Inspiratory Muscle Training Program Using the PowerBreath®: Does It Have Ergogenic Potential for Respiratory and/or Athletic Performance? A Systematic Review with Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Selection of Articles: Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Data Analysis
2.5. Quality Assessment
3. Results
3.1. Selection of Studies
3.2. Descriptive Information of the Selected Articles Included in the Systematic Review
3.3. Results of the Quality Assessment
3.4. Performance Measures
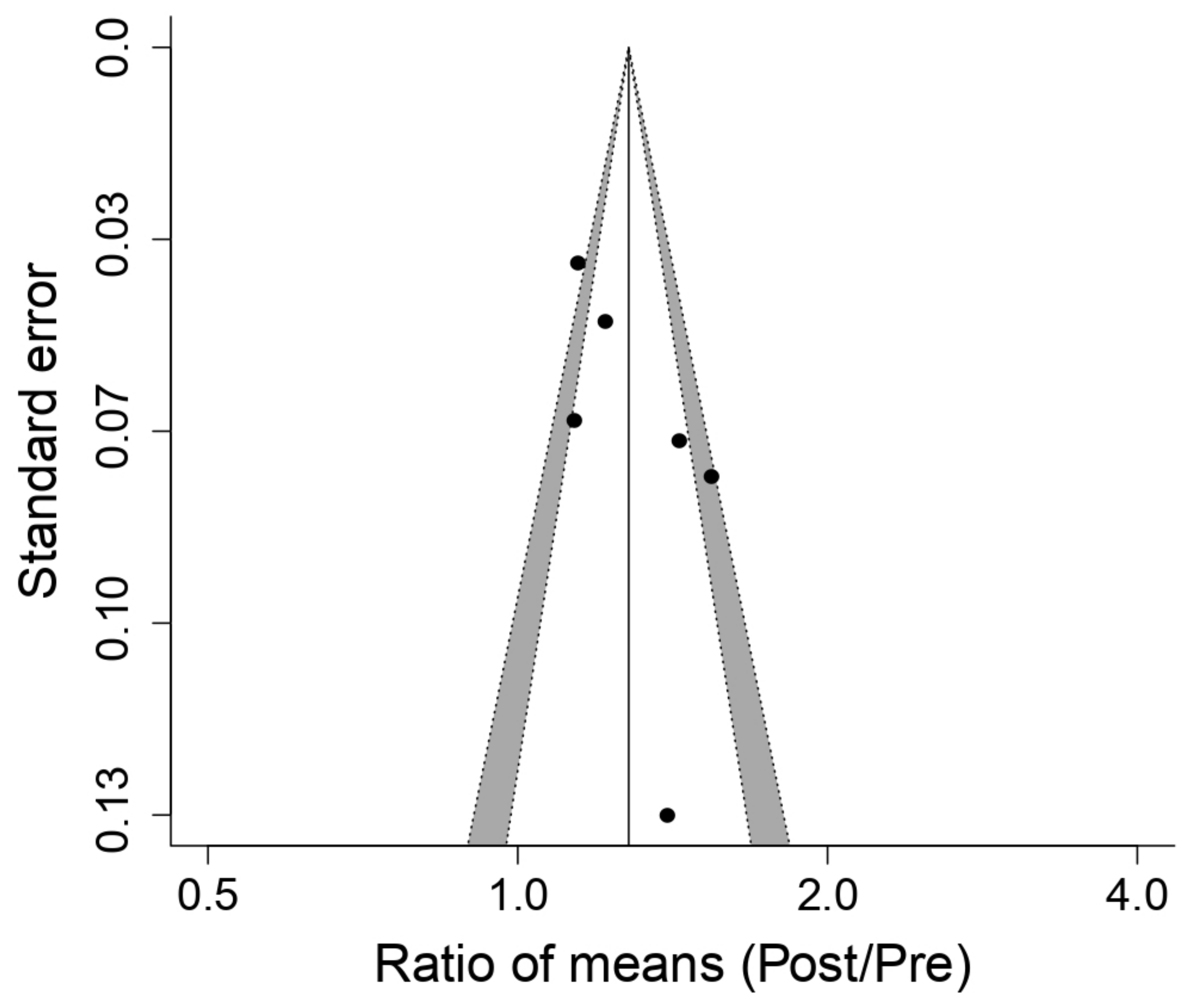
3.5. Meta-Analysis
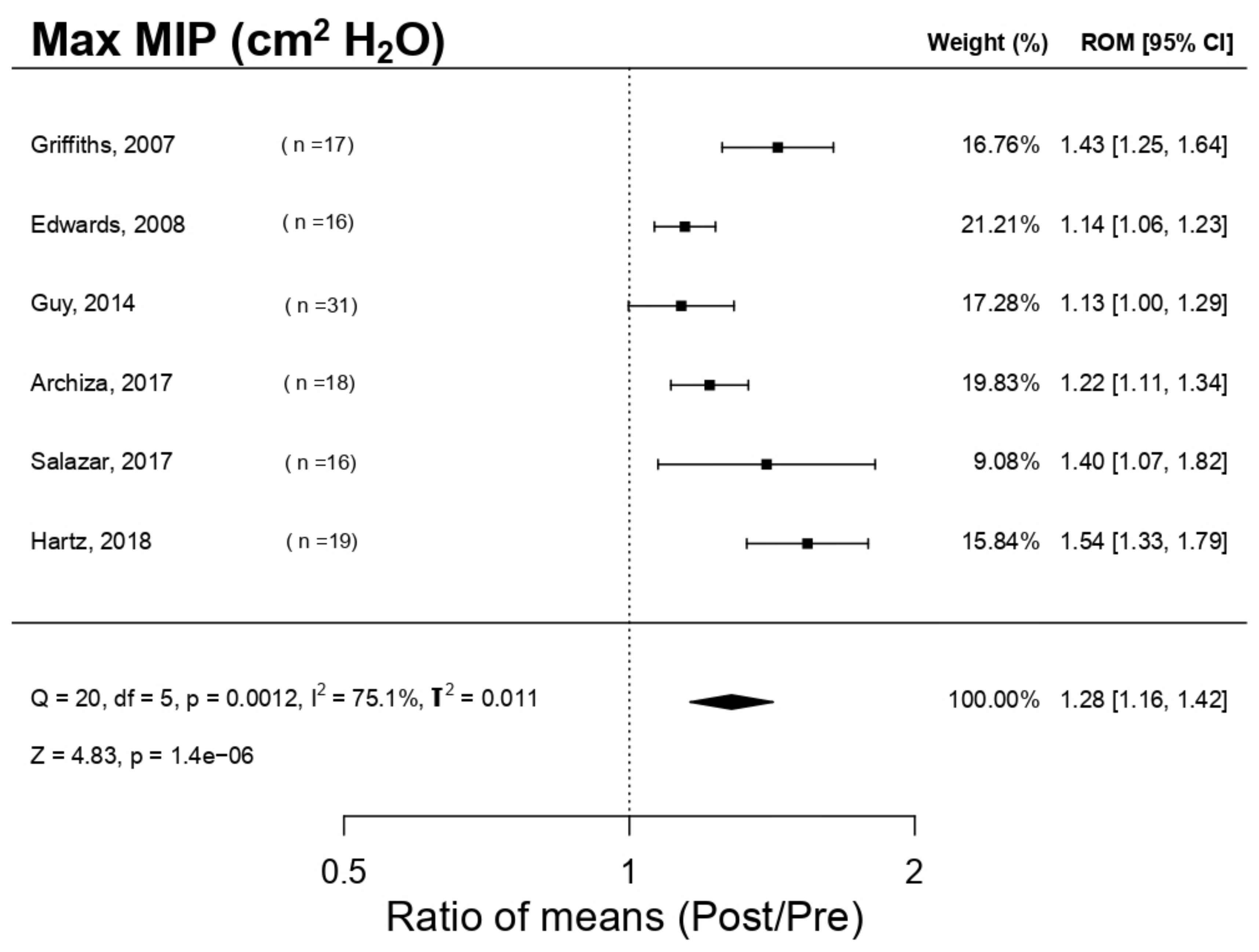
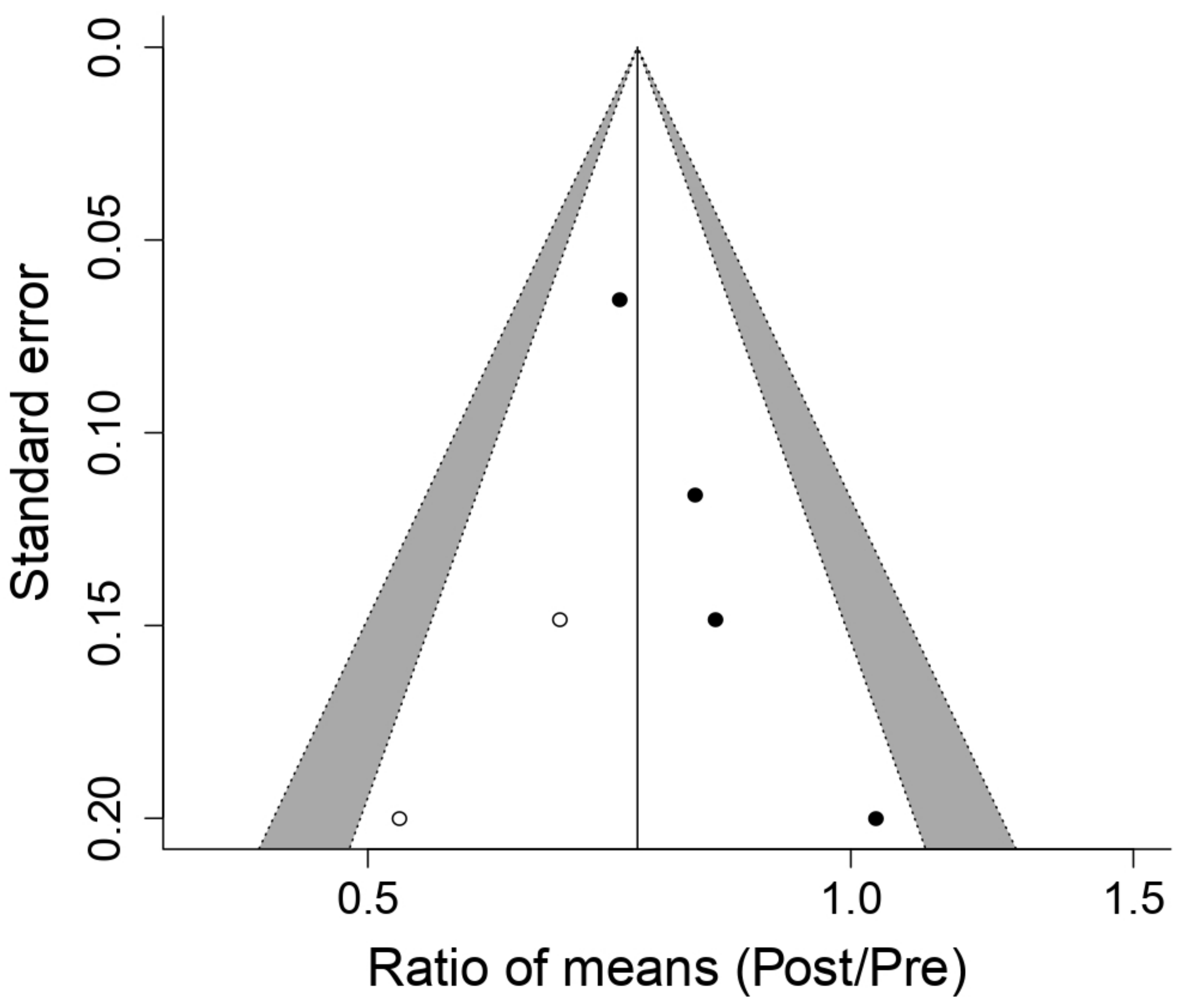
3.5.1. Effect on Maximal Inspiratory Pressure (MIP)
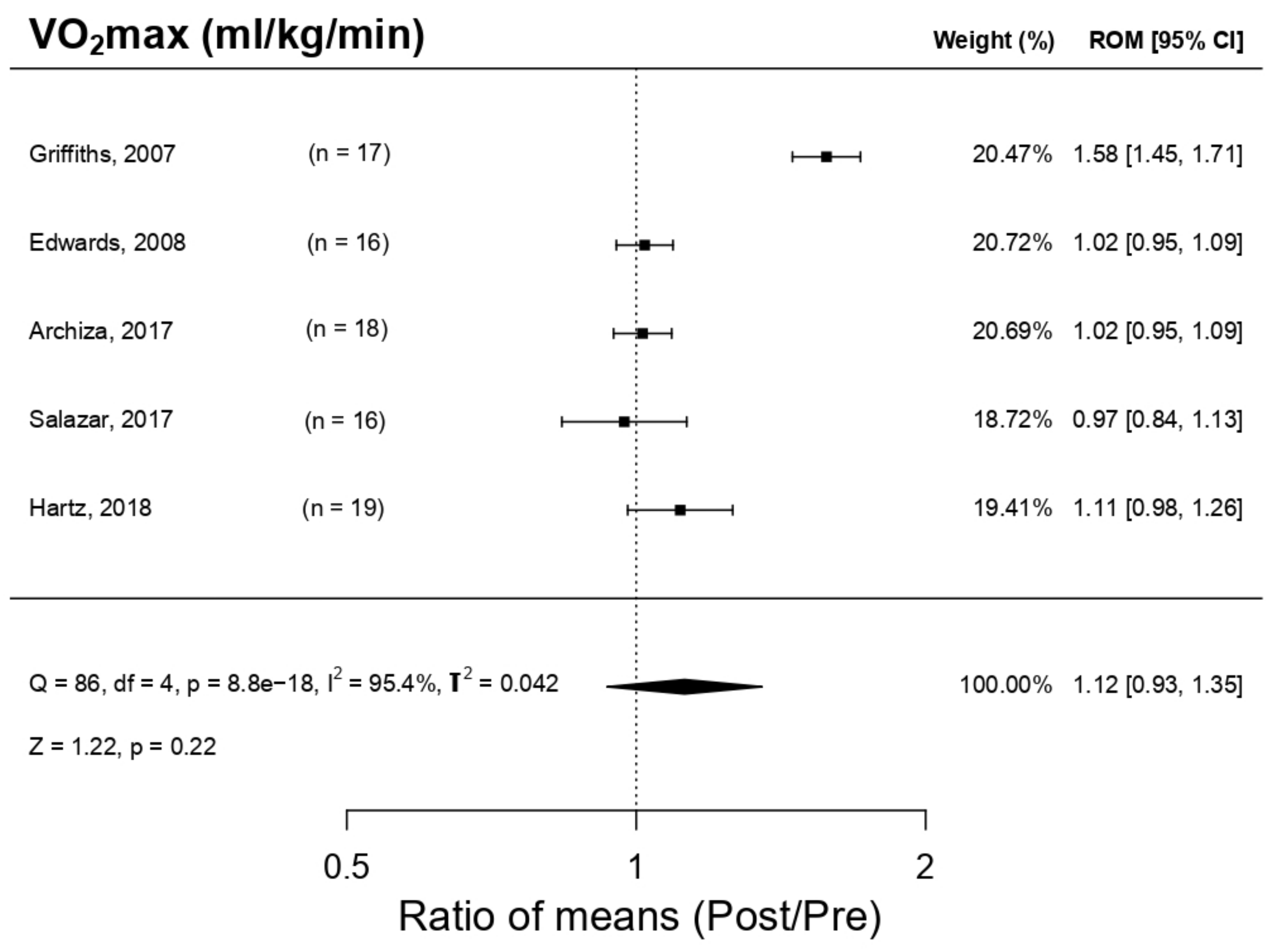
3.5.2. Effect on Maximal Oxygen Volume (VO2max)
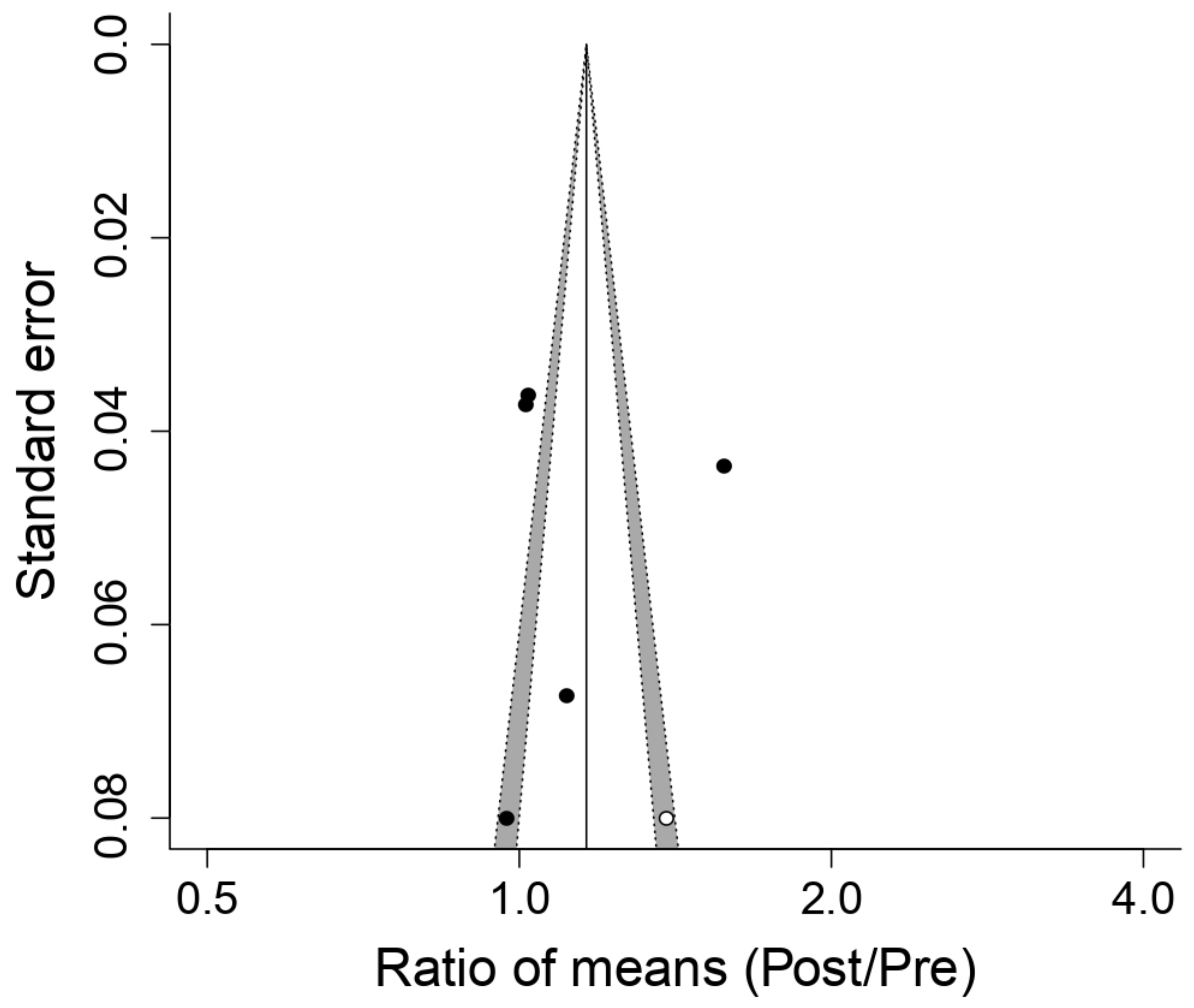
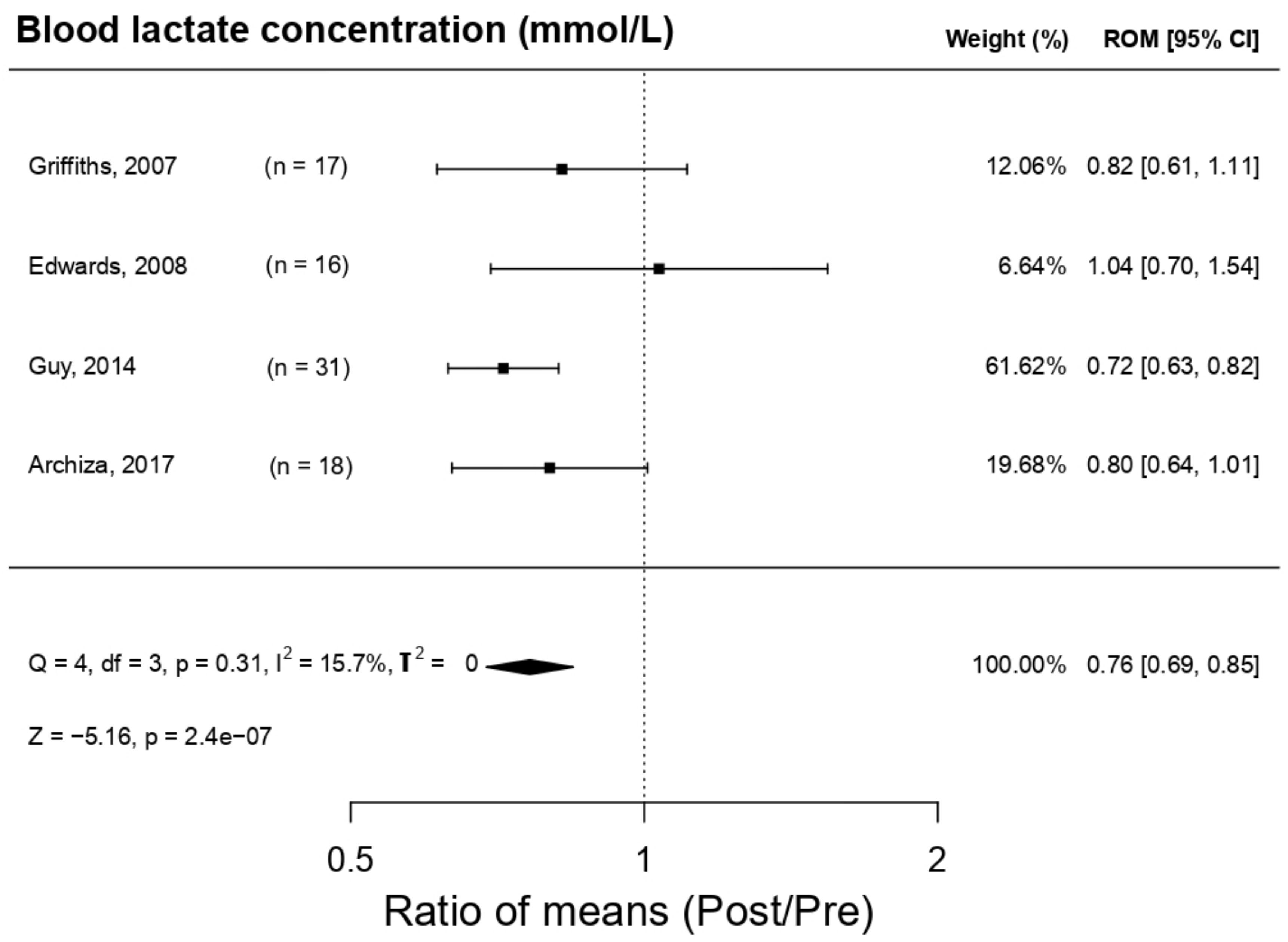
3.5.3. Effect on Blood Lactate Concentration (LA)
4. Discussion
4.1. Athletic Methods and PowerBreath® (PwB) Models
4.2. PowerBreath® (PwB) Appliance Method
4.3. Ergo-Respiratory Parameters Evaluation
4.4. Maximal Respiratory Pressure
4.5. Evaluation of Physical Parameters
5. Application of PowerBreath® (PwB) as a Non-Nutritional Supplement in Sports
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| O2 | Oxygen |
| PA | Physical activity |
| RM | Respiratory muscles |
| RMRM | Metabolic reflex mechanism of the respiratory muscles “metaboreflex” |
| IMT | Inspiratory muscle training |
| LA | Blood lactate concentration |
| CO2 | Carbon dioxide |
| MIP | Maximal inspiratory pressure |
| PwB | Power-Breathe |
| cmH2O | Centimetres of water |
| SL | Standard of living |
| PRISMA | Preferred Reporting Items for Systematic Review and Meta-Analyses |
| P | Population |
| I | Intervention |
| C | Comparators |
| O | Outcome |
| S | Studies design |
| MeSH | Titles of medical matter |
| S2 | Within-study variance |
| τ2 | Tau squared |
| ES | Effect size |
| ROM | Ratio of Means |
| VO2max | Maximal oxygen volume |
| MEP | Maximal expiratory pressure |
| RER | Respiratory effort ratio |
| RE | Respiratory efficiency |
| FEV1 | Forced expiratory volume in a second |
References
- Janssens, L.; Brumagne, S.; McConnell, A.K.; Raymaekers, J.; Goossens, N.; Gayan-Ramirez, G.; Hermans, G.; Troosters, T. The assessment of inspiratory muscle fatigue in healthy individuals: A systematic review. Respir. Med. 2013, 107, 331–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forster, H.V.; Haouzi, P.; Dempsey, J.A. Control of breathing during exercise. Compr. Physiol. 2012, 2, 743–777. [Google Scholar]
- Fernández-Lázaro, D. Ergogenic Strategies for Optimizing Performance and Health in Regular Physical Activity Participants: Evaluation of the Efficacy of Compressive Cryotherapy, Exposure to Intermittent Hypoxia at Rest and Sectorized Training of the Inspiratory Muscles. Doctoral Thesis, Faculty of Health Sciences. University of León, León, Spain, 2020. [Google Scholar]
- Hartz, C.S.; Sindorf, M.A.G.; Lopes, C.R.; Batista, J.; Moreno, M.A. Effect of Inspiratory Muscle Training on Performance of Handball Athletes. J. Hum. Kinet. 2018, 63, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Romer, L.M.; Polkey, M.I. Exercise-induced respiratory muscle fatigue: Implications for performance. J. Appl. Physiol. 2008, 104, 879–888. [Google Scholar] [CrossRef] [Green Version]
- HajGhanbari, B.; Yamabayashi, C.; Buna, T.R.; Coelho, J.D.; Freedman, K.D.; Morton, T.A.; Palmer, S.A.; Toy, M.A.; Walsh, C.; William, S.A.; et al. Effects of respiratory muscle training on performance in athletes: A systematic review with meta-analyses. J. Strength Cond. Res. 2013, 27, 1643–1663. [Google Scholar] [CrossRef] [PubMed]
- Richard, P.; Billaut, F. Combining Chronic Ischemic Preconditioning and Inspiratory Muscle Warm-Up to Enhance On-Ice Time-Trial Performance in Elite Speed Skaters. Front. Physiol. 2018, 9, 1036. [Google Scholar] [CrossRef] [Green Version]
- Edwards, A.M.; Cooke, C.B. Oxygen uptake kinetics and maximal aerobic power are unaffected by inspiratory muscle training in healthy subjects where time to exhaustion is extended. Eur. J. Appl. Physiol. 2004, 93, 139–144. [Google Scholar] [CrossRef]
- Archiza, B.; Andaku, D.K.; Caruso, F.C.R.; Bonjorno, J.C., Jr.; Oliveira, C.R.; de Ricci, P.A.; do Amaral, A.C.; Mattiello, S.M.; Libardi, C.A.; Phillips, S.A.; et al. Effects of inspiratory muscle training in professional women football players: A randomized sham-controlled trial. J. Sports Sci. 2018, 36, 771–780. [Google Scholar] [CrossRef] [PubMed]
- McConnell, A.K. The role of inspiratory muscle function and training in the genesis of dyspnoea in asthma and COPD. Prim. Care Respir. J. 2005, 14, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Salazar-Martínez, E.; Gatterer, H.; Burtscher, M.; Naranjo Orellana, J.; Santalla, A. Influence of inspiratory muscle training on ventilatory efficiency and cycling performance in normoxia and hypoxia. Front. Physiol. 2017, 8, 133. [Google Scholar] [CrossRef] [Green Version]
- Naranjo-Orellana, J.; Santalla, A. Long-term combined training in idiopathic pulmonary fibrosis: A case study. Int. J. Environ. Res. Public Health 2020, 17, 5091. [Google Scholar] [CrossRef]
- Gosselink, R.; De Vos, J.; Van Den Heuvel, S.P.; Segers, J.; Decramer, M.; Kwakkel, G. Impact of inspiratory muscle training in patients with COPD: What is the evidence? Eur. Respir. J. 2011, 37, 416–425. [Google Scholar] [CrossRef]
- Madariaga, V.B.; Iturri, J.B.G.; Manterola, A.G.; Buey, J.C.; Sebastián, N.T.; Peña, V.S. Comparison of 2 methods of inspiratory muscle training in COPD patients. Arch. Bronconeumol. 2007, 43, 431–438. [Google Scholar] [CrossRef]
- Menzes, K.K.P.; Nascimento, L.R.; Avelino, P.R.; Polese, J.C.; Salmela, L.F.T. A Review on Respiratory Muscle Training Devices. J. Pulm. Respir. Med. 2018, 8, 2. [Google Scholar]
- Guy, J.H.; Edwards, A.M.; Deakin, G.B. Inspiratory muscle training improves exercise tolerance in recreational soccer players without concomitant gain in soccer-specific fitness. J. Strength Cond. Res. 2014, 28, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Gething, A.D.; Williams, M.; Davies, B. Inspiratory resistive loading improves cycling capacity: A placebo controlled trial. Br. J. Sports Med. 2004, 38, 730–736. [Google Scholar] [CrossRef]
- Abeijon, B. Effects of Diaphragm and Accessory Muscles of Inspiration Training in Elite Athletes. Doctoral Thesis, Faculty of Medicine, Autonomous University, Barcelona, Spain, 2007. [Google Scholar]
- Edwards, A.M.; Wells, C.; Butterly, R. Concurrent inspiratory muscle and cardiovascular training differentially improves both perceptions of effort and 5000 m running performance compared with cardiovascular training alone. Br. J. Sports Med. 2008, 42, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Klusiewicz, A.; Starczewski, M.; Sadowska, D.; Ładyga, M. Effect of high-and low-resistance inspiratory muscle training on physiological response to exercise in cross-country skiers. J. Sports Med. Phys. Fitness 2019, 59, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Law, M.; Stewart, C.; Pollock, N.; Letts, L.; Bosch, J.; Westmorland, M. Guidelines for Critical Review of Qualitative Studies; McMaster University Occupational Therapy Evidence-Based Practice Research Group: Hamilton, ON, Canada, 1998; pp. 1–9. [Google Scholar]
- Griffiths, L.A.; McConnell, A.K. The influence of inspiratory and expiratory muscle training upon rowing performance. Eur. J. Appl. Physiol. 2007, 99, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Shei, R.-J. Recent advancements in our understanding of the ergogenic effect of respiratory muscle training in healthy humans: A systematic review. J. Strength Cond. Res. 2018, 32, 2665–2676. [Google Scholar] [CrossRef]
- Duruturk, N.; Acar, M.; Doğrul, M.I. Effect of inspiratory muscle training in the management of patients with asthma. J. Cardiopulm. Rehabil. Prev. 2018, 38, 198–203. [Google Scholar] [CrossRef]
- González-Saiz, L.; Fiuza-Luces, C.; Sanchis-Gomar, F.; Santos-Lozano, A.; Quezada-Loaiza, C.A.; Flox-Camacho, A.; Izquierdo, D.M.; Ara, I.; Santalla, A.; Morán, M.; et al. Benefits of skeletal-muscle exercise training in pulmonary arterial hypertension: The WHOLEi+ 12 trial. Int. J. Cardiol. 2017, 231, 277–283. [Google Scholar] [CrossRef]
- Lawton, T.W.; Cronin, J.B.; McGuigan, M.R. Strength testing and training of rowers. Sport Med. 2011, 41, 413–432. [Google Scholar] [CrossRef]
- González-Montesinos, J.L.; Pardal, C.V.; Santos, J.R.F.; Muñoz, A.A.; Sepúlveda, J.L.C.; de los Monteros, R.G.E. Effects of respiratory muscle training on performance. Literature review. Rev. Andal. Med. Deport. 2012, 5, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Martin, B.J.; Stager, J.M. Ventilatory endurance in athletes and non-athletes. Med. Sci. Sports Exerc. 1981, 13, 21–26. [Google Scholar] [CrossRef]
- De Jesús Mora-Romero, U.; Gochicoa-Rangel, L.; Guerrero-Zúñiga, S.; Cid-Juárez, S.; Silva-Cerón, M.; Salas-Escamilla, I.; Torre-Bouscoulet, L. Maximal inspiratory and expiratory pressures: Recommendations and procedure. NCT Neumol. y Cirugía Tórax 2014, 73, 247–253. [Google Scholar]
- Powers, S.K.; Lawler, J.; Criswell, D.; Dodd, S.; Grinton, S.; Bagby, G.; Silverman, H. Endurance-training-induced cellular adaptations in respiratory muscles. J. Appl. Physiol. 1990, 68, 2114–2118. [Google Scholar] [CrossRef]
- Aubier, M.; Trippenbach, T.; Roussos, C.H. Respiratory muscle fatigue during cardiogenic shock. J. Appl. Physiol. 1981, 51, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Adams, D.P.; González-Bernal, J.J.; Araque, A.F.; García, A.C.; Fernández-Lázaro, C. Electromyography: A simple and accessible tool to assess physical performance and health during hypoxia training. A systematic review. Sustain. 2020, 12, 9137. [Google Scholar]
- Fernández-Lázaro, D.; González-Bernal, J.J.; Sánchez-Serrano, N.; Navascués, L.J.; Del Río, A.A.; Mielgo-Ayuso, J. Physical exercise as a multimodal tool for COVID-19: Could it be used as a preventive strategy? Int. J. Environ. Res. Public Health 2020, 17, 8496. [Google Scholar] [CrossRef] [PubMed]
- Harms, C.A.; Wetter, T.J.; McClaran, S.R.; Pegelow, D.F.; Nickele, G.A.; Nelson, W.B.; Hanson, P.; Dempsey, J.A. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J. Appl. Physiol. 1998, 85, 609–618. [Google Scholar] [CrossRef]
- McConnell, A.K.; Lomax, M. The influence of inspiratory muscle work history and specific inspiratory muscle training upon human limb muscle fatigue. J. Physiol. 2006, 577, 445–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harms, C.A.; Babcock, M.A.; McClaran, S.R.; Pegelow, D.F.; Nickele, G.A.; Nelson, W.B.; Dempsey, J.A. Respiratory muscle work compromises leg blood flow during maximal exercise. J. Appl. Physiol. 1997, 82, 1573–1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McConnell, A.K.; Caine, M.P.; Lacy, G.K. Inspiratory muscle training device with variable loading. Google Patents. US Patent 6,554,746, 29 April 2003. [Google Scholar]
- Volianitis, S.; McConnell, A.K.; Jones, D.A. Assessment of maximum inspiratory pressure. Respiration 2001, 68, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Jurić, I.; Labor, S.; Plavec, D.; Labor, M. Inspiratory muscle strength affects anaerobic endurance in professional athletes. Arh. Hig. Rada Toksikol. 2019, 70, 42–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
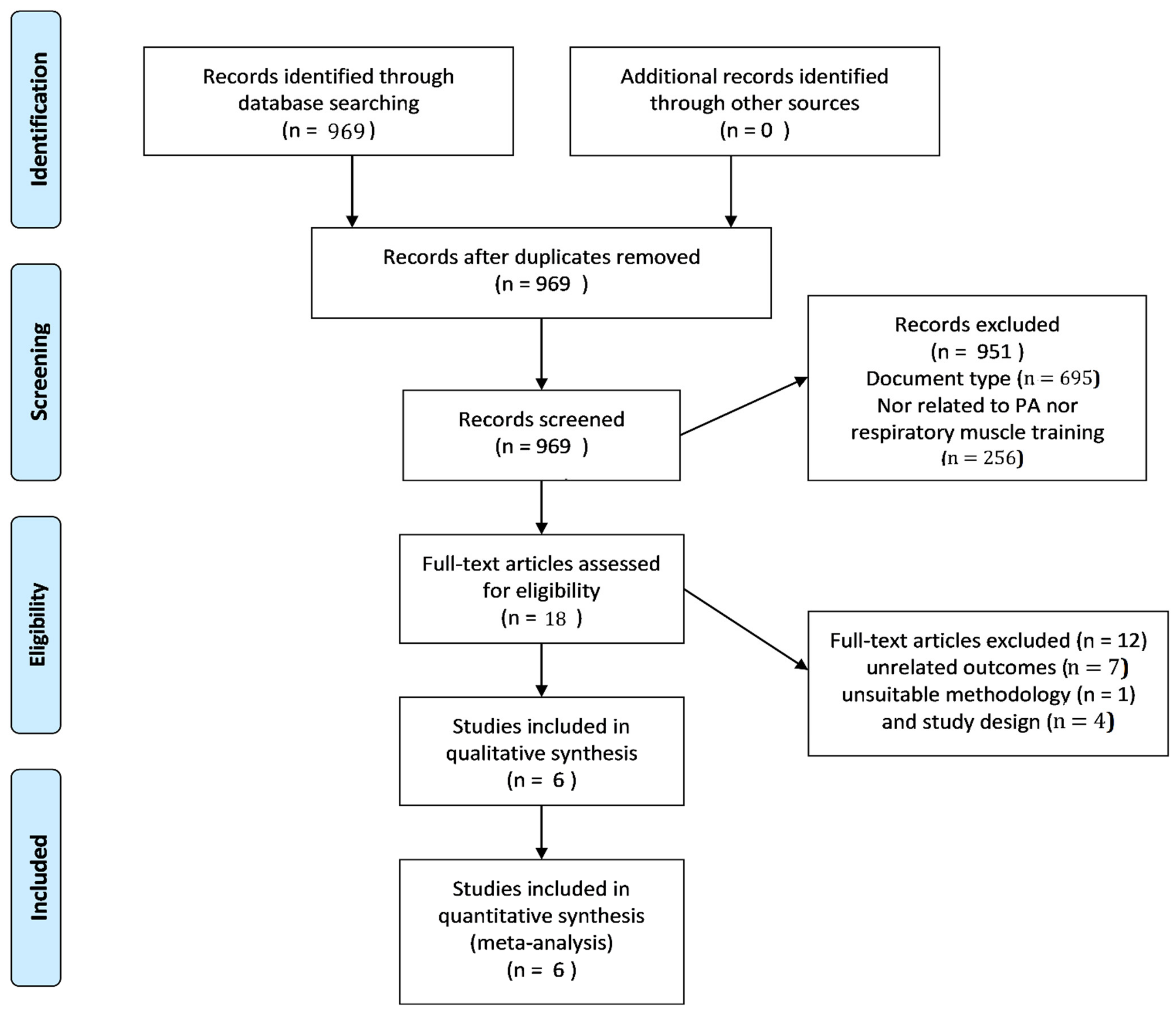
| Subject’s level | Professional | 3 studies [4,9,25] |
| Amateur | 3 studies [11,16,19] | |
| Subject’s age | Senior (20–30) | 5 studies [4,9,11,16,25] |
| N/A | 1 study [19] | |
| Training method | 2 daily sessions, 5 days/week (30 inspirations 50% MIP) | 3 studies [4,9,11] |
| 4 weeks IMT + 6 combined weeks (IMT + EMT) | 1 study [25] | |
| 2 running weekly sessions + IMT (30 inspirations) | 1 study [19] | |
| 2 days/weeks regular football training + IMT (2 times/day 30 inspirations at the subject’s own pace) | 1 study [16] | |
| 4 weeks | 1 study [19] | |
| 6 weeks | 3 studies [9,11,16] | |
| 10 weeks | 1 study [25] | |
| 12 weeks | 1 study [4] |
| Reference | ITEMS | TE | % | MC | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||||
| Hartz el al. 2018 [4] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 14 | 87.5 | VG |
| Griffiths et al. 2007 [25] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 12 | 75.0 | G |
| Salazar-Martínez et al. 2017 [11] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 13 | 81.3 | VG |
| Edwars et al. 2015 [19] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 14 | 87.5 | VG |
| Archiza et al. 2017 [9] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 13 | 81.3 | VG |
| Guy et al. 2014 [16] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 14 | 87.5 | VG |
| T | 7 | 7 | 7 | 7 | 2 | 7 | 7 | 7 | 7 | 7 | 5 | 1 | 3 | 7 | 7 | 3 | |||
| Characteristics of the Studies Included in the Systematic Review | |||||||
|---|---|---|---|---|---|---|---|
| Authors/Year | Population | Study Design | PowerBreaht® | Respiratory Muscle Training | Analysed Parameters | Results | Conclusion |
| Hartz et al. 2018 [4] | 19 ♂ (20 ± 3 yo) professional handball players. Group PwB: n = 10, 19 ± 4 yo Placebo group: n = 9, 22 ± 1 yo | Random, with Placebo | PwB Plus Heavy Resistance Sports Model | 2 h/session 5 sessions/weeks For 12 weeks RES increment 50–70% MIP | MIP MEP MVV PP VO2 max | ↑MIP * ↑MEP ↑MVV ↑PP ↑VO2max * | IMT produces a relevant increment in the strength and resistance and, therefore, endurance |
| Griffiths et al. 2007 [25] | 17 ♂ professional rowers. Group A: (n = 10; 24.9 ± 5.6 yo). Gruop B: n = 7; age, 28.7 ± 9.1 yo) | Random, with Placebo | Group A: IMT PwB Group B: EMT Powerlung (IMT inactivated) | 4 weeks; 30 respirations * 2/day Group A: IMT Group B: EMT RES 50% MIP | MIP MEP HR VO2 max LAB RRE | ↑MIP * ↑MEP HR X ↑VO2 max * ↓LAB * ↓RRE | IMT (using PwB) improved the performance of rowers, but EMT (using Powerlung) does not. |
| Salazar-Martínez et al. 2017 [11] | 16 amateurs’ cyclists (23.05 ± 4.7 yo). 9 ♂ (23.44 ± 2.7) and 7 ♀ (25.37 ± 3.24) | Random, controlled, without Placebo | PwB K3 | 6 weeks; 30 respirations * 5 days/week; 2 sessions/day. RES 50% MIP | MIP ER En VO2 max FEV1 OUES | ↑MIP * ↑ER ↑En * ↓VO2 max * ↓FEV1 * ↑OUES * | IMT has a positive effect in the performance of cyclists |
| Edwards et al. 2015 [19] | 16 ♂ endurance amateur athletes | Random, with Placebo | PwB | 4 weeks; in each week: IMT: 30 respirations * 1 session/day RES 15% MIP + (CV1: 5 × 1000 m; CV2: 3 × 1600 m, SP1: 20′ running) | MIP VO2 max HR LAB RPE Test duration 5000 m FEV1 | ↑MIP * VO2 max X HR X ↓ LAB ↓ RPE * ↑Test duration 5000 m * ↑FEV1 | IMT combined with cardiovascular training increases the MIP, improves significantly capability/endurance in 5000 m test; it does not affect VO2max |
| Archiza et al. 2017 [9] | 18 ♀ professional footballers. Group PwB: (n = 10; 22.0 ± 3.9 yo). Simulation Group: (n = 8; 20.1 ± 2.0 yo) | Double random blind controlled by a simulation (placebo). | PwB K5 | 6 weeks; 5 days/week; 30 respirations * 2 sessions/day RES50% MIP | Antropometrics, pulmonal function, respiratory musculature strength, incremental maximal exercise test + “Tlim” test + repeated sprints | ↑MIP * ↓VE ↓(44)B * ↑RSA ↑VO2max * ↓FEV1 | IMP increases the strength of inspiratory muscles, exercise tolerance and recovering process post repeated sprints in footballers |
| Guy et al. 2014 [16] | 31 ♂ amateur footballers. Group PwB: n = 12; 28.4 ± 8.2 yo). Group Placebo: n = 9; 23.9 ± 6.7 yo). Control Group: n = 10; 21.3 ± 4.9 yo) | Random, with Placebo and control group | PwB K1 | 6 weeks 2 sessions/weeks of normal training + 2 * 30 inspirations IMT/session RES 55% | MIP FVC FEV1 MSFT LAB SSFT | ↑MIP * FVC X ↓FEV1 ↑MSFT * ↓LAB * SSFT X | IMT increases the endurance of the footballers’ preseason |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Lázaro, D.; Gallego-Gallego, D.; Corchete, L.A.; Fernández Zoppino, D.; González-Bernal, J.J.; García Gómez, B.; Mielgo-Ayuso, J. Inspiratory Muscle Training Program Using the PowerBreath®: Does It Have Ergogenic Potential for Respiratory and/or Athletic Performance? A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 6703. https://doi.org/10.3390/ijerph18136703
Fernández-Lázaro D, Gallego-Gallego D, Corchete LA, Fernández Zoppino D, González-Bernal JJ, García Gómez B, Mielgo-Ayuso J. Inspiratory Muscle Training Program Using the PowerBreath®: Does It Have Ergogenic Potential for Respiratory and/or Athletic Performance? A Systematic Review with Meta-Analysis. International Journal of Environmental Research and Public Health. 2021; 18(13):6703. https://doi.org/10.3390/ijerph18136703
Chicago/Turabian StyleFernández-Lázaro, Diego, David Gallego-Gallego, Luis A. Corchete, Darío Fernández Zoppino, Jerónimo J. González-Bernal, Blanca García Gómez, and Juan Mielgo-Ayuso. 2021. "Inspiratory Muscle Training Program Using the PowerBreath®: Does It Have Ergogenic Potential for Respiratory and/or Athletic Performance? A Systematic Review with Meta-Analysis" International Journal of Environmental Research and Public Health 18, no. 13: 6703. https://doi.org/10.3390/ijerph18136703
APA StyleFernández-Lázaro, D., Gallego-Gallego, D., Corchete, L. A., Fernández Zoppino, D., González-Bernal, J. J., García Gómez, B., & Mielgo-Ayuso, J. (2021). Inspiratory Muscle Training Program Using the PowerBreath®: Does It Have Ergogenic Potential for Respiratory and/or Athletic Performance? A Systematic Review with Meta-Analysis. International Journal of Environmental Research and Public Health, 18(13), 6703. https://doi.org/10.3390/ijerph18136703











