in the EU/EEA and the UK—Eleventh Update. Available online: https://www.zva.gov.lv/sites/default/files/inline-files/05_07_covid-19-rapid-risk-assessment-coronavirus-disease-2019-ninth-update-23-april-2020-1.pdf (accessed on 26 March 2021).
- WHO/World Health Organization. Available online: https://www.who.int/ (accessed on 26 March 2021).
- Alwan, N.A. A Negative COVID-19 Test Does Not Mean Recovery. Nature 2020, 584, 170. [Google Scholar] [CrossRef]
- Carlsen, E.; Giwercman, A.; Keiding, N.; Skakkebaek, N.E. Evidence for Decreasing Quality of Semen during Past 50 Years. BMJ 1992, 305, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Mindlis, I.; Pinotti, R.; Swan, S.H. Temporal Trends in Sperm Count: A Systematic Review and Meta-Regression Analysis. Hum. Reprod. Update 2017, 23, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Dutta, S.; Krajewska-Kulak, E. The Disappearing Sperms: Analysis of Reports Published Between 1980 and 2015. Am. J. Mens. Health 2017, 11, 1279–1304. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Negi, M.P.S.; Srivastava, M.; Singh, K.; Rajender, S. Decline in Seminal Quality in Indian Men over the Last 37 Years. Reprod. Biol. Endocrinol. 2018, 16. [Google Scholar] [CrossRef]
- Safarinejad, M.R. Infertility among Couples in a Population-Based Study in Iran: Prevalence and Associated Risk Factors. Int. J. Androl. 2008, 31, 303–314. [Google Scholar] [CrossRef]
- Huang, C.; Li, B.; Xu, K.; Liu, D.; Hu, J.; Yang, Y.; Nie, H.; Fan, L.; Zhu, W. Decline in Semen Quality among 30,636 Young Chinese Men from 2001 to 2015. Fertil. Steril. 2017, 107, 83–88.e2. [Google Scholar] [CrossRef]
- Yuan, H.-F.; Shangguan, H.-F.; Zheng, Y.; Meng, T.-Q.; Xiong, C.-L.; Guan, H.-T. Decline in Semen Concentration of Healthy Chinese Adults: Evidence from 9357 Participants from 2010 to 2015. Asian J. 2018, 20, 379–384. [Google Scholar] [CrossRef]
- Siqueira, S.; Ropelle, A.C.; Nascimento, J.A.A.; Fazano, F.A.T.; Bahamondes, L.G.; Gabiatti, J.R.; Costa-Paiva, L.; Baccaro, L.F. Changes in Seminal Parameters among Brazilian Men between 1995 and 2018. Sci. Rep. 2020, 10, 6430. [Google Scholar] [CrossRef]
- Rehman, S.; Usman, Z.; Rehman, S.; AlDraihem, M.; Rehman, N.; Rehman, I.; Ahmad, G. Endocrine Disrupting Chemicals and Impact on Male Reproductive Health. Transl. Urol. 2018, 7, 490–503. [Google Scholar] [CrossRef]
- Lymperi, S.; Giwercman, A. Endocrine Disruptors and Testicular Function. Metab. Clin. Exp. 2018, 86, 79–90. [Google Scholar] [CrossRef]
- Pironti, C.; Ricciardi, M.; Proto, A.; Bianco, P.M.; Montano, L.; Motta, O. Endocrine-Disrupting Compounds: An Overview on Their Occurrence in the Aquatic Environment and Human Exposure. Water 2021, 13, 1347. [Google Scholar] [CrossRef]
- Deng, Z.; Chen, F.; Zhang, M.; Lan, L.; Qiao, Z.; Cui, Y.; An, J.; Wang, N.; Fan, Z.; Zhao, X.; et al. Association between Air Pollution and Sperm Quality: A Systematic Review and Meta-Analysis. Environ. Pollut. 2016, 208, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Carré, J.; Gatimel, N.; Moreau, J.; Parinaud, J.; Léandri, R. Does Air Pollution Play a Role in Infertility? A Systematic Review. Environ. Health 2017, 16, 82. [Google Scholar] [CrossRef]
- Wei, Y.; Cao, X.-N.; Tang, X.-L.; Shen, L.-J.; Lin, T.; He, D.-W.; Wu, S.-D.; Wei, G.-H. Urban Fine Particulate Matter (PM2.5) Exposure Destroys Blood-Testis Barrier (BTB) Integrity through Excessive ROS-Mediated Autophagy. Toxicol. Mech. Methods 2018, 28, 302–319. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Ma, J.; Bao, W.; Li, J.; Zhou, T.; Cui, X.; Peng, Z.; Zhang, H.; Feng, M.; et al. Inverse Association between Ambient Sulfur Dioxide Exposure and Semen Quality in Wuhan, China. Environ. Sci. Technol. 2017, 51, 12806–12814. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, A. Modeling Exposure-Lag-Response Associations with Distributed Lag Non-Linear Models. Stat. Med. 2014, 33, 881–899. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, J.; Cao, W.; Lu, W.; Zheng, T.; Zeng, Q. Identifying Critical Exposure Windows for Ambient Air Pollution and Semen Quality in Chinese Men. Environ. Res. 2020, 189, 109894. [Google Scholar] [CrossRef]
- Bosco, L.; Notari, T.; Ruvolo, G.; Roccheri, M.C.; Martino, C.; Chiappetta, R.; Carone, D.; Lo Bosco, G.; Carrillo, L.; Raimondo, S.; et al. Sperm DNA Fragmentation: An Early and Reliable Marker of Air Pollution. Environ. Toxicol. Pharmacol. 2018, 58, 243–249. [Google Scholar] [CrossRef]
- Vecoli, C.; Montano, L.; Andreassi, M.G. Environmental Pollutants: Genetic Damage and Epigenetic Changes in Male Germ Cells. Env. Sci. Pollut. Res. Int. 2016, 23, 23339–23348. [Google Scholar] [CrossRef]
- Eisenberg, M.L.; Li, S.; Behr, B.; Pera, R.R.; Cullen, M.R. Relationship between Semen Production and Medical Comorbidity. Fertil. Steril. 2015, 103, 66–71. [Google Scholar] [CrossRef]
- Choy, J.T.; Eisenberg, M.L. Male Infertility as a Window to Health. Fertil. Steril. 2018, 110, 810–814. [Google Scholar] [CrossRef]
- Glazer, C.H.; Bonde, J.P.; Eisenberg, M.L.; Giwercman, A.; Hærvig, K.K.; Rimborg, S.; Vassard, D.; Pinborg, A.; Schmidt, L.; Bräuner, E.V. Male Infertility and Risk of Nonmalignant Chronic Diseases: A Systematic Review of the Epidemiological Evidence. Semin. Reprod. Med. 2017, 35, 282–290. [Google Scholar] [CrossRef]
- Jensen, T.K.; Jacobsen, R.; Christensen, K.; Nielsen, N.C.; Bostofte, E. Good Semen Quality and Life Expectancy: A Cohort Study of 43,277 Men. Am. J. Epidemiol. 2009, 170, 559–565. [Google Scholar] [CrossRef]
- Šrám, R.J.; Binková, B.; Rössner, P.; Rubeš, J.; Topinka, J.; Dejmek, J. Adverse Reproductive Outcomes from Exposure to Environmental Mutagens. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 1999, 428, 203–215. [Google Scholar] [CrossRef]
- Rubes, J.; Selevan, S.G.; Evenson, D.P.; Zudova, D.; Vozdova, M.; Zudova, Z.; Robbins, W.A.; Perreault, S.D. Episodic Air Pollution Is Associated with Increased DNA Fragmentation in Human Sperm without Other Changes in Semen Quality. Hum. Reprod. 2005, 20, 2776–2783. [Google Scholar] [CrossRef] [PubMed]
- Jurewicz, J.; Radwan, M.; Sobala, W.; Polańska, K.; Radwan, P.; Jakubowski, L.; Ulańska, A.; Hanke, W. The Relationship between Exposure to Air Pollution and Sperm Disomy. Environ. Mol. Mutagen. 2015, 56, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, G.; D’Agostino, G.; Mele, E.; Cardito, C.; Esposito, R.; Cimmino, A.; Giarra, A.; Trifuoggi, M.; Raimondo, S.; Notari, T.; et al. Discovery of the Involvement in DNA Oxidative Damage of Human Sperm Nuclear Basic Proteins of Healthy Young Men Living in Polluted Areas. Int. J. Mol. Sci. 2020, 21, 4198. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ren, L.; Wei, J.; Zhang, J.; Zhu, Y.; Li, X.; Jing, L.; Duan, J.; Zhou, X.; Sun, Z. Fine Particle Matter Disrupts the Blood-Testis Barrier by Activating TGF-Β3/P38 MAPK Pathway and Decreasing Testosterone Secretion in Rat. Environ. Toxicol. 2018, 33, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Chen, M.; Wang, X.; Qin, X.; Chen, S.; Qian, Y.; Liu, Z.; Cao, Q.; Ying, Z. Exposure to Concentrated Ambient PM2.5 Compromises Spermatogenesis in a Mouse Model: Role of Suppression of Hypothalamus-Pituitary-Gonads Axis. Toxicol. Sci. 2018, 162, 318–326. [Google Scholar] [CrossRef]
- Aitken, R.J.; Gibb, Z.; Baker, M.A.; Drevet, J.; Gharagozloo, P. Causes and Consequences of Oxidative Stress in Spermatozoa. Reprod. Fertil. Dev. 2016, 28, 1–10. [Google Scholar] [CrossRef]
- Bergamo, P.; Volpe, M.G.; Lorenzetti, S.; Mantovani, A.; Notari, T.; Cocca, E.; Cerullo, S.; Di Stasio, M.; Cerino, P.; Montano, L. Human Semen as an Early, Sensitive Biomarker of Highly Polluted Living Environment in Healthy Men: A Pilot Biomonitoring Study on Trace Elements in Blood and Semen and Their Relationship with Sperm Quality and RedOx Status. Reprod. Toxicol. 2016, 66, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jurewicz, J.; Dziewirska, E.; Radwan, M.; Hanke, W. Air Pollution from Natural and Anthropic Sources and Male Fertility. Reprod. Biol. Endocrinol. 2018, 16, 109. [Google Scholar] [CrossRef] [PubMed]
- Montano, L.; Bergamo, P.; Andreassi, M.G.; Vecoli, C.; Volpe, M.G.; Lorenzetti, S.; Mantovani, A.; Notari, T. The Role of Human Semen for Assessing Environmental Impact on Human Health in Risk Areas: Novels and Early Biomarkers of Environmental Pollution. EcoFoodFertility Project. Reprod. Toxicol. 2017, 72, 44–45. [Google Scholar] [CrossRef]
- Vecoli, C.; Montano, L.; Borghini, A.; Notari, T.; Guglielmino, A.; Mercuri, A.; Turchi, S.; Andreassi, M.G. Effects of Highly Polluted Environment on Sperm Telomere Length: A Pilot Study. Int. J. Mol. Sci. 2017, 18, 1703. [Google Scholar] [CrossRef]
- Nowicka-Bauer, K.; Nixon, B. Molecular Changes Induced by Oxidative Stress That Impair Human Sperm Motility. Antioxid. 2020, 9, 134. [Google Scholar] [CrossRef]
- Lin, C.-I.; Tsai, C.-H.; Sun, Y.-L.; Hsieh, W.-Y.; Lin, Y.-C.; Chen, C.-Y.; Lin, C.-S. Instillation of Particulate Matter 2.5 Induced Acute Lung Injury and Attenuated the Injury Recovery in ACE2 Knockout Mice. Int. J. Biol. Sci. 2018, 14, 253–265. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Chen, H.; Guo, J.; Wang, C.; Luo, F.; Yu, X.; Zhang, W.; Li, J.; Zhao, D.; Xu, D.; Gong, Q.; et al. Clinical Characteristics and Intrauterine Vertical Transmission Potential of COVID-19 Infection in Nine Pregnant Women: A Retrospective Review of Medical Records. Lancet 2020, 395, 809–815. [Google Scholar] [CrossRef]
- Garolla, A.; Pizzol, D.; Bertoldo, A.; Menegazzo, M.; Barzon, L.; Foresta, C. Sperm Viral Infection and Male Infertility: Focus on HBV, HCV, HIV, HPV, HSV, HCMV, and AAV. J. Reprod. Immunol. 2013, 100, 20–29. [Google Scholar] [CrossRef]
- Lyu, Z.; Feng, X.; Li, N.; Zhao, W.; Wei, L.; Chen, Y.; Yang, W.; Ma, H.; Yao, B.; Zhang, K.; et al. Human Papillomavirus in Semen and the Risk for Male Infertility: A Systematic Review and Meta-Analysis. BMC Infect. Dis. 2017, 17, 714. [Google Scholar] [CrossRef]
- Heurich, A.; Hofmann-Winkler, H.; Gierer, S.; Liepold, T.; Jahn, O.; Pöhlmann, S. TMPRSS2 and ADAM17 Cleave ACE2 Differentially and Only Proteolysis by TMPRSS2 Augments Entry Driven by the Severe Acute Respiratory Syndrome Coronavirus Spike Protein. J. Virol. 2014, 88, 1293–1307. [Google Scholar] [CrossRef]
- SARS-CoV-2 Related Proteins—The Human Protein. Atlas. Available online: https://www.proteinatlas.org/humanproteome/sars-cov-2 (accessed on 16 May 2021).
- Sheikhzadeh Hesari, F.; Hosseinzadeh, S.S.; Asl Monadi Sardroud, M.A. Review of COVID-19 and Male Genital Tract. Andrologia 2021, 53. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Lee, M.-S.; Lucht, A.; Chou, F.-P.; Huang, W.; Havighurst, T.C.; Kim, K.; Wang, J.-K.; Antalis, T.M.; Johnson, M.D.; et al. TMPRSS2, a Serine Protease Expressed in the Prostate on the Apical Surface of Luminal Epithelial Cells and Released into Semen in Prostasomes, Is Misregulated in Prostate Cancer Cells. Am J Pathol 2010, 176, 2986–2996. [Google Scholar] [CrossRef]
- Tur-Kaspa, I.; Tur-Kaspa, T.; Hildebrand, G.; Cohen, D. COVID-19 May Affect Male Fertility but Is Not Sexually Transmitted: A Systematic Review. FS Rev. 2021, 2, 140–149. [Google Scholar] [CrossRef]
- Borges, E.; Setti, A.S.; Iaconelli, A.; de Almeida Ferreira Braga, D.P. Current Status of the COVID-19 and Male Reproduction: A Review of the Literature. Andrology 2021. [Google Scholar] [CrossRef]
- Kadihasanoglu, M.; Aktas, S.; Yardimci, E.; Aral, H.; Kadioglu, A. SARS-CoV-2 Pneumonia Affects Male Reproductive Hormone Levels: A Prospective, Cohort Study. J. Sex Med. 2021, 18, 256–264. [Google Scholar] [CrossRef]
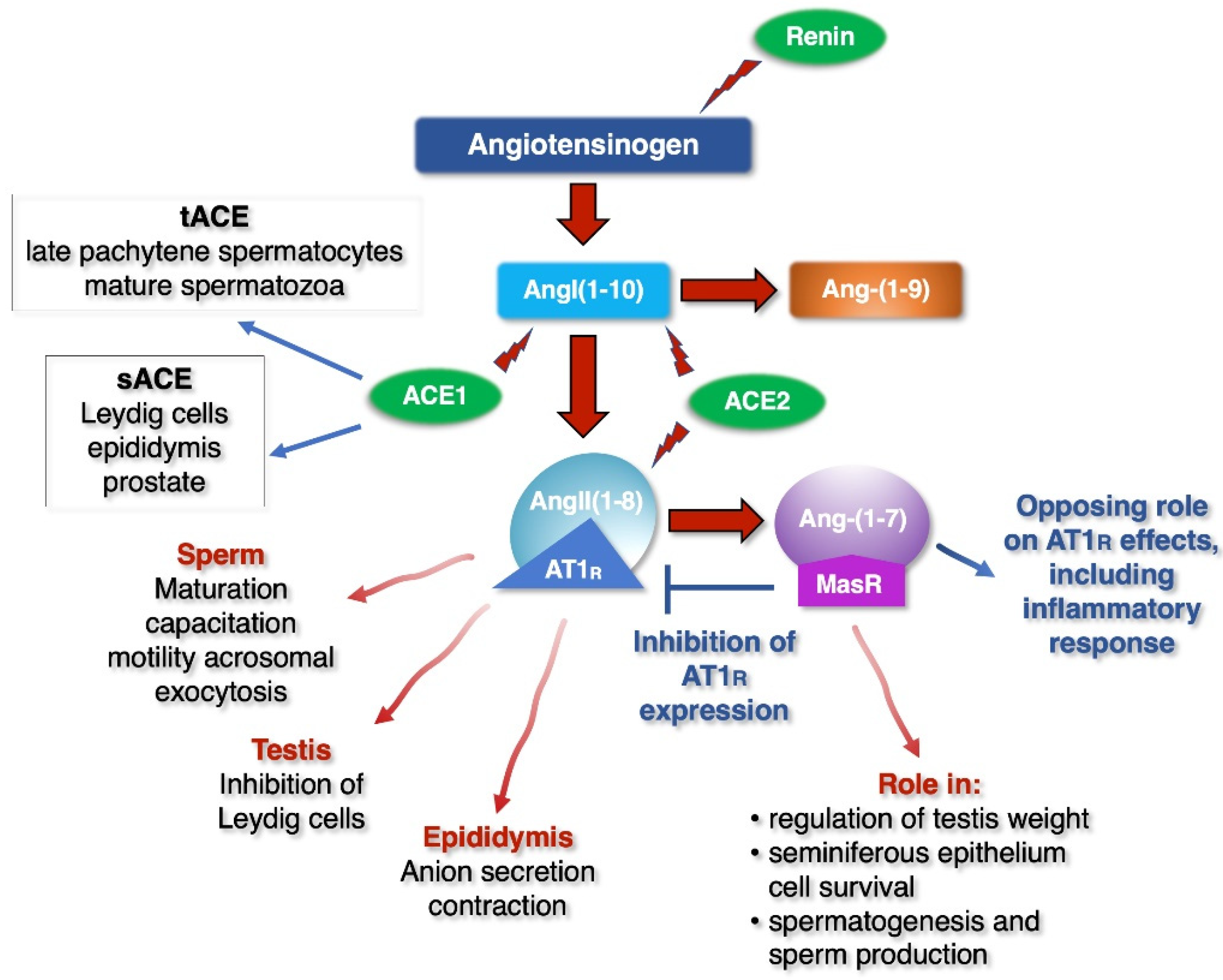
- Leung, P.S.; Sernia, C. The Renin-Angiotensin System and Male Reproduction: New Functions for Old Hormones. J. Mol. Endocrinol. 2003, 30, 263–270. [Google Scholar] [CrossRef]
- Hagaman, J.R.; Moyer, J.S.; Bachman, E.S.; Sibony, M.; Magyar, P.L.; Welch, J.E.; Smithies, O.; Krege, J.H.; O’Brien, D.A. Angiotensin-Converting Enzyme and Male Fertility. Proc Natl Acad Sci USA 1998, 95, 2552–2557. [Google Scholar] [CrossRef]
- Muhanna, D.; Arnipalli, S.R.; Kumar, S.B.; Ziouzenkova, O. Osmotic Adaptation by Na+-Dependent Transporters and ACE2: Correlation with Hemostatic Crisis in COVID-19. Biomedicines 2020, 8, 460. [Google Scholar] [CrossRef]
- Leal, M.C.; Pinheiro, S.V.B.; Ferreira, A.J.; Santos, R.A.S.; Bordoni, L.S.; Alenina, N.; Bader, M.; França, L.R. The Role of Angiotensin-(1–7) Receptor Mas in Spermatogenesis in Mice and Rats. J. Anat. 2009, 214, 736–743. [Google Scholar] [CrossRef]
- Reis, A.B.; Araújo, F.C.; Pereira, V.M.; Dos Reis, A.M.; Santos, R.A.; Reis, F.M. Angiotensin (1-7) and Its Receptor Mas Are Expressed in the Human Testis: Implications for Male Infertility. J. Mol. Histol. 2010, 41, 75–80. [Google Scholar] [CrossRef]
- Valdivia, A.; Cortés, L.; Beitia, M.; Totorikaguena, L.; Agirregoitia, N.; Corcostegui, B.; Casis, L.; Matorras, R.; Irazusta, J.; Agirregoitia, E. Role of Angiotensin-(1-7) via MAS Receptor in Human Sperm Motility and Acrosome Reaction. Reproduction 2020, 159, 241–249. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM Structure of the 2019-NCoV Spike in the Prefusion Conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Qi, L.; Chi, X.; Yang, J.; Wei, X.; Gong, E.; Peh, S.; Gu, J. Orchitis: A Complication of Severe Acute Respiratory Syndrome (SARS). Biol. Reprod. 2006, 74, 410–416. [Google Scholar] [CrossRef]
- Villarreal, L.Y.B.; Brandão, P.E.; Chacón, J.L.; Assayag, M.S.; Maiorka, P.C.; Raffi, P.; Saidenberg, A.B.S.; Jones, R.C.; Ferreira, A.J.P. Orchitis in Roosters with Reduced Fertility Associated with Avian Infectious Bronchitis Virus and Avian Metapneumovirus Infections. Avian Dis. 2007, 51, 900–904. [Google Scholar] [CrossRef][Green Version]
- Yang, M.; Chen, S.; Huang, B.; Zhong, J.-M.; Su, H.; Chen, Y.-J.; Cao, Q.; Ma, L.; He, J.; Li, X.-F.; et al. Pathological Findings in the Testes of COVID-19 Patients: Clinical Implications. Eur. Urol. Focus 2020, 6, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Bendayan, M.; Boitrelle, F. Covid-19 and Impairment of Spermatogenesis: What If Fever Was the Only Cause? EClinicalMedicine 2020, 29. [Google Scholar] [CrossRef]
- Ma, X.; Guan, C.; Chen, R.; Wang, Y.; Feng, S.; Wang, R.; Qu, G.; Zhao, S.; Wang, F.; Wang, X.; et al. Pathological and Molecular Examinations of Postmortem Testis Biopsies Reveal SARS-CoV-2 Infection in the Testis and Spermatogenesis Damage in COVID-19 Patients. Cell Mol. Immunol. 2021, 18, 487–489. [Google Scholar] [CrossRef]
- Hsu, A.L.; Finlinson, A.; Warncke, K. Mechanisms by Which SARS-CoV-2 May Impact Male Fertility. Reprod. Sci. 2021, 28, 332–333. [Google Scholar] [CrossRef]
- Shastri, A.; Wheat, J.; Agrawal, S.; Chaterjee, N.; Pradhan, K.; Goldfinger, M.; Kornblum, N.; Steidl, U.; Verma, A.; Shastri, J. Delayed Clearance of SARS-CoV2 in Male Compared to Female Patients: High ACE2 Expression in Testes Suggests Possible Existence of Gender-Specific Viral Reservoirs. Infectious Diseases (except HIV/AIDS). MedRxiv 2020. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
- Moshrefi, M.; Ghasemi-Esmailabad, S.; Ali, J.; Findikli, N.; Mangoli, E.; Khalili, M.A. The Probable Destructive Mechanisms behind COVID-19 on Male Reproduction System and Fertility. J. Assist. Reprod. Genet 2021. [Google Scholar] [CrossRef] [PubMed]
- Jung, A.; Schuppe, H.-C. Influence of Genital Heat Stress on Semen Quality in Humans. Andrologia 2007, 39, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Illiano, E.; Trama, F.; Costantini, E. Could COVID-19 Have an Impact on Male Fertility? Andrologia 2020, 52, e13654. [Google Scholar] [CrossRef] [PubMed]
- Younis, J.S.; Abassi, Z.; Skorecki, K. Is There an Impact of the COVID-19 Pandemic on Male Fertility? The ACE2 Connection. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E878–E880. [Google Scholar] [CrossRef] [PubMed]
- Archana, S.S.; Selvaraju, S.; Binsila, B.K.; Arangasamy, A.; Krawetz, S.A. Immune Regulatory Molecules as Modifiers of Semen and Fertility: A Review. Mol. Reprod. Dev. 2019, 86, 1485–1504. [Google Scholar] [CrossRef]
- Mahmudpour, M.; Roozbeh, J.; Keshavarz, M.; Farrokhi, S.; Nabipour, I. COVID-19 Cytokine Storm: The Anger of Inflammation. Cytokine 2020, 133, 155151. [Google Scholar] [CrossRef]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative Stress and Sperm Function: A Systematic Review on Evaluation and Management. Arab. J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef]
- Sengupta, P.; Dutta, S. Does SARS-CoV-2 Infection Cause Sperm DNA Fragmentation? Possible Link with Oxidative Stress. Eur. J. Contracept. Reprod. Health Care 2020, 25, 405–406. [Google Scholar] [CrossRef]
- Sun, J. The Hypothesis That SARS-CoV-2 Affects Male Reproductive Ability by Regulating Autophagy. Med Hypotheses 2020, 143, 110083. [Google Scholar] [CrossRef]
- Effect of SARS-CoV-2 Infection upon Male Gonadal Function: A Single Center-Based Study/MedRxiv. Available online: https://www.medrxiv.org/content/10.1101/2020.03.21.20037267v2 (accessed on 28 November 2020).
- Mussa, B.M.; Srivastava, A.; Verberne, A.J.M. COVID-19 and Neurological Impairment: Hypothalamic Circuits and Beyond. Viruses 2021, 13, 498. [Google Scholar] [CrossRef]
- Selvaraj, K.; Ravichandran, S.; Krishnan, S.; Radhakrishnan, R.K.; Manickam, N.; Kandasamy, M. Testicular Atrophy and Hypothalamic Pathology in COVID-19: Possibility of the Incidence of Male Infertility and HPG Axis Abnormalities. Reprod. Sci. 2021, 7, 1–8. [Google Scholar] [CrossRef]
- Kang, X.; Xie, Q.; Zhou, X.; Li, F.; Huang, J.; Liu, D.; Huang, T. Effects of Hepatitis B Virus S Protein Exposure on Sperm Membrane Integrity and Functions. PLoS ONE 2012, 7, e33471. [Google Scholar] [CrossRef]
- Root-Bernstein, R.S.; Hobbs, S.H. Does HIV “Piggyback” on CD4-like Surface Proteins of Sperm, Viruses, and Bacteria? Implications for Co-Transmission, Cellular Tropism and the Induction of Autoimmunity in AIDS. J. Theor. Biol. 1993, 160, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Scofield, V.L.; Clisham, R.; Bandyopadhyay, L.; Gladstone, P.; Zamboni, L.; Raghupathy, R. Binding of Sperm to Somatic Cells via HLA-DR. Modulation by Sulfated Carbohydrates. J. Immunol. 1992, 148, 1718–1724. [Google Scholar] [PubMed]
- Bart, S.M.; Cohen, C.; Dye, J.M.; Shorter, J.; Bates, P. Enhancement of Ebola Virus Infection by Seminal Amyloid Fibrils. Proc Natl. Acad. Sci. USA 2018, 115, 7410–7415. [Google Scholar] [CrossRef] [PubMed]
- Dejucq, N.; Jégou, B. Viruses in the Mammalian Male Genital Tract and Their Effects on the Reproductive System. Microbiol. Mol. Biol. Rev. 2001, 65, 208–231. [Google Scholar] [CrossRef]
- Pan, F.; Xiao, X.; Guo, J.; Song, Y.; Li, H.; Patel, D.P.; Spivak, A.M.; Alukal, J.P.; Zhang, X.; Xiong, C.; et al. No Evidence of Severe Acute Respiratory Syndrome–Coronavirus 2 in Semen of Males Recovering from Coronavirus Disease 2019. Fertil. Steril. 2020, 113, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Holtmann, N.; Edimiris, P.; Andree, M.; Doehmen, C.; Baston-Buest, D.; Adams, O.; Kruessel, J.-S.; Bielfeld, A.P. Assessment of SARS-CoV-2 in Human Semen—a Cohort Study. Fertil. Steril. 2020, 114, 233–238. [Google Scholar] [CrossRef]
- Li, D.; Jin, M.; Bao, P.; Zhao, W.; Zhang, S. Clinical Characteristics and Results of Semen Tests Among Men with Coronavirus Disease 2019. JAMA Netw. Open 2020, 3, e208292. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, S.M.; Bendayan, M.; Huyghe, É.; Soufir, J.C.; Amar, E.; El Osta, R.; Plotton, I.; Delalande, C.; Perrin, J.; Leroy, C.; et al. COVID-19 and Andrology: Recommendations of the French-Speaking Society of Andrology (Société d’Andrologie de Langue Française SALF). Basic Clin. Androl. 2020, 30. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Tang, W.; Zhang, L.; Chen, W.; Yan, Z.; Yuan, P.; Yang, M.; Kong, S.; Yan, L.; et al. Single-Cell Transcriptome Analysis of the Novel Coronavirus (SARS-CoV-2) Associated Gene ACE2 Expression in Normal and Non-Obstructive Azoospermia (NOA) Human Male Testes. Sci. China Life Sci. 2020, 63, 1006–1015. [Google Scholar] [CrossRef]
- Salute, M. Della Covid-19 Weekly Monitoring, Report of August 10–16. Available online: http://www.salute.gov.it/portale/news/p3_2_1_1_1.jsp?lingua=italiano&menu=notizie&p=dalministero&id=5028 (accessed on 28 November 2020).
- Domingo, J.L.; Rovira, J. Effects of Air Pollutants on the Transmission and Severity of Respiratory Viral Infections. Env. Res. 2020, 187, 109650. [Google Scholar] [CrossRef] [PubMed]
- Frontera, A.; Martin, C.; Vlachos, K.; Sgubin, G. Regional Air Pollution Persistence Links to COVID-19 Infection Zoning. J. Infect. 2020, 81, 318–356. [Google Scholar] [CrossRef] [PubMed]
- Bontempi, E. Commercial Exchanges Instead of Air Pollution as Possible Origin of COVID-19 Initial Diffusion Phase in Italy: More Efforts Are Necessary to Address Interdisciplinary Research. Environ. Res. 2020, 188, 109775. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Yee-tak Fong, d.; Zhu, b.; Karlberg, j. Environmental Factors on the SARS Epidemic: Air Temperature, Passage of Time and Multiplicative Effect of Hospital Infection. Epidemiol. Infect. 2006, 134, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Liang, P.; Wu, C.; Wang, G.; Xu, Q.; Xiong, X.; Wang, T.; Zolfo, M.; Segata, N.; Qin, H.; et al. Longitudinal Survey of Microbiome Associated with Particulate Matter in a Megacity. Genome Biol. 2020, 21, 55. [Google Scholar] [CrossRef]
- Tung, N.T.; Cheng, P.-C.; Chi, K.-H.; Hsiao, T.-C.; Jones, T.; BéruBé, K.; Ho, K.-F.; Chuang, H.-C. Particulate Matter and SARS-CoV-2: A Possible Model of COVID-19 Transmission. Sci. Total. Env. 2021, 750, 141532. [Google Scholar] [CrossRef]
- Ram, K.; Thakur, R.C.; Singh, D.K.; Kawamura, K.; Shimouchi, A.; Sekine, Y.; Nishimura, H.; Singh, S.K.; Pavuluri, C.M.; Singh, R.S.; et al. Why Airborne Transmission Hasn’t Been Conclusive in Case of COVID-19? An Atmospheric Science Perspective. Sci. Total Environ. 2021, 773, 145525. [Google Scholar] [CrossRef]
- Wu, X.; Nethery, R.C.; Sabath, M.B.; Braun, D.; Dominici, F. Air Pollution and COVID-19 Mortality in the United States: Strengths and Limitations of an Ecological Regression Analysis. Sci. Adv. 2020, 6. [Google Scholar] [CrossRef]
- Ali, N.; Islam, F. The Effects of Air Pollution on COVID-19 Infection and Mortality—A Review on Recent Evidence. Front. Public Health 2020, 8. [Google Scholar] [CrossRef]
- Comunian, S.; Dongo, D.; Milani, C.; Palestini, P. Air Pollution and COVID-19: The Role of Particulate Matter in the Spread and Increase of COVID-19′s Morbidity and Mortality. Int. J. Environ. Res. Public Health 2020, 17, 4487. [Google Scholar] [CrossRef]
- Mukherjee, S.; Boral, S.; Siddiqi, H.; Mishra, A.; Meikap, B.C. Present Cum Future of SARS-CoV-2 Virus and Its Associated Control of Virus-Laden Air Pollutants Leading to Potential Environmental Threat–A Global Review. J. Environ. Chem. Eng. 2021, 9, 104973. [Google Scholar] [CrossRef] [PubMed]
- Martelletti, L.; Martelletti, P. Air Pollution and the Novel Covid-19 Disease: A Putative Disease Risk Factor. SN Compr. Clin. Med. 2020, 2, 383–387. [Google Scholar] [CrossRef]
- Fattorini, D.; Regoli, F. Role of the Chronic Air Pollution Levels in the Covid-19 Outbreak Risk in Italy. Environ. Pollut. 2020, 264, 114732. [Google Scholar] [CrossRef]
- Ogen, Y. Assessing Nitrogen Dioxide (NO2) Levels as a Contributing Factor to Coronavirus (COVID-19) Fatality. Sci. Total Environ. 2020, 726, 138605. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Z.-F.; Froines, J.; Zhao, J.; Wang, H.; Yu, S.-Z.; Detels, R. Air Pollution and Case Fatality of SARS in the People’s Republic of China: An Ecologic Study. Env. Health 2003, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Pan, J.; Liu, Z.; Meng, X.; Wang, W.; Kan, H.; Wang, W. Temporal Association between Particulate Matter Pollution and Case Fatality Rate of COVID-19 in Wuhan. Environ. Res. 2020, 189, 109941. [Google Scholar] [CrossRef]
- Sarkodie, S.A.; Owusu, P.A. Global Effect of City-to-City Air Pollution, Health Conditions, Climatic & Socio-Economic Factors on COVID-19 Pandemic. Sci. Total Env. 2021, 778, 146394. [Google Scholar] [CrossRef]
- Pozzer, A.; Dominici, F.; Haines, A.; Witt, C.; Münzel, T.; Lelieveld, J. Regional and Global Contributions of Air Pollution to Risk of Death from COVID-19. Cardiovasc. Res. 2020, 116, 2247–2253. [Google Scholar] [CrossRef] [PubMed]
- Brini, I.; Bhiri, S.; Ijaz, M.; Bouguila, J.; Nouri-Merchaoui, S.; Boughammoura, L.; Sboui, H.; Hannachi, N.; Boukadida, J. Temporal and Climate Characteristics of Respiratory Syncytial Virus Bronchiolitis in Neonates and Children in Sousse, Tunisia, during a 13-Year Surveillance. Env. Sci. Pollut. Res. 2020, 27, 23379–23389. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Zhao, B. Estimating Mortality Derived from Indoor Exposure to Particles of Outdoor Origin. PLoS ONE 2015, 10, e0124238. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.S.; Key, T.J.; Norat, T.; Scoccianti, C.; Cecchini, M.; Berrino, F.; Boutron-Ruault, M.-C.; Espina, C.; Leitzmann, M.; Powers, H.; et al. European Code against Cancer 4th Edition: Obesity, Body Fatness and Cancer. Cancer Epidemiol. 2015, 39 (Suppl. 1), S34–S45. [Google Scholar] [CrossRef]
- Hadei, M.; Naddafi, K. Cardiovascular Effects of Airborne Particulate Matter: A Review of Rodent Model Studies. Chemosphere 2020, 242, 125204. [Google Scholar] [CrossRef] [PubMed]
- Tsai, D.-H.; Riediker, M.; Berchet, A.; Paccaud, F.; Waeber, G.; Vollenweider, P.; Bochud, M. Effects of Short- and Long-Term Exposures to Particulate Matter on Inflammatory Marker Levels in the General Population. Env. Sci. Pollut. Res. Int. 2019, 26, 19697–19704. [Google Scholar] [CrossRef]
- Araujo, J.A.; Barajas, B.; Kleinman, M.; Wang, X.; Bennett, B.J.; Gong, K.W.; Navab, M.; Harkema, J.; Sioutas, C.; Lusis, A.J.; et al. Ambient Particulate Pollutants in the Ultrafine Range Promote Early Atherosclerosis and Systemic Oxidative Stress. Circ. Res. 2008, 102, 589–596. [Google Scholar] [CrossRef]
- Horan, T.S.; Marre, A.; Hassold, T.; Lawson, C.; Hunt, P.A. Germline and Reproductive Tract Effects Intensify in Male Mice with Successive Generations of Estrogenic Exposure. PLoS Genet. 2017, 13, e1006885. [Google Scholar] [CrossRef]
- Lettieri, G.; Marra, F.; Moriello, C.; Prisco, M.; Notari, T.; Trifuoggi, M.; Giarra, A.; Bosco, L.; Montano, L.; Piscopo, M. Molecular Alterations in Spermatozoa of a Family Case Living in the Land of Fires. A First Look at Possible Transgenerational Effects of Pollutants. Int. J. Mol. Sci. 2020, 21, 6710. [Google Scholar] [CrossRef]
- Post, C.M.; Boule, L.A.; Burke, C.G.; O’Dell, C.T.; Winans, B.; Lawrence, B.P. The Ancestral Environment Shapes Antiviral CD8+ T Cell Responses across Generations. iScience 2019, 20, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Soubry, A. POHaD: Why We Should Study Future Fathers. Env. Epigenet. 2018, 4. [Google Scholar] [CrossRef]
- Xavier, M.J.; Roman, S.D.; Aitken, R.J.; Nixon, B. Transgenerational Inheritance: How Impacts to the Epigenetic and Genetic Information of Parents Affect Offspring Health. Hum. Reprod. Update 2019, 25, 518–540. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.D.; Simmons, C.P.; Thanh, T.T.; Hien, V.M.; Smith, G.J.D.; Chau, T.N.B.; Hoang, D.M.; Van Vinh Chau, N.; Khanh, T.H.; Dong, V.C.; et al. Fatal Outcome of Human Influenza A (H5N1) Is Associated with High Viral Load and Hypercytokinemia. Nat. Med. 2006, 12, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Short, K.R.; Veeris, R.; Leijten, L.M.; van den Brand, J.M.; Jong, V.L.; Stittelaar, K.; Osterhaus, A.D.M.E.; Andeweg, A.; van Riel, D. Proinflammatory Cytokine Responses in Extra-Respiratory Tissues During Severe Influenza. J. Infect. Dis. 2017, 216, 829–833. [Google Scholar] [CrossRef]
- Van Riel, D.; Verdijk, R.; Kuiken, T. The Olfactory Nerve: A Shortcut for Influenza and Other Viral Diseases into the Central Nervous System. J. Pathol. 2015, 235, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, M.; Kortekaas, J.; Herden, C.; Hoffmann, B.; Tappe, D.; Trebst, C.; Griffin, D.E.; Brindle, H.E.; Solomon, T.; Brown, A.S.; et al. Neurotropic Virus Infections as the Cause of Immediate and Delayed Neuropathology. Acta Neuropathol. 2016, 131, 159–184. [Google Scholar] [CrossRef] [PubMed]
- Probert, L. TNF and Its Receptors in the CNS: The Essential, the Desirable and the Deleterious Effects. Neuroscience 2015, 302, 2–22. [Google Scholar] [CrossRef]
- Libbey, J.E.; Kennett, N.J.; Wilcox, K.S.; White, H.S.; Fujinami, R.S. Interleukin-6, Produced by Resident Cells of the Central Nervous System and Infiltrating Cells, Contributes to the Development of Seizures Following Viral Infection. J. Virol. 2011, 85, 6913–6922. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Unfer, V.; Garzon, S.; Bizzarri, M. Role of Inositol to Improve Surfactant Functions and Reduce IL-6 Levels: A Potential Adjuvant Strategy for SARS-CoV-2 Pneumonia? Med. Hypotheses 2020, 144, 110262. [Google Scholar] [CrossRef]
- Bizzarri, M.; Laganà, A.S.; Aragona, D.; Unfer, V. Inositol and Pulmonary Function. Could Myo-Inositol Treatment Downregulate Inflammation and Cytokine Release Syndrome in SARS-CoV-2? Eur. Rev. Med. Pharm. Sci. 2020, 24, 3426–3432. [Google Scholar] [CrossRef]
- Craigie, R.; Bushman, F.D. HIV DNA Integration. Cold Spring Harb. Perspect. Med. 2012, 2, a006890. [Google Scholar] [CrossRef]
- Caso, F.; Costa, L.; Ruscitti, P.; Navarini, L.; Del Puente, A.; Giacomelli, R.; Scarpa, R. Could Sars-Coronavirus-2 Trigger Autoimmune and/or Autoinflammatory Mechanisms in Genetically Predisposed Subjects? Autoimmun. Rev. 2020, 19, 102524. [Google Scholar] [CrossRef] [PubMed]
- Masters, S.L.; Simon, A.; Aksentijevich, I.; Kastner, D.L. Horror Autoinflammaticus: The Molecular Pathophysiology of Autoinflammatory Disease. Annu. Rev. Immunol. 2009, 27, 621–668. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- El-Maadawy, E.A.; Talaat, R.M.; Ahmed, M.M.; El-Shenawy, S.Z. Interleukin-6 Promotor Gene Polymorphisms and Susceptibility to Chronic Hepatitis B Virus in Egyptians. Hum. Immunol. 2019, 80, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Saper, V.E.; Chen, G.; Deutsch, G.H.; Guillerman, R.P.; Birgmeier, J.; Jagadeesh, K.; Canna, S.; Schulert, G.; Deterding, R.; Xu, J.; et al. Emergent High Fatality Lung Disease in Systemic Juvenile Arthritis. Ann. Rheum. Dis. 2019, 78, 1722–1731. [Google Scholar] [CrossRef]
- Schulert, G.S.; Yasin, S.; Carey, B.; Chalk, C.; Do, T.; Schapiro, A.H.; Husami, A.; Watts, A.; Brunner, H.I.; Huggins, J.; et al. Systemic Juvenile Idiopathic Arthritis-Associated Lung Disease: Characterization and Risk Factors. Arthritis Rheumatol. 2019, 71, 1943–1954. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider Cytokine Storm Syndromes and Immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brito-Zerón, P.; López-Guillermo, A.; Khamashta, M.A.; Bosch, X. Adult Haemophagocytic Syndrome. Lancet 2014, 383, 1503–1516. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.-M.; Yu, X.-H.; Tang, S.-L.; Tang, C.-K. Coronavirus Disease 2019 (COVID-19): Current Status and Future Perspectives. Int. J. Antimicrob. Agents 2020, 55, 105951. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial Cell Infection and Endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Zhou, N.; Cui, Z.; Yang, S.; Han, X.; Chen, G.; Zhou, Z.; Zhai, C.; Ma, M.; Li, L.; Cai, M.; et al. Air Pollution and Decreased Semen Quality: A Comparative Study of Chongqing Urban and Rural Areas. Environ. Pollut. 2014, 187, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Nordkap, L.; Joensen, U.N.; Blomberg Jensen, M.; Jørgensen, N. Regional Differences and Temporal Trends in Male Reproductive Health Disorders: Semen Quality May Be a Sensitive Marker of Environmental Exposures. Mol. Cell. Endocrinol. 2012, 355, 221–230. [Google Scholar] [CrossRef]
- Le Moal, J.; Rolland, M.; Goria, S.; Wagner, V.; De Crouy-Chanel, P.; Rigou, A.; De Mouzon, J.; Royère, D. Semen Quality Trends in French Regions Are Consistent with a Global Change in Environmental Exposure. Reproduction 2014, 147, 567–574. [Google Scholar] [CrossRef]
- Casas, M.; Chevrier, C.; Hond, E.D.; Fernandez, M.F.; Pierik, F.; Philippat, C.; Slama, R.; Toft, G.; Vandentorren, S.; Wilhelm, M.; et al. Exposure to Brominated Flame Retardants, Perfluorinated Compounds, Phthalates and Phenols in European Birth Cohorts: ENRIECO Evaluation, First Human Biomonitoring Results, and Recommendations. Int. J. Hyg. Environ. Health 2013, 216, 230–242. [Google Scholar] [CrossRef]
- Bergman, A.; Heindel, J.J.; Kasten, T.; Kidd, K.A.; Jobling, S.; Neira, M.; Zoeller, R.T.; Becher, G.; Bjerregaard, P.; Bornman, R.; et al. The Impact of Endocrine Disruption: A Consensus Statement on the State of the Science. Environ. Health Perspect. 2013, 121, A104–A106. [Google Scholar] [CrossRef] [PubMed]
- Sajadi, M.M.; Habibzadeh, P.; Vintzileos, A.; Shokouhi, S.; Miralles-Wilhelm, F.; Amoroso, A. Temperature, Humidity, and Latitude Analysis to Estimate Potential Spread and Seasonality of Coronavirus Disease 2019 (COVID-19). JAMA Netw. Open 2020, 3. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, G.; Mollo, V.; Ambrosino, A.; Caccavale, F.; Troisi, J.; Febbraio, F.; Piscopo, M. Molecular Effects of Copper on the Reproductive System of Mytilus Galloprovincialis. Mol. Reprod. Dev. 2019, 86, 1357–1368. [Google Scholar] [CrossRef]
- Piscopo, M.; Notariale, R.; Rabbito, D.; Ausió, J.; Olanrewaju, O.S.; Guerriero, G. Mytilus Galloprovincialis (Lamarck, 1819) Spermatozoa: Hsp70 Expression and Protamine-like Protein Property Studies. Env. Sci. Pollut. Res. Int. 2018, 25, 12957–12966. [Google Scholar] [CrossRef]
- Piscopo, M.; Trifuoggi, M.; Notariale, R.; Labar, S.; Troisi, J.; Giarra, A.; Rabbito, D.; Puoti, R.; de Benedictis, D.; Brundo, M.V.; et al. Protamine-like Proteins’ Analysis as an Emerging Biotechnique for Cadmium Impact Assessment on Male Mollusk Mytilus Galloprovincialis (Lamarck 1819). Acta Biochim. Pol. 2018, 65, 259–267. [Google Scholar] [CrossRef] [PubMed]
- De Guglielmo, V.; Puoti, R.; Notariale, R.; Maresca, V.; Ausió, J.; Troisi, J.; Verrillo, M.; Basile, A.; Febbraio, F.; Piscopo, M. Alterations in the Properties of Sperm Protamine-like II Protein after Exposure of Mytilus Galloprovincialis (Lamarck 1819) to Sub-Toxic Doses of Cadmium. Ecotoxicol. Environ. Saf. 2019, 169, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Montano, L.; Donato, F.; Bianco, P.M.; Lettieri, G.; Guglielmino, A.; Motta, O.; Bonapace, I.M.; Piscopo, M. Semen Quality as a Potential Susceptibility Indicator to SARS-CoV-2 Insults in Polluted Areas. Environ. Sci. Pollut. Res. 2021. [CrossRef]
- Montano, L.; Bonapace, I.M.; Donato, F.; Bianco, P.M.; Guglielmino, A.; Piscopo, M. Semen Quality as a Potential Susceptibility Indicator to SARS-CoV-2 Insults in Polluted Areas. Preprints 2020, 2020060314. [Google Scholar] [CrossRef]
- Borro, M.; Di Girolamo, P.; Gentile, G.; De Luca, O.; Preissner, R.; Marcolongo, A.; Ferracuti, S.; Simmaco, M. Evidence-Based Considerations Exploring Relations between SARS-CoV-2 Pandemic and Air Pollution: Involvement of PM2.5-Mediated Up-Regulation of the Viral Receptor ACE-2. Int. J. Environ. Res. Public. Health 2020, 17, 5573. [Google Scholar] [CrossRef]
- Frontera, A.; Cianfanelli, L.; Vlachos, K.; Landoni, G.; Cremona, G. Severe Air Pollution Links to Higher Mortality in COVID-19 Patients: The “Double-Hit” Hypothesis. J. Infect. 2020, 81, 255–259. [Google Scholar] [CrossRef]
- Shen, Q.; Xiao, X.; Aierken, A.; Yue, W.; Wu, X.; Liao, M.; Hua, J. The ACE2 Expression in Sertoli Cells and Germ Cells May Cause Male Reproductive Disorder after SARS-CoV-2 Infection. J. Cell Mol. Med. 2020. [Google Scholar] [CrossRef]
- Glencross, D.A.; Ho, T.-R.; Camiña, N.; Hawrylowicz, C.M.; Pfeffer, P.E. Air Pollution and Its Effects on the Immune System. Free Radic. Biol. Med. 2020, 151, 56–68. [Google Scholar] [CrossRef]
- Breton, C.V.; Song, A.Y.; Xiao, J.; Kim, S.-J.; Mehta, H.H.; Wan, J.; Yen, K.; Sioutas, C.; Lurmann, F.; Xue, S.; et al. Effects of Air Pollution on Mitochondrial Function, Mitochondrial DNA Methylation, and Mitochondrial Peptide Expression. Mitochondrion 2019, 46, 22–29. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montano, L.; Donato, F.; Bianco, P.M.; Lettieri, G.; Guglielmino, A.; Motta, O.; Bonapace, I.M.; Piscopo, M. Air Pollution and COVID-19: A Possible Dangerous Synergy for Male Fertility. Int. J. Environ. Res. Public Health 2021, 18, 6846. https://doi.org/10.3390/ijerph18136846
Montano L, Donato F, Bianco PM, Lettieri G, Guglielmino A, Motta O, Bonapace IM, Piscopo M. Air Pollution and COVID-19: A Possible Dangerous Synergy for Male Fertility. International Journal of Environmental Research and Public Health. 2021; 18(13):6846. https://doi.org/10.3390/ijerph18136846
Chicago/Turabian StyleMontano, Luigi, Francesco Donato, Pietro Massimiliano Bianco, Gennaro Lettieri, Antonino Guglielmino, Oriana Motta, Ian Marc Bonapace, and Marina Piscopo. 2021. "Air Pollution and COVID-19: A Possible Dangerous Synergy for Male Fertility" International Journal of Environmental Research and Public Health 18, no. 13: 6846. https://doi.org/10.3390/ijerph18136846
APA StyleMontano, L., Donato, F., Bianco, P. M., Lettieri, G., Guglielmino, A., Motta, O., Bonapace, I. M., & Piscopo, M. (2021). Air Pollution and COVID-19: A Possible Dangerous Synergy for Male Fertility. International Journal of Environmental Research and Public Health, 18(13), 6846. https://doi.org/10.3390/ijerph18136846









