Abstract
This article investigates the effects of continuous positive airway pressure (CPAP) on hearing impairment in sensorineural hearing loss (SNHL) patients with sleep-disordered breathing (SDB). This retrospective and observational study took place from September 2016 to February 2021, accumulating 77 subjects with SNHL and SDB (60.7 ± 11.1 years). Of which, 28 received CPAP treatment (63.0 ± 8.5 years). In our methodology, hearing thresholds at low, medium, high, and average frequencies are assessed by pure-tone audiometry at baseline (BL), three (3 m), six (6 m), and 12 (12 m) months. Our results show that the BL of at least three frequencies in all subjects is positively associated with old age, males, smoking, alcohol, coronary artery disease, hypertension, and apnea-hypopnea index [AHI] (all p < 0.05). Moreover, low, medium, and average frequencies are negatively correlated at CPAP-6 m (−5.60 ± 2.33, −5.82 ± 2.56, and −5.10 ± 2.26 dB; all p < 0.05) and CPAP-12 m (−7.97 ± 2.74, −8.15 ± 2.35, and −6.67 ± 2.37 dB; all p < 0.01) against corresponding measures of CPAP-BL. High, medium, and average frequencies positively correlated with age (p < 0.001 for high and average frequencies and <0.01 for medium frequencies). We conclude that in SNHL patients with SDB, hearing thresholds at low and medium frequencies improves under CPAP use after six months, which persists at least to the end of one year.
1. Introduction
Sensorineural hearing loss (SNHL), resulting from cochlear hair cell loss and associated auditory nerve dysfunction [1], is one of the most common geriatric disorders. Hearing loss resulting in impaired verbal communication could be linked to social isolation, anxiety, depression, cognitive impairment, and reduced quality of life [2,3]. SNHL has been cited [4] in recent years as the third leading cause of disability worldwide by the World Health Organization. SNHL is associated with genetic [5], vascular, hormonal, toxic, neoplastic, infectious, traumatic, and iatrogenic factors, as well as smoking and alcohol habits, systemic diseases, environmental noise, and socioeconomic status [6,7,8]. SNHL patients present with degenerative changes (at least partially caused by aging), earlier in the central than the peripheral auditory system [9,10]. It is crucial to find a way to slow or prevent the worsening of hearing impairment in geriatric individuals to maintain their quality of life.
Sleep-disordered breathing (SDB) is characterized by repetitive complete or partial upper airway obstruction during sleep, possibly together with symptoms of excessive daytime somnolence, decreased alertness, memory deficits, and depression [11]. It affects 24% of middle-aged males and 9% of middle-aged females [12], and is more prevalent in the elderly. The repeated episodes of apnea and hypopnea may trigger intermittent hypoxia, promote reactive oxygen species production and the inflammatory cascade, and advance vascular endothelial injury. SDB is also associated with hypertension, cardiovascular diseases, neurocognitive disorder, peripheral neuropathy [13], and even ocular neuropathy [14]. Therefore, growing evidence has unsurprisingly supported the relationship between SDB and hearing impairment [15,16,17,18]. SDB specifically, rather than simply snoring [19,20], has been independently associated with hearing impairment [17], sudden SNHL [21], and central auditory dysfunction in the elderly [22]. Continuous positive airway pressure (CPAP), the primary therapy for SDB, has been shown to prevent intermittent hypoxemia, recover sleep quality, and improve health-related quality of life [23]. Intriguingly, uvulopalatopharyngoplasty may reverse [24] the low prevalence of transient-evoked otoacoustic emissions and reduced distortion product otoacoustic emissions, which both are associated with SDB. When CPAP was used for six months, it was shown to mitigate reduced hearing ability from Meniere disease [25]. Based on pathophysiological rationales, CPAP therapy for a specific period of time should potentially have a beneficial effect on the hearing impairment of patients with SNHL and SDB. Nevertheless, the effect of CPAP therapy on hearing impairment in SNHL patients has not been investigated.
We hypothesized that for patients with subacute or chronic hearing impairment and coexistent SDB, CPAP therapy would improve the pure tone audiometry threshold, regardless of age and SDB severity.
2. Materials and Methods
2.1. Study Design
This retrospective and observational study was conducted at a single hospital from September 2016 to February 2021. The study was approved by the Institutional Review Board of the Tsao-tun Psychiatric Center, Taichung, Taiwan (No. 108002).
2.2. Study Population
One hundred forty-eight consecutive patients applying for social subsidies and/or clinical services with chronic hearing impairment or sudden hearing loss were recruited from our Otorhinolaryngology clinics (Figure 1).

Figure 1.
Flow chart showing recruitment methodology. Hearing thresholds were measured by pure-tone audiometry (PTA) at baseline (BL), three (3 m), six (6 m), and 12 months (12 m) in the 77 patients. Abbreviations: SNHL, sensorineural hearing loss; SDB, sleep-disordered breathing; PSG, sleep polysomnography study; CPAP continuous positive airway pressure therapy group.
The inclusion criteria were SNHL patients of both gender who were over 18 years old, had symptoms of SDB, and were willing to participate in our study. SNHL was defined as hearing thresholds ≥ 25 decibels (dB) uni- or bilaterally at any tested frequency by pure-tone audiometry (PTA). Air conduction and bone conduction were calculated, and conductive hearing impairment was excluded from our study. Symptoms of SDB [11,26] include loud snoring, excessive daytime sleepiness, nocturia, sleep interruption, and mouth breathing when sleeping.
The exclusion criteria were (1) a history of any condition potentially leading to conductive hearing loss [27] (external or middle ear infectious disease, eardrum perforation, otosclerosis, external or middle ear tumor, or ossicular chain dislocation); the air-bone gap was also calculated to exclude conductive hearing impairment precisely. (2) Hearing loss induced by ototoxic drugs or sudden deafness under high dose steroid treatment or hyperbaric oxygen (HBO) therapy. We excluded those etiologies of SNHL which might be affected by the medications [27,28], such as steroid, aminoglycosides, cisplatin, furosemide, and so on. (3) Disorders or administration of medications or substances that could affect neurocognitive function; or (4) current or prior SDB treatment with CPAP or surgery.
2.3. Protocol
Information related to anthropometric measurements, seated blood pressure, current medications, general health data, hearing-related history, sleep symptoms, as well as otorhinolaryngological examinations and services, time points for otoscopy, and audiometry testing, and the night of the polysomnography (PSG) study were obtained on the chart. Relevant covariates [5,29] of interest for SNHL and SDB were also recorded: age, gender, body mass index, cigarette smoking, alcohol consumption, coronary artery disease, hypertension, diabetes mellitus, hypercholesterolemia, depression, sudden deafness, and the duration of hearing impairment. PTA was conducted in the otorhinolaryngological lab within three days after the first clinical visit (baseline measure; BL). The PSG study was performed in the sleep lab within 10 days for those who fulfilled the inclusion criteria, and had no exclusion criteria.
All eligible subjects were further separated into two groups, CPAP and non-CPAP treatment. The former included those with good primary adherence to CPAP and regular medications for personal, systemic illnesses based on the chart record. However, regardless of receiving the CPAP treatment or not, all the patients received sleep hygiene education and adequate medications [30], such as nasal spray for nasal obstruction before sleep and regular regimens for individual systemic diseases. In all participants, PTA hearing thresholds were re-evaluated at three (3 m), six (6 m), and 12 (12 m) months. CPAP compliance was defined as using the therapy for at least an average of four hours per night, as determined by the card reader.
2.4. Pure-Tone Audiometry (PTA)
PTA was performed to determine individual hearing thresholds using an annually calibrated GSI 61 audiometer (Grason-Stadler, Eden Prairie, MN 55344, USA) equipped with earphones (Telephonics, Farmingdale, New York, NY, USA). Hearing thresholds were tested at six pure-tone frequencies (250, 500, 1000, 2000, 4000, and 8000 Hz for air conduction). A qualified audiologist blinded to the treatment assignments performed all the auditory testing procedures in a double-walled, soundproof booth. An ear specialist (Dr. Chi) supervised all data collection and interpretation of audiometry studies.
To quantify the severity of hearing impairment, the hearing threshold frequencies of interest were categorized as low frequency threshold (low; 250 Hz), medium frequency (medium; mean of 500, 1000, and 2000 Hz), high frequency (high; mean of 4000 and 8000 Hz), and the mean of these six frequencies (average). To avoid bias, hearing threshold measurements were always taken from the same ear, selected by the likelihood of responding to treatment. If the patient had sudden unilateral SNHL, we chose that affected ear after two weeks of standard steroid treatment.
To further distinguish the effect of time, all subjects received three follow-up pure-tone audiometry assessments at three, six, and 12 months (3 m, 6 m, and 12 m) in addition to a baseline (BL).
2.5. Polysomnography (PSG)
Participants were asked to abstain from caffeinated food and drinks after lunchtime on the day of sleep testing as our routine. They arrived at the sleep center at 9:00 p.m. to complete anthropometric assessments, questionnaires for chronic psychophysiological illnesses, and physical exams. Sleep was evaluated by PSG between 10:30 p.m. and 6:00 a.m. As in our previous studies [31], the procedures, skilled professionals, electroencephalography dependent sleep staging, and scoring criteria of the sleep variables were defined according to the 2007 American Academy of Sleep Medicine Manual guidelines. The sleep variables [32] consisted of sleep efficiency, total sleep time, apnea-hypopnea index (AHI), arousal index, lowest oxygen saturation (miniSpO2), percentage of the total period for which oxygen saturation was less than 90%, rapid eye movement (REM), non-REM (stage 1, 2, and slow-wave sleep). Hypopnea was defined as a 30 to 90% reduction in oronasal flow for at least 10 s, followed by at least a 3% decrease in arterial oxygen saturation. A sleep study was considered effective if there was more than four hours of recording and more than three hours of total sleep time recorded.
2.6. Continuous Positive Airway Pressure (CPAP)
The patients tried CPAP therapy at home with either the Auto A-Flex (Philips Respironics System One; “AutoSet”, Murrysville, PA, USA) or the SleepStyle Auto (Fisher and Paykel Healthcare SleepStyle, Auckland, New Zealand) machines with a pressure range of 4 to 20 cm H2O. For automatic pressure titration during initiative therapy in the first week, the pressure was set at 4 to 15 cm H2O and then adjusted to an individualized optimal constant pressure based on a review of CPAP use. All subjects were provided humidifiers to avoid upper airway dryness. A nasal or full-face mask was used based on user preference, final titrated pressure, and bed partner’s observation. Site staff contacted users three times within the first week after starting CPAP to encourage usage adherence and manage relevant problems.
2.7. Illnesses and Their Definitions
The covariates for hearing impairment [5,6,7,8,9,10] of interest consisted of age, gender, obesity, and the presence of various chronic psychophysiological illnesses. Coronary artery disease and depression were defined using self-reported history and current regular medicines. Hypertension was defined [33] as having a systolic or diastolic blood pressure ≥ 140/90 mmHg or current use of antihypertensive medications. Diabetes mellitus was defined [34,35] as having a fasting glucose ≥ 126 mg/dL, non-fasting glucose ≥ 200 mg/dL, hemoglobin A1C ≥ 6.5%, or current diabetes medication use. Hypercholesterolemia [36] was defined as having a low-density lipoprotein cholesterol ≥ 160 mg/dL, high-density lipoprotein cholesterol ≤ 40 mg/dL, triglycerides ≥ 200 mg/dL, or current relevant medication use.
2.8. Statistical Analyses
All continuous data were presented as mean ± standard deviation. The Student’s t-test and chi-squared test were used for continuous and categorical data, respectively, to compare the differences in various parameters between the non-CPAPand CPAP groups. Univariate analyses to examine relationships between continuous or categorical variables and baseline hearing thresholds of various frequencies were conducted using Pearson’s correlation and one-way analysis of variance (ANOVA) [37], respectively.
The traditional statistics and a generalized estimating equation (GEE) [38,39] were used to examine changes or relationships between hearing thresholds in the pure tone audiometry at various frequencies and the participants’ data in the CPAP group with adjusting the covariate factors.
Statistical analysis was performed using SPSS 25.0 (Armonk, NY, USA: IBM Corp). Two-sided p values < 0.05 were considered statistically significant.
3. Results
One hundred forty-eight consecutive patients with SNHL and SDB symptoms were initially selected, 71 patients were excluded because of incomplete chart data (Figure 1). Of these, 48 individuals were excluded, due to loss follow-up and an incomplete PTA or PSG examination, 19 were not SDB sufferers, and four had prior upper airway surgeries. Seventy-seven subjects were recruited to our observational study.
Of these 77 subjects (Table 1; 54.5% male, 60.7 ± 11.1 years, 25.2 ± 4.2 kg/m2, 28.0 ± 22.8 events/hour in AHI, 82.5 ± 8.7% in miniSpO2, 32.9 ± 19.6, 32.3 ± 22.4, 54.4 ± 24.6 and 40.2 ± 21.3 dB in BL hearing thresholds at low, medium, high, and average frequencies, respectively), 28 received CPAP therapy with good compliance throughout the study. The remaining participants were in the non-CPAP treatment group (N = 49). However, because the patients could decide to receive CPAP treatment or not by themselves, most of the patients who had mild to moderate SDB didn’t want to receive CPAP treatment. That’s why there was a significant difference in AHI between these two groups. Additionally, there was a significant difference in BMI, miniSpO2, gender, cigarette smoking, alcoholic drinking, and hypertension in these two groups. Those factors were correlated with the severity of sleep apnea. Because of the severity of SDB and some parameters not being similar, it was not possible to compare them as intervention and control groups. Therefore, to discuss the correlation between PTA threshold and CPAP treatment in SNHL patients, we discussed CPAP groups (N = 28) only. There was attrition of participants in 6 M and 12 M because of poor CPAP compliance. To be more specific, there were still 28 participates in three months. In six months, one participate was excluded because of poor CPAP compliance (N = 27). In twelve months, four participates were excluded, due to the same reason (N = 24).

Table 1.
Differences between non-CPAP group and CPAP group in anthropometric variables, comorbid diseases, history and severity of hearing impairment, and severity of sleep-disordered breathing.
To discuss the relationship between covariate factors and hearing thresholds, among 77 participants, the covariate factors of SNHL (Old age, male, smoking, alcohol, coronary artery disease, hypertension, and AHI; all p < 0.05), were positively associated with baseline hearing thresholds at more than three frequencies among all subjects (Table 2). Interestingly, high, medium, and average frequencies positively correlated with age (p < 0.001 for high and average frequencies and <0.01 for medium frequencies). Concerning AHI, it was related to low, medium, high, and average frequencies all. Remarkably, depression seemed to be related to thresholds at medium frequency. However, the sample size was too small (N = 5) to conduct further statistical analyses. In Table 2, the mean ± SD was shown in the nominal scale, and Pearson product-moment correlation coefficient (r) was calculated in the ratio scale.

Table 2.
The relationship between baseline measures of hearing thresholds at various frequencies and anthropometric variables, comorbid diseases, hearing-specific history, and severities of hearing impairment and sleep-disordered breathing by univariate analysis.
To reiterate, to avoid potential bias in the CPAP and non-CPAP groups with significant differences, the PTA thresholds were analyzed in the CPAP group (N = 28) only at low, medium, high, and average frequencies in the baseline, 3 months, 6 months, and 12 months after CPAP treatment. The traditional statistics and a generalized estimating equation (GEE) were used to examine changes or relationships between hearing thresholds in the pure tone audiometry at various frequencies and the participants’ data in the CPAP group with adjusting the covariate factors. (Table 3 and Figure 2). The covariate factors, which were showed in Table 2, such as age, gender, smoking, alcohol, coronary artery disease, hypertension, and AHI, were adjusted. Low, medium, and average frequencies were negatively associated at CPAP-6 m (−5.60 ± 2.33, −5.82 ± 2.56, and −5.10 ± 2.26 dB, respectively; all p < 0.05) and CPAP-12 m (−7.97 ± 2.74, −8.15 ± 2.35, and −6.67 ± 2.37 dB, respectively; all p < 0.01) with corresponding measures of CPAP-BL).

Table 3.
The effect of CPAP therapy on hearing thresholds by a generalized estimating equation with adjusting potential confounding factors.
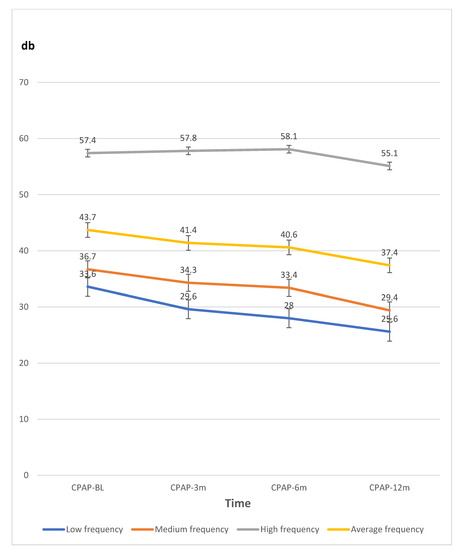
Figure 2.
The line graph data of hearing thresholds by pure tone audiometry (dB) at various frequencies measured at baseline (BL), three (3 m), six (6 m), and 12 months (12 m) in the CPAP therapy group. Abbreviations: CPAP continuous positive airway pressure therapy; db, decibels.
4. Discussion
Our study shows that for SNHL patients with coexistent SDB, good compliance with CPAP treatment for 6 to 12 months may improve pure tone audiometry threshold at low, medium, and average frequencies, with adjusting age, gender, smoking, alcohol, coronary artery disease, hypertension, and AHI. While low, medium, high, and average frequencies were significantly correlated with AHI, which indicated the severity of SDB; high, medium, and average frequencies were significantly related to age. We postulate that treating SDB might reverse hearing deficit on the apex and middle turns of the cochlea, rather than the basal turn, which is mainly worsened by the aging process [40].
Indeed, SDB had been reported associated with hearing problems and possibly sudden deafness [41]. Although the lowest oxyhemoglobin saturation at night sleep possibly the factor is affecting hearing impairment [16], AHI exhibited dose-responsively associated with hearing impairment at various frequencies in a previous huge study (~14,000 participants) [17]. High frequency hearing loss was detected in adults with severe SDB with prolonged latencies of waves I and V of auditory brainstem evoked response [42]. Another longitudinal observational study [43] of 6797 individuals, showed that SDB increases the odds of a future hearing impairment by 21%. Some authors also noted that hearing threshold values in the patients with SDB were significantly higher compared to the control group at 500, 1000, 2000, 4000, and 8000 Hz in both ears [44]. The finding of the present study that AHI is the only factor beyond age-related to the hearing threshold after adjusting for all anthropometric factors, is quite consistent with previous cross-sectional studies [17,42]. However, it is still a question if treating SDB effectively could attenuate the hearing impairment in SNHL patients, or even improve the hearing threshold. The results of our study might offer the preliminary data in this issue, though no consistent effect was found in another small retrospective study (n = 12 and 10 in CPAP and non-CPAP groups, respectively) [45]. Nevertheless, future randomized control study is warranted.
A murine model of four-week long sleep apnea [46] leaded to extensive damage over the outer hair cells of the organ of Corti. The community study mentioned above [17] has shown that patients with moderate to severe SDB had hearing impairment, particularly at high frequency, regardless of age. The moderate level of SDB affected high frequency hearing abilities, whereas the severe level affected all hearing functions [18]. High frequency hearing loss in severe SDB was caused by cochlear damage [15]. Severe OSA is independently associated with cochlear function impairment in patients with no significant co-morbidities [47]. All these findings might support hair cells in the basal turn of the cochlea being more susceptible to SDB-associated oxidative stress [48], probably due to the lower activity of glutathione-related antioxidant enzymes [49]. In contrast, the present finding that high frequency hearing impairment is independently correlated with age [40,49]. We inferred that in addition to aging effects, the cochlear base in our patients was damaged irreversibly by SDB, while the cochlear apex and middle turns (corresponding to low frequency hearing) were relatively reversible after 6 to 12 month long CPAP therapy.
To date, there are no effective treatments for SNHL [50], with the exception of hearing aids, cochlear implants, and glucocorticoid or hyperbaric oxygen treatment for sudden SNHL. Insulin-like growth factor-1 (IGF-1) [50] recently emerged as a possible factor for treatment of SNHL because it depletes by the mutation of a corresponding human homozygous gene resulting SNHL, negatively correlated with SDB-related hypoxemia [51], and increased in secretion after three months and six months of CPAP use for SDB patients. In our study, the improvement by CPAP treatment of hearing at 6 m and 12 m may possibly reflect restorations of the IGF-1 axis, as well as the apical cochlear function. Undoubtedly, further relevant clinical studies are needed to prove.
Auditory evoked potentials would be affected at somewhat higher centers than at the cochlear level [52]. The prefrontal cortex [53] also appears vulnerable to sleep fragmentation and intermittent hypoxia caused by SDB. Apart from higher pure-tone thresholds, severe SDB subjects were likely to experience ponto-mesencephalopathy [54], higher speech recognition thresholds, and lower speech discrimination ability [18]. In fact, hearing thresholds, speech recognition thresholds, and speech discrimination disability were all positively correlated with AHI [22] and desaturation index [55], and negatively correlated with minimum oxygen saturation [18]. Further clinical and basic science studies focusing on the mechanism are warranted.
Limitations
The single-hospital data collection of a relatively small number of Taiwanese subjects with a wide range of body mass index and AHI limits its generalizability. The significant difference in demographic measures between CPAP and non-CPAP groups could lead to a bias. Thereafter, the case series study was chosen for data processing. Further randomized control cohort studies of a larger number of participants were expected in the future. Additionally, the reliability of self-reported lifestyles and the presence of illnesses might bias the results.
Some strengths are also noteworthy: Sleep and hearing impairment measured by standardized protocols [56,57], accurate CPAP follow-up, findings adjusted for confounding variables, the duration of treatment, and follow-up longer than most previous studies.
5. Conclusions
In summary, CPAP application to SNHL patients with comorbid SDB improved hearing threshold at low, medium, and average frequencies after three months, reliably present at six months, and the effects persist throughout the first year. CPAP therapy is a potentially important treatment for individuals with SNHL and SDB. Further investigation is required to determine whether early application of CPAP alone or combined with topical administration of any neurotrophic factor, such as IGF-1, can serve as a valid strategy for preventive or therapeutic purposes.
Author Contributions
J.C.-Y.C., conception of study design, data interpretation, literature review, statistical analysis, figure and table creation, manuscript writing, and critical revision; S.-D.L., conception of study design, data interpretation, and critical revision; R.-J.H., conception of study design, data interpretation, and critical revision; C.-H.L., conception of study design, data interpretation, statistical analysis, figure and table creation, critical revision, and study supervision; S.Y.L., conception of study design, data interpretation, manuscript writing, and critical revision; Y.-J.T., conception of study design, data interpretation, and critical revision; P.-H.F., conception of study design, data interpretation, and critical revision; H.T., conception of study design, data interpretation, literature review, statistical analysis, figure and table creation, manuscript writing, critical revision, and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Taichung Hospital, Ministry of Health and Welfare, Taichung, Taiwan, grant number: TAIC107-04.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of the Institutional Review Board of the Tsao-tun Psychiatric Center, Taichung, Taiwan (protocol code: No. 108002; Date of approval: 21 March 2019).
Informed Consent Statement
Patient consent was waived due to the retrospective study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available because we will try to do a retrospective cohort study in the future.
Acknowledgments
The authors would like to thank all colleagues who contributed to this study. My thanks go to Juen-Haur Hwang and Cheng-Yu Lin for help with the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Peter, C.; Weber, S.K. Anatomy and Physiology of Hearing. In Byron J. Bailey’s Head and Neck Surgery. Otolaryngology, 4th ed; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Lin, F.R. Hearing Loss and Cognition Among Older Adults in the United States. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2011, 66, 1131–1136. [Google Scholar] [CrossRef]
- Cacioppo, J.T.; Hawkley, L.C.; Norman, G.J.; Berntson, G.G. Social isolation. Ann. N. Y. Acad. Sci. 2011, 1231, 17–22. [Google Scholar] [CrossRef]
- Yang, Z.; Cosetti, M. Safety and outcomes of cochlear implantation in the elderly: A review of recent literature. J. Otol. 2016, 11, 1–6. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Wu, C.-C.; Hsu, C.-J.; Hwang, J.-H.; Liu, T.-C. The grainyhead-like 2 gene (GRHL2) single nucleotide polymorphism is not associated with age-related hearing impairment in Han Chinese. Laryngoscope 2011, 121, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Arts, H.A. Sensorineural Hearing Loss in Adults. In Cummings Otolaryngology Head and Neck Surgery, 4th ed.; Mosby: St. Louis, MO, USA, 2004. [Google Scholar]
- Guimaraes, P.; Frisina, S.T.; Mapes, F.; Tadros, S.F.; Frisina, D.R.; Frisina, R.D. Progestin negatively affects hearing in aged women. Proc. Natl. Acad. Sci. USA 2006, 103, 14246–14249. [Google Scholar] [CrossRef] [PubMed]
- Van Eyken, E.; Van Camp, G.; Van Laer, L. The complexity of age-related hearing impairment: Contributing environmental and genetic factors. Audiol. Neurotol. 2007, 12, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Snell, K.B.; Frisina, D.R. Relationships among age-related differences in gap detection and word recognition. J. Acoust. Soc. Am. 2000, 107, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-H.; Li, C.-W.; Wu, C.W.; Chen, J.-H.; Liu, T.-C. Aging Effects on the Activation of the Auditory Cortex during Binaural Speech Listening in White Noise: An fMRI Study. Audiol. Neurotol. 2007, 12, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Flemons, W.; Tsai, W. Quality of life consequences of sleep-disordered breathing. J. Allergy Clin. Immunol. 1997, 99, S750–S756. [Google Scholar] [CrossRef]
- Young, T.; Palta, M.; Dempsey, J.; Skatrud, J.; Weber, S.; Badr, S. The Occurrence of Sleep-Disordered Breathing among Middle-Aged Adults. N. Engl. J. Med. 1993, 328, 1230–1235. [Google Scholar] [CrossRef]
- Gu, X.; Luo, X.; Wang, X.; Tang, J.; Yang, W.; Cai, Z. The correlation between obstructive sleep apnea and diabetic neuropathy: A meta-analysis. Prim. Care Diabetes 2018, 12, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Mentek, M.; Aptel, F.; Godin-Ribuot, D.; Tamisier, R.; Pepin, J.-L.; Chiquet, C. Diseases of the retina and the optic nerve associated with obstructive sleep apnea. Sleep Med. Rev. 2018, 38, 113–130. [Google Scholar] [CrossRef]
- Martines, F.; Ballacchino, A.; Sireci, F.; Mucia, M.; La Mattina, E.; Rizzo, S.; Salvago, P. Audiologic profile of OSAS and simple snoring patients: The effect of chronic nocturnal intermittent hypoxia on auditory function. Eur. Arch. Oto-Rhino-Laryngol. 2015, 273, 1419–1424. [Google Scholar] [CrossRef]
- Seo, Y.J.; Park, S.Y.; Chung, H.J.; Kim, C.-H.; Lee, J.-G.; Kim, S.H.; Cho, H.-J. Lowest Oxyhemoglobin Saturation May Be an Independent Factor Influencing Auditory Function in Severe Obstructive Sleep Apnea. J. Clin. Sleep Med. 2016, 12, 653–658. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chopra, A.; Jung, M.; Kaplan, R.C.; Appel, D.W.; Dinces, E.A.; Dhar, S.; Zee, P.C.; Gonzalez, F.; Lee, D.J.; Ramos, A.R.; et al. Sleep Apnea Is Associated with Hearing Impairment: The Hispanic Community Health Study/Study of Latinos. J. Clin. Sleep Med. 2016, 12, 719–726. [Google Scholar] [CrossRef]
- Kayabasi, S.; Hizli, O.; Yildirim, G. The association between obstructive sleep apnea and hearing loss: A cross-sectional analysis. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 2215–2221. [Google Scholar] [CrossRef]
- Spinosi, M.C.; D’Amico, F.; Passàli, G.; Cingi, C.; Rodriguez, H.; Passàli, D. Hearing loss in mild OSAS and simple snoring patients. Otolaryngol. Polska 2017, 71, 11–15. [Google Scholar] [CrossRef]
- Hoffstein, V.; Haight, J.; Cole, P.; Zamel, N. Does Snoring Contribute to Presbycusis? Am. J. Respir. Crit. Care Med. 1999, 159, 1351–1354. [Google Scholar] [CrossRef]
- Sheu, J.-J.; Wu, C.-S.; Lin, H.-C. Association between Obstructive Sleep Apnea and Sudden Sensorineural Hearing Loss: A Population-Based Case-Control Study. Arch. Otolaryngol. Head Neck Surg. 2012, 138, 55–59. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Chen, J.-C.; Hsu, C.-J.; Liu, T.-C. Association of Obstructive Sleep Apnea and Auditory Dysfunctions in Older Subjects. Otolaryngol. Neck Surg. 2010, 144, 114–119. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Wang, R.; Gleason, K.J.; Lewis, E.F.; Quan, S.F.; Toth, C.M.; Morrical, M.; Rueschman, M.; Weng, J.; Ware, J.H.; et al. Effect of Continuous Positive Airway Pressure Treatment on Health-Related Quality of Life and Sleepiness in High Cardiovascular Risk Individuals with Sleep Apnea: Best Apnea Interventions for Research (BestAIR) Trial. Sleep 2017, 40. [Google Scholar] [CrossRef]
- She, W.D.; Zhang, Q.; Chen, F.; Jiang, P.; Wang, J. Peri-uvulopalatopharyngoplasty otoacoustic emissions in patients with obstructive sleep apnea-hypopnea syndrome. Zhonghua Er Bi Yan Hou Ke Za Zhi 2004, 39, 48–51. [Google Scholar]
- Nakayama, M.; Masuda, A.; Ando, K.B.; Arima, S.; Kabaya, K.; Inagaki, A.; Nakamura, Y.; Suzuki, M.; Brodie, H.; Diaz, R.C.; et al. A Pilot Study on the Efficacy of Continuous Positive Airway Pressure on the Manifestations of Meniere’s Disease in Patients with Concomitant Obstructive Sleep Apnea Syndrome. J. Clin. Sleep Med. 2015, 11, 1101–1107. [Google Scholar] [CrossRef]
- Kauta, S.R.; Keenan, B.T.; Goldberg, L.; Schwab, R.J. Diagnosis and Treatment of Sleep Disordered Breathing in Hospitalized Cardiac Patients: A Reduction in 30-Day Hospital Readmission Rates. J. Clin. Sleep Med. 2014, 10, 1051–1059. [Google Scholar] [CrossRef]
- Zahnert, T. The Differential Diagnosis of Hearing Loss. Dtsch. Aerzteblatt Online 2011, 108, 433–444. [Google Scholar] [CrossRef]
- Leung, M.A.; Flaherty, A.; Zhang, J.A.; Hara, J.; Barber, W.; Burgess, L. Sudden Sensorineural Hearing Loss: Primary Care Update. Hawaii J. Med. Public Health 2016, 75, 172–174. [Google Scholar]
- Punjabi, N.M.; Caffo, B.S.; Goodwin, J.L.; Gottlieb, D.J.; Newman, A.B.; O’Connor, G.T.; Rapoport, D.M.; Redline, S.; Resnick, H.E.; Robbins, J.A.; et al. Sleep-Disordered Breathing and Mortality: A Prospective Cohort Study. PLoS Med. 2009, 6, e1000132. [Google Scholar] [CrossRef]
- Irish, L.A.; Kline, C.; Gunn, H.E.; Buysse, D.J.; Hall, M.H. The role of sleep hygiene in promoting public health: A review of empirical evidence. Sleep Med. Rev. 2015, 22, 23–36. [Google Scholar] [CrossRef]
- Ting, H.; Lo, H.-S.; Chang, S.-Y.; Chung, A.-H.; Kuan, P.-C.; Yuan, S.-C.; Huang, C.-N.; Lee, S.-D. Post- to pre-overnight sleep systolic blood pressures are associated with sleep respiratory disturbance, pro-inflammatory state and metabolic situation in patients with sleep-disordered breathing. Sleep Med. 2009, 10, 720–725. [Google Scholar] [CrossRef]
- Hertenstein, E.; Gabryelska, A.; Spiegelhalder, K.; Nissen, C.; Johann, A.F.; Umarova, R.; Riemann, D.; Baglioni, C.; Feige, B. Reference Data for Polysomnography-Measured and Subjective Sleep in Healthy Adults. J. Clin. Sleep Med. 2018, 14, 523–532. [Google Scholar] [CrossRef]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation; World Health Organization: Geneva, Switzerland, 2006; p. 21. ISBN 978-92-4-159493-6. [Google Scholar]
- Vijan, S. Type 2 Diabetes. Ann. Intern. Med. 2010, 152, ITC3-1. [Google Scholar] [CrossRef] [PubMed]
- National Cholesterol Education Program. ATP III Guidelines At.-A-Glance Quick Desk Reference; National Cholesterol Education Program: Bethesda, MD, USA, 2013. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 5th ed.; Wiley: New York, NY, USA, 2001; ISBN 9780471316497. [Google Scholar]
- Liang, K.-Y.; Zeger, S.L. Longitudinal data analysis using generalized linear models. Biometrika 1986, 73, 13–22. [Google Scholar] [CrossRef]
- Hardin, J.; Hilbe, J. Generalized Estimating Equations; Chapman and Hall/CRC: London, UK, 2003; ISBN 978-1-58488-307-4. [Google Scholar]
- Johnsson, L.-G.; Hawkins, J.E. Sensory and Neural Degeneration with Aging, as Seen in Microdissections of the Human Inner Ear. Ann. Otol. Rhinol. Laryngol. 1972, 81, 179–193. [Google Scholar] [CrossRef]
- Fischer, Y.; Yakinthou, A.; Mann, W.J. Prevalence of obstructive sleep apnea syndrome (OSA) in patients with sudden hearing loss. A pilot study. HNO 2003, 51, 462–466. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.-J.; Zhang, X.-Y.; Liang, S.-C.; Guo, Z.-P.; Lu, M.-L.; Ye, J.-Y. Inner ear function in patients with obstructive sleep apnea. Sleep Breath. 2019, 24, 65–69. [Google Scholar] [CrossRef]
- Lisan, Q.; Van Sloten, T.; Climie, R.E.; Boutouyrie, P.; Guibout, C.; Thomas, F.; Danchin, N.; Jouven, X.; Empana, J. Sleep apnoea is associated with hearing impairment: The Paris prospective study 3. Clin. Otolaryngol. 2020, 45, 681–686. [Google Scholar] [CrossRef]
- Gozeler, M.S.; Sengoz, F.; Kilic, K.; Sakat, M.S. Auditory Function of Patients with Obstructive Sleep Apnea Syndrome: A Study. Eurasian J. Med. 2020, 52, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Deniz, M.; Ersözlü, T. Evaluation of the changes in the hearing system over the years among patients with OSAS using a CPAP device. CRANIO®® 2020, 1–4. [Google Scholar] [CrossRef]
- Seo, Y.J.; Ju, H.M.; Lee, S.H.; Kwak, S.H.; Kang, M.J.; Yoon, J.-H.; Kim, C.-H.; Cho, H.-J. Damage of Inner Ear Sensory Hair Cells via Mitochondrial Loss in a Murine Model of Sleep Apnea with Chronic Intermittent Hypoxia. Sleep 2017, 40. [Google Scholar] [CrossRef]
- Matsumura, E.; Matas, C.G.; Sanches, S.G.G.; Magliaro, F.C.L.; Pedreño, R.M.; Genta, P.R.; Lorenzi-Filho, G.; Carvallo, R.M.M. Severe obstructive sleep apnea is associated with cochlear function impairment. Sleep Breath. 2017, 22, 71–77. [Google Scholar] [CrossRef]
- Seidman, M.D.; Ahmad, N.; Joshi, D.; Seidman, J.; Thawani, S.; Quirk, W.S. Age-related hearing loss and its association with reactive oxygen species and mitochondrial DNA damage. Acta Oto-Laryngol. 2004, 124, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.-H.; Taylor, R.; Forge, A.; Schacht, J. Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear. Res. 2001, 155, 1–8. [Google Scholar] [CrossRef]
- Yamahara, K.; Yamamoto, N.; Nakagawa, T.; Ito, J. Insulin-like growth factor 1: A novel treatment for the protection or regeneration of cochlear hair cells. Hear. Res. 2015, 330, 2–9. [Google Scholar] [CrossRef]
- Hoyos, C.M.; Killick, R.; Keenan, D.M.; Baxter, R.; Veldhuis, J.D.; Liu, P.Y. Continuous Positive Airway Pressure Increases Pulsatile Growth Hormone Secretion and Circulating Insulin-like Growth Factor-1 in a Time-Dependent Manner in Men with Obstructive Sleep Apnea: A Randomized Sham-Controlled Study. Sleep 2014, 37, 733–741. [Google Scholar] [CrossRef]
- Matsuura, S.; Kuno, M.; Nakamura, T. Intracranial Pressure and Auditory Evoked Responses of the Cat. Acta Oto-Laryngol. 1986, 102, 12–19. [Google Scholar] [CrossRef]
- Beebe, D.W.; Gozal, D. Obstructive sleep apnea and the prefrontal cortex: Towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J. Sleep Res. 2002, 11, 1–16. [Google Scholar] [CrossRef]
- Kotterba, S.; Rasche, K. Acoustic evoked potentials (AEP) in obstructive sleep apnea syndrome. Pneumologie 1996, 50, 924–926. [Google Scholar]
- Duzlu, M.; Köktürk, O.; Kemaloglu, Y.K.; Eravcı, F.C.; Küçükünal, I.S.; Karamert, R.; İriz, A. The effect of obstructive sleep apnea syndrome on the central auditory system. Turk. J. Med. Sci. 2018, 48, 5–9. [Google Scholar] [CrossRef]
- Laratta, C.R.; Ayas, N.T.; Povitz, M.; Pendharkar, S.R. Diagnosis and treatment of obstructive sleep apnea in adults. Can. Med. Assoc. J. 2017, 189, E1481–E1488. [Google Scholar] [CrossRef]
- Tucci, D.L.; Merson, M.H.; Wilson, B.S. A Summary of the Literature on Global Hearing Impairment. Otol. Neurotol. 2010, 31, 31–41. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).