Effects of Diacutaneous Fibrolysis on Passive Neuromuscular Response and Mechanosensitivity in Athletes with Hamstring Shortening: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Size Calculation
2.3. Sample Selection Criteria
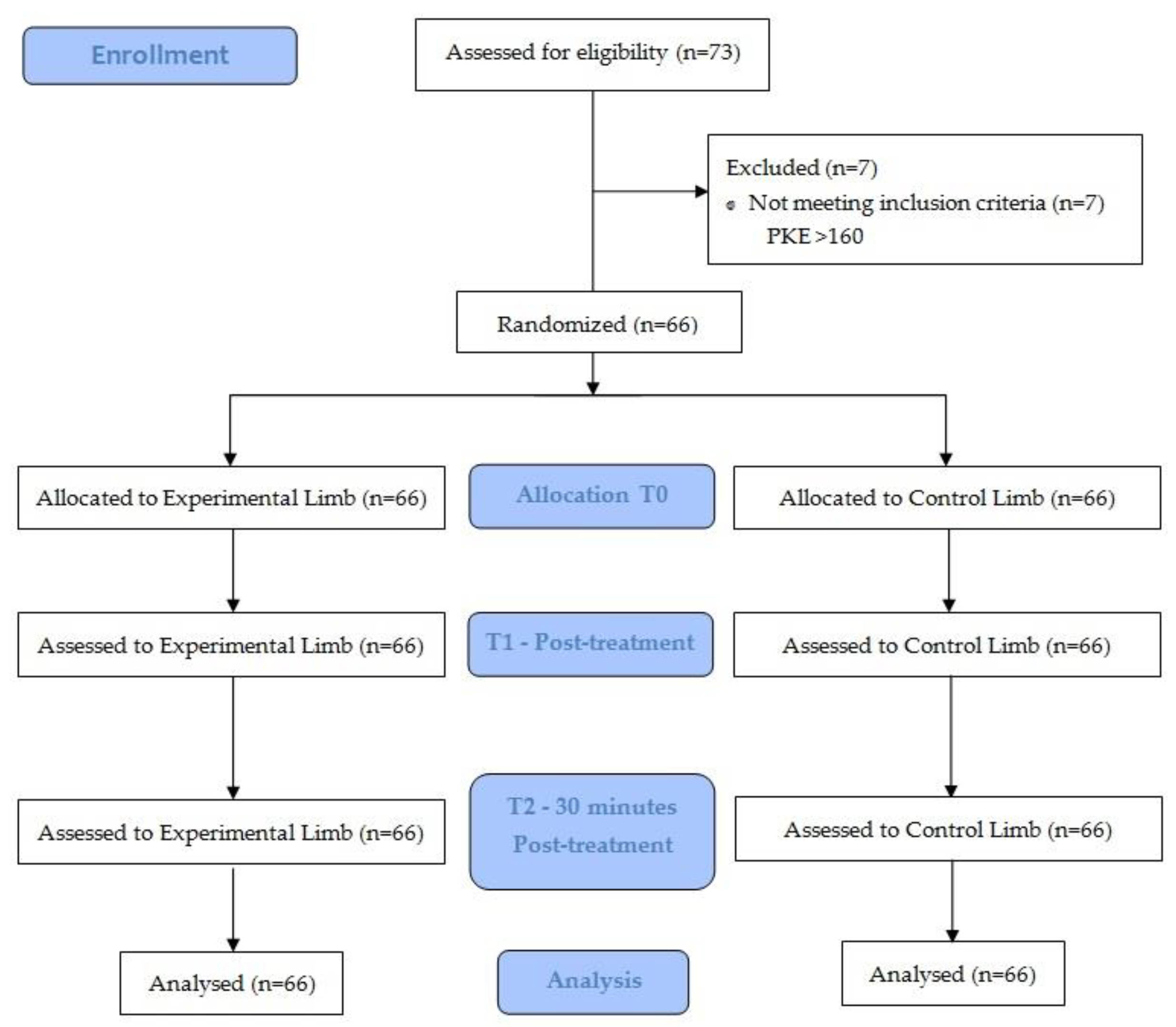
2.4. Randomization and Allocation
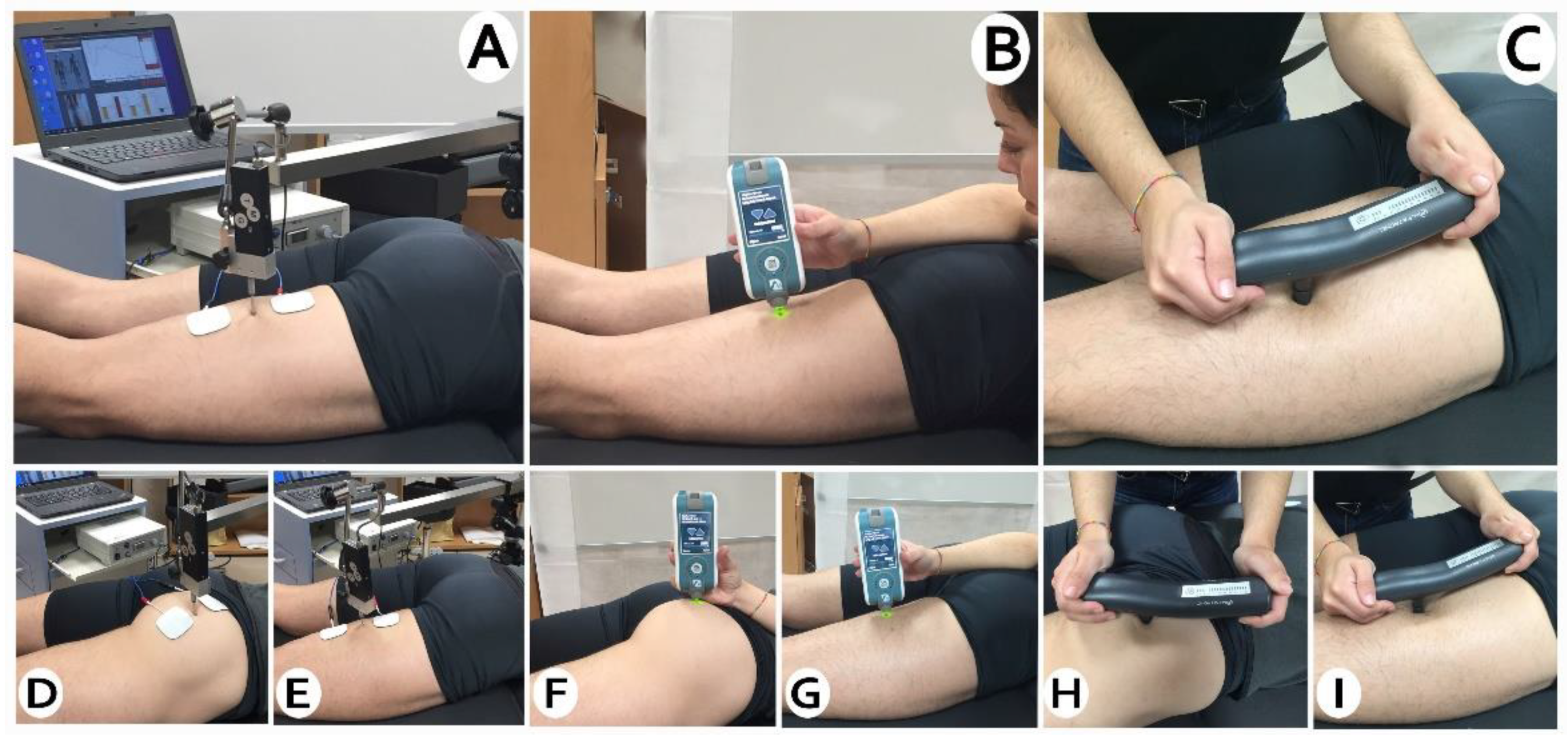
2.5. Measurements
2.6. Outcomes
2.6.1. Viscoelastic Properties
2.6.2. Muscle Contractile Properties
2.6.3. Mechanosensibility
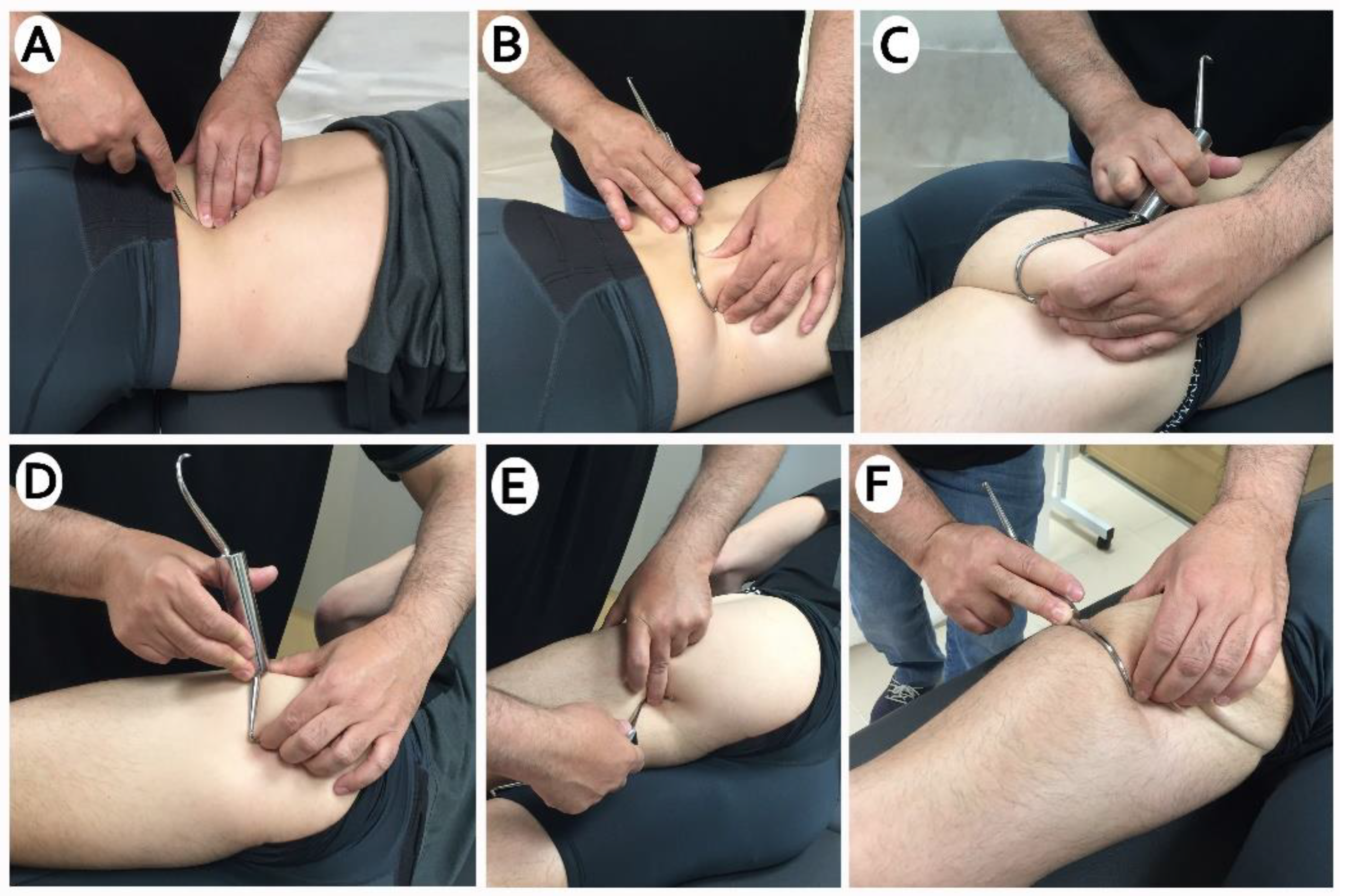
2.7. Intervention
2.8. Statistical Analysis
3. Results
3.1. Viscoelastic Properties
3.2. Contractile Properties
3.3. Mechanosensibility
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tricás, J.M.; Lucha, O.; Duby, P. Fibrolisis Diacutánea Según el Concepto de Kurt Ekman; Asociación Española de Fibrolisis Diacutánea: Zaragoza, Spain, 2010. [Google Scholar]
- Barra, M.; López, C.; Fernández, G.; Murillo, E.; Villar, E.; Raya, L. The immediate effects of diacutaneous fibrolysis on pain and mobility in patients suffering from painful shoulder: A randomized placebo-controlled pilot study. Clin. Rehabil. 2011, 25, 339–348. [Google Scholar] [CrossRef]
- Jiménez del Barrio, S.; Estébanez de Miguel, E.; Bueno Gracia, E.; Haddad Garay, M.; Tricás Moreno, J.M.; Hidalgo García, C. Effects of diacutaneous fibrolysis in patients with mild to moderate symptomatic carpal tunnel syndrome: A randomized controlled trial. Clin. Rehabil. 2018, 32, 1645–1655. [Google Scholar] [CrossRef]
- Leite, W.B.; Oliveira, M.L.; Ferreira, I.C.; Anjos, C.F.; Barbosa, M.A.; Barbosa, A.C. Effects of 4-Week Diacutaneous Fibrolysis on Myalgia, Mouth Opening, and Level of Functional Severity in Women with Temporomandibular Disorders: A Randomized Controlled Trial. J. Manip. Physiol. Ther. 2020, 43. [Google Scholar] [CrossRef]
- Cadellans-Arróniz, A.; Llurda-Almuzara, L.; Campos-Laredo, B.; Cabanas-Valdés, R.; Garcia-Sutil, A.; López-De-Celis, C. The effectiveness of diacutaneous fibrolysis on pain, range of motion and functionality in musculoskeletal disorders: A systematic review and meta-analysis. Clin. Rehabil. 2020. [Google Scholar] [CrossRef] [PubMed]
- Veszely, M.; Guissard, N.; Duchateau, J. Contribution à l’étude des effets de la fibrolyse diacutanée sur le triceps sural. Ann. Kinésithérapie 2000, 2, 54–59. [Google Scholar]
- Pérez-bellmunt, A.; Llurda, L.; Simon, M.; Navarro, R.; Casasayas, O.; López-de-celis, C. Review Article Neuromuscular Response What is it and How to Measure it? Phys. Med. Rehabil. J. 2019, 2, 1–7. [Google Scholar]
- Labata-Lezaun, N.; López-De-Celis, C.; Llurda-Almuzara, L.; González-Rueda, V.; Cadellans-Arróniz, A.; Pérez-Bellmunt, A. Correlation between maximal radial muscle displacement and stiffness in gastrocnemius muscle. Physiol. Meas. 2020, 41, 125013. [Google Scholar] [CrossRef] [PubMed]
- López-De-Celis, C.; Pérez-Bellmunt, A.; Bueno-Gracia, E.; Fanlo-Mazas, P.; Zárate-Tejero, C.A.; Llurda-Almuzara, L.; Arróniz, A.C.; Rodriguez-Rubio, P.R. Effect of diacutaneous fibrolysis on the muscular properties of gastrocnemius muscle. PLoS ONE 2020, 15, e0243225. [Google Scholar] [CrossRef]
- Arner, J.W.; McClincy, M.P.; Bradley, J.P. Hamstring Injuries in Athletes. J. Am. Acad. Orthop. Surg. 2019, 27, 868–877. [Google Scholar] [CrossRef]
- Yıldırım, M.Ş.; Tuna, F.; Demirbağ Kabayel, D.; Süt, N. The Cut-off Values for the Diagnosis of Hamstring Shortness and Related Factors. Balkan Med. J. Int. 2018, 35, 388–393. [Google Scholar] [CrossRef]
- Kim, D.-H.; Lee, J.J.; You, J.; Sung, H. Effects of instrument-assisted soft tissue mobilization technique on strength, knee joint passive stiffness, and pain threshold in hamstring shortness. J. Back Musculoskelet. Rehabil. 2018, 31, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Opar, D.A.; Williams, M.D.; Shield, A.J. Hamstring strain injuries: Factors that Lead to injury and re-Injury. Sport Med. 2012, 42, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, N.; Okawa, N.; Kishi, M.; Nakatani, K.; Nishikawa, K.; Tokumura, D.; Matsui, Y.; Moriyama, H. Effects of long-term self-massage at the musculotendinous junction on hamstring extensibility, stiffness, stretch tolerance, and structural indices: A randomized controlled trial. Phys. Ther. Sport 2016, 21, 38–45. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Albert, P.; Labata-lezaun, N.; Llurda-almuzara, L.; Rodr, J. Effects of a Massage Protocol in Tensiomyographic and Myotonometric Proprieties. Int. J. Environ. Res. Public Health 2021, 18, 3891. [Google Scholar]
- Lucha López, M.; López De Celis, C.; Fanlo Mazas, P.; Barra López, M.; Hidalgo García, C.; Tricás Moreno, J. Efectos inmediatos de la fibrolisis diacutánea en deportistas con dolor anterior en la rodilla. Cuest. Fisioter Rev. Univ. Inf. Investig. Fisioter 2015, 44, 33–40. [Google Scholar]
- Maniar, N.; Shield, A.; Williams, M.D.; Timmins, R.G.; Opar, D. Hamstring strength and flexibility after hamstring strain injury: A systematic review and meta-analysis. Br. J. Sports Med. 2016, 50, 909–920. [Google Scholar] [CrossRef]
- García-Pinillos, F.; Ruiz-Ariza, A.; Del Castillo, R.M.; Latorre-Román, P.Á. Impact of limited hamstring flexibility on vertical jump, kicking speed, sprint, and agility in young football players. J. Sports Sci. 2015, 33, 1293–1297. [Google Scholar] [CrossRef]
- Wan, X.; Qu, F.; Garrett, W.E.; Liu, H.; Yu, B. Relationships among hamstring muscle optimal length and hamstring flexibility and strength. J. Sport Health Sci. 2017, 6, 275–282. [Google Scholar] [CrossRef]
- Álvarez-Díaz, P.; Alentorn-Geli, E.; Ramon, S.; Marín, M.; Steinbacher, G.; Rius, M.; Seijas, R.; Ballester, J.; Cugat, R. Effects of anterior cruciate ligament reconstruction on neuromuscular tensiomyographic characteristics of the lower extremity in competitive male soccer players. Knee Surg. Sports Traumatol. Arthrosc. 2014, 23, 3407–3413. [Google Scholar] [CrossRef]
- Reurink, G.; Goudswaard, G.J.; Oomen, H.G.; Moen, M.H.; Tol, J.L.; Verhaar, J.A.; Weir, A. Reliability of the Active and Passive Knee Extension Test in Acute Hamstring Injuries. Am. J. Sports Med. 2013, 41, 1757–1761. [Google Scholar] [CrossRef]
- Davidson, M.J.; Bryant, A.L.; Bower, W.F.; Frawley, H.C. Myotonometry Reliably Measures Muscle Stiffness in the Thenar and Perineal Muscles. Physiother. Can. 2017, 69, 104–112. [Google Scholar] [CrossRef]
- Bizzini, M.; Mannion, A.F. Reliability of a new, hand-held device for assessing skeletal muscle stiffness. Clin. Biomech. 2003, 18, 459–461. [Google Scholar] [CrossRef]
- Šimunič, B. Between-day reliability of a method for non-invasive estimation of muscle composition. J. Electromyogr. Kinesiol. 2012, 22, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Tous-Fajardo, J.; Moras, G.; Jiménez, S.R.; Usach, R.; Doutres, D.M.; Maffiuletti, N.A. Inter-rater reliability of muscle contractile property measurements using non-invasive tensiomyography. J. Electromyogr. Kinesiol. 2010, 20, 761–766. [Google Scholar] [CrossRef]
- Pérez-Bellmunt, A.; Simon, M.; López-De-Celis, C.; Ortiz-Miguel, S.; González-Rueda, V.; Fernandez-De-Las-Peñas, C. Effects on Neuromuscular Function after Ischemic Compression in Latent Trigger Points in the Gastrocnemius Muscles: A Randomized Within-Participant Clinical Trial. J. Manip. Physiol. Ther. 2021. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, E.K.; Ozdincler, A.R. Reliability and responsiveness of algometry for measuring pressure pain threshold in patients with knee osteoarthritis. J. Phys. Ther. Sci. 2015, 27, 1961–1965. [Google Scholar] [CrossRef]
- Nussbaum, E.L.; Downes, L. Reliability of Clinical Pressure-Pain Algometric Measurements Obtained on Consecutive Days. Phys. Ther. 1998, 78, 160–169. [Google Scholar] [CrossRef]
- Marshall, P.W.; Lovell, R.; Siegler, J.C.; Siefler, J.C. Changes in Passive Tension of the Hamstring Muscles during a Simulated Soccer Match. Int. J. Sports Physiol. Perform. 2016, 11, 594–601. [Google Scholar] [CrossRef]
- Ho, C.-S.; Lee, M.-C.; Chang, C.-Y.; Chen, W.-C.; Huang, W.-C. Beneficial effects of a negative ion patch on eccentric exercise-induced muscle damage, inflammation, and exercise performance in badminton athletes. Chin. J. Physiol. 2020, 63, 35–42. [Google Scholar] [CrossRef]
- Ikeda, N.; Otsuka, S.; Kawanishi, Y.; Kawakami, Y. Effects of Instrument-assisted Soft Tissue Mobilization on Musculoskeletal Properties. Med. Sci. Sports Exerc. 2019, 51, 2166–2172. [Google Scholar] [CrossRef]
- Leite, W.B.; De Oliveira, M.L.; Barbosa, M.A.; Ferreira, I.C.; Mesquita, G.; Baumgarth, H.; Barbosa, A.C. Muscle excitation, force response, and efficiency during explosive force production after diacutaneous fibrolysis on lateral gastrocnemius of recreational athletes. J. Bodyw. Mov. Ther. 2020, 24, 554–560. [Google Scholar] [CrossRef]
- MacGregor, L.J.; Fairweather, M.M.; Bennett, R.M.; Hunter, A.M. The Effect of Foam Rolling for Three Consecutive Days on Muscular Efficiency and Range of Motion. Sports Med. Open 2018, 4, 26. [Google Scholar] [CrossRef]
- Sato, K.; Nimura, A.; Yamaguchi, K.; Akita, K. Anatomical study of the proximal origin of hamstring muscles. J. Orthop. Sci. 2012, 17, 614–618. [Google Scholar] [CrossRef] [PubMed]
- O’Rahilly, R.; Müller, F.; Meyer, D.B. The human vertebral column at the end of the embryonic period proper. 4. The sacrococcygeal region. J. Anat. 1990, 168, 95–111. [Google Scholar] [PubMed]
- Willard, F.H.; Vleeming, A.; Schuenke, M.D.; Danneels, L.; Schleip, R. The thoracolumbar fascia: Anatomy, function and clinical considerations. J. Anat. 2012, 221, 507–536. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bellmunt, A.; Miguel-Pérez, M.; Brugué, M.B.; Cabús, J.B.; Casals, M.; Martinoli, C.; Kuisma, R. An anatomical and histological study of the structures surrounding the proximal attachment of the hamstring muscles. Man. Ther. 2015, 20, 445–450. [Google Scholar] [CrossRef]
- Seffrin, M.C.B.; Cattano, N.M.; Reed, M.A.; Gardiner-Shires, A.M. Instrument-Assisted Soft Tissue Mobilization: A Systematic Review and Effect-Size Analysis. J. Athl. Train. 2019, 54, 808–821. [Google Scholar] [CrossRef]
- Babatunde, O.O.; Jordan, J.L.; Van Der Windt, D.A.; Hill, J.C.; Foster, N.E.; Protheroe, J. Effective treatment options for musculoskeletal pain in primary care: A systematic overview of current evidence. PLoS ONE 2017, 12, e0178621. [Google Scholar] [CrossRef]
| Clinical Features | Mean ± SD or n (%) (n = 66) |
|---|---|
| Age (years) | 21.7 ± 3.5 |
| Sex | |
| Men | 46 (69.7%) |
| Women | 20 (30.3%) |
| Height (cm) | 175.5 ± 8.34 |
| Weight (kg) | 70 ± 11.89 |
| BMI (kg/m2) | 22.71 ± 2.86 |
| Dominance | |
| Right | 50 (75.8%) |
| Left | 16 (24.2%) |
| Variables | T0 | T1 | Difference T0–T1 | T2 | Difference T0–T2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean | 95% CI | p | ES | Mean ± SD | Mean | 95% CI | p | ES | ||
| Experimental Limbs | ||||||||||||
| Gluteus | Tone (Hz) | 11.01 ± 0.84 | 10.75 ± 1.55 | −0.26 | [−0.657; 0.133] | 0.325 | 0.21 | 10.81 ± 0.74 | −0.21 | [−0.388; −0.024] | 0.021 | 0.25 |
| Stiffness (N/m) | 159.70 ± 30.76 | 149.54 ± 29.07 | −10.16 | [−20.033; −0.286] | 0.042 | 0.34 | 150.61 ± 19.80 | −9.09 | [−17.467; −0.715] | 0.029 | 0.35 | |
| Relaxation (m/s) | 31.33 ± 4.09 | 31.79 ± 5.18 | 0.46 | [−1.021; 1.938] | 1.000 | 0.10 | 31.42 ± 4.59 | 0.08 | [−1.340; 1.510] | 1.000 | 0.02 | |
| Biceps Femoris | Tone (Hz) | 15.85 ± 1.73 | 15.47 ± 1.56 | −0.39 | [−0.702; −0.072] | 0.011 | 0.23 | 15.70 ± 1.53 | −0.15 | [−0.463; 0.268] | 0.764 | 0.09 |
| Stiffness (N/m) | 286.09 ± 39.19 | 272.18 ± 36.30 | −13.91 | [−19.417; −8.401] | 0.001 | 0.37 | 279 59 ± 38.83 | −6.50 | [−12.751; −0.249] | 0.039 | 0.17 | |
| Relaxation (m/s) | 18.98 ± 2.64 | 19.76 ± 2.81 | 0.78 | [0.381; 1.180] | 0.001 | 0.29 | 19.32 ± 2.70 | 0.34 | [−0.032; 0.714] | 0.084 | 0.13 | |
| Semitendinosus | Tone (Hz) | 15.23 ± 1.89 | 15.06 ± 1.71 | −0.17 | [−0.510; 0.164] | 0.638 | 0.09 | 15.22 ± 1.69 | −0.02 | [−0.375; 0.345] | 1.000 | 0.01 |
| Stiffness (N/m) | 269.52 ± 51.75 | 259.80 ±46.03 | −9.72 | [−18.917; −0.513] | 0.035 | 0.20 | 259.12 ± 42.83 | −10.40 | [−20.521; −0.273] | 0.042 | 0.22 | |
| Relaxation (m/s) | 19.62 ± 4.96 | 20.35 ± 4.06 | 0.74 | [−0.066; 1.537] | 0.083 | 0.16 | 20.12 ± 3.35 | 0.50 | [−0.431; 1.440] | 0.570 | 0.12 | |
| Control Limbs | ||||||||||||
| Gluteus | Tone (Hz) | 10.95 ± 0.93 | 10.87 ± 0.84 | −0.08 | [−0.302; 0.138] | 1.000 | 0.09 | 10.75 ± 0.76 | −0.20 | [−0.443; 0.052] | 0.170 | 0.24 |
| Stiffness (N/m) | 156.74 ± 24.99 | 154.21 ± 20.49 | −2.53 | [−9.443; 4.382] | 1.000 | 0.11 | 150.48 ± 21.33 | −6.26 | [−13.765; 1.250] | 0.134 | 0.27 | |
| Relaxation (m/s) | 31.67 ± 3.48 | 31.72 ± 3.10 | 0.05 | [−0.777; 0.877] | 1.000 | 0.02 | 31.65 ± 3.11 | −0.02 | [−0.880; 0.847] | 1.000 | 0.01 | |
| Biceps Femoris | Tone (Hz) | 15.96 ± 1.78 | 15.96 ± 1.72 | 0.00 | [−0.306; 0.310] | 1.000 | 0.00 | 15.72 ± 1.60 | −0.24 | [−0.544; 0.065] | 0.174 | 0.19 |
| Stiffness (N/m) | 289.33 ± 42.27 | 281.85 ± 42.40 | −7.48 | [−16.873; 1.903] | 0.163 | 0.18 | 281.88 ± 36.10 | −7.45 | [−14.584; −0.325] | 0.037 | 0.20 | |
| Relaxation (m/s) | 18.86 ± 2.95 | 19.25 ± 3.18 | 0.39 | [−0.975; 0.194] | 0.316 | 0.13 | 19.16 ± 2.79 | 0.30 | [−0.169; 0.763] | 0.367 | 0.10 | |
| Semitendinosus | Tone (Hz) | 15.51 ± 2.13 | 15.33 ± 1.98 | −0.18 | [−0.551; 0.200] | 0.764 | 0.09 | 15.48 ± 2.00 | −0.03 | [−0.438; −0.371] | 1.000 | 0.02 |
| Stiffness (N/m) | 273.11 ± 56.04 | 265.70 ± 49.10 | −7.41 | [−18.392; 3.574] | 0.307 | 0.14 | 266.53 ± 49.04 | −6.58 | [−17.374; 4.222] | 0.418 | 0.13 | |
| Relaxation (m/s) | 19.60 ± 4.28 | 19.83 ± 3.61 | 0.24 | [−0.537; 1.014] | 1.000 | 0.06 | 19.57 ± 3.83 | −0.02 | [−0.880; 0.834] | 1.000 | 0.01 | |
| Variables | T0 | T1 | Difference T0–T1 | T2 | Difference T0–T2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean | 95% CI | p | ES | Mean ± SD | Mean | 95% CI | p | ES | ||
| Experimental Limbs | ||||||||||||
| Gluteus | Tc (ms) | 39.85 ± 16.74 | 43.35 ± 22.32 | 3.50 | [−4.110; 11.112] | 0.787 | 0.18 | 35.68 ± 12.43 | −4.17 | [−8.876; 0.536] | 0.099 | 0.28 |
| Dm (mm) | 5.41 ± 3.16 | 4.88 ± 3.14 | −0.53 | [−1.427; 0.361] | 0.443 | 0.17 | 4.44 ± 2.86 | −0.97 | [1.760; 0.178] | 0.011 | 0.32 | |
| Biceps Femoris | Tc (ms) | 34.13 ± 14.29 | 32.26 ± 12.27 | −1.87 | [−5.772; 2.034] | 0.731 | 0.14 | 32.16 ± 14.26 | −1.97 | [−6.230; 2.298] | 0.784 | 0.14 |
| Dm (mm) | 4.71 ± 2.88 | 4.61 ± 2.72 | −0.10 | [−0.816; 0.621] | 1.000 | 0.04 | 4.46 ± 2.84 | −0.24 | [−0.916; 0.428] | 1.000 | 0.09 | |
| Semitendinosus | Tc (ms) | 40.32 ± 12.11 | 38.95 ± 12.40 | −1.37 | [−4.530; 1.794] | 0.875 | 0.11 | 40.97 ± 12.05 | 0.65 | [−2.431; 3.734] | 1.000 | 0.05 |
| Dm (mm) | 6.70 ± 2.80 | 6.43 ± 3.08 | −0.27 | [−0.805; 0.266] | 0.663 | 0.09 | 6.37 ± 3.11 | −0.34 | [−0.954; 0.280] | 0.554 | 0.11 | |
| Control Limbs | ||||||||||||
| Gluteus | Tc (ms) | 44.03 ± 27.19 | 43.93 ± 25.93 | −0.10 | [−10.389; 10.186] | 1.000 | 0.00 | 39.93 ± 16.10 | −4.10 | [−13.480; 5.284] | 0.861 | 0.28 |
| Dm (mm) | 6.24 ± 3.75 | 5.55 ± 3.50 | −0.69 | [−1.470; 0.098] | 0.106 | 0.19 | 5.28 ± 3.32 | −0.96 | [−1.705; −0.212] | 0.007 | 0.27 | |
| Biceps Femoris | Tc (ms) | 34.63 ± 13.33 | 32.42 ± 14.17 | −2.21 | [−6.697; 2.278] | 0.692 | 0.16 | 35.28 ± 16.85 | 0.65 | [−4.760; 6.058] | 1.000 | 0.04 |
| Dm (mm) | 5.12 ± 2.83 | 4.44 ± 3.07 | −0.67 | [−1.453; 0.107] | 0.113 | 0.23 | 4.51 ± 2.62 | −0.60 | [−1.162; −0.044] | 0.030 | 0.22 | |
| Semitendinosus | Tc (ms) | 41.36 ± 12.64 | 41.73 ± 12.05 | 0.38 | [−3.340; 4.094] | 1.000 | 0.03 | 42.81 ± 10.66 | 1.45 | [−2.465; 5.369] | 1.000 | 0.12 |
| Dm (mm) | 6.83 ± 2.86 | 6.60 ± 2.94 | −0.23 | [−0.961; 0.507] | 1.000 | 0.08 | 6.38 ± 3.02 | −0.45 | [−1.280; 0.377] | 0.556 | 0.15 | |
| T0 | T1 | Difference T0–T1 | T2 | Difference T0–T2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean | 95% CI | p | ES | Mean ± SD | Mean | 95% CI | p | ES | |
| Experimental Limb | |||||||||||
| Gluteus (NPRS 0–10) | 1.17 ± 1.83 | 1.27 ± 1.85 | 0.11 | [−0.160; 0.372] | 0.992 | 0.05 | 1.21 ± 1.84 | 0.05 | [−0.275; 0.366] | 1.000 | 0.02 |
| Biceps Femoris (NPRS 0–10) | 1.02 ± 1.55 | 0.83 ± 1.63 | −0.18 | [−0.441; 0.078] | 0.269 | 0.14 | 0.85 ± 1.56 | −0.17 | [−0.450; 0.117] | 0.461 | 0.11 |
| Semitendinosus (NPRS 0–10) | 1.00 ± 1.36 | 0.94 ± 1.46 | −0.06 | [−0.391; 0.270] | 1.000 | 0.04 | 0.92 ± 1.44 | −0.08 | [−0.445; 0.293] | 1.000 | 0.06 |
| Control Limb | |||||||||||
| Gluteus (NPRS 0–10) | 1.17 ± 1.67 | 1.20 ± 1.65 | 0.03 | [−0.182; 0.242] | 1.000 | 0.02 | 1.17 ± 1.79 | −0.00 | [−0.276; 0.276] | 1.000 | 0.00 |
| Biceps Femoris (NPRS 0–10) | 1.05 ± 1.47 | 0.91 ± 1.43 | −0.14 | [−0.354; 0.082] | 0.388 | 0.10 | 1.05 ± 1.65 | −0.00 | [−0.286; 0.286] | 1.000 | 0.00 |
| Semitendinosus (NPRS 0–10) | 0.88 ± 1.23 | 0.85 ± 1.37 | −0.03 | [−0.311; 0.250] | 1.000 | 0.02 | 0.89 ± 1.30 | 0.02 | [−0.278; 0.308] | 1.000 | 0.01 |
| Variable | Difference T0–T1 | Difference T0–T2 | |||||
|---|---|---|---|---|---|---|---|
| Experimental Limbs | Control Limbs | Experimental Limbs | Control Limbs | ||||
| Mean ± SD | Mean ± SD | p | Mean ± SD | Mean ± SD | p | ||
| Gluteus | Tone (Hz) | −0.26 ± 1.31 | −0.08 ± 0.73 | 0.283 | −0.21 ± 0.60 | −0.20 ± 0.82 | 0.829 |
| Stiffness (N/m) | −10.16 ± 32.64 | −2.53 ± 22.85 | 0.048 | −9.09 ± 27.69 | −6.26 ± 24.82 | 0.289 | |
| Relaxation (m/s) | 0.46 ± 4.89 | 0.05 ± 2.73 | 0.633 | 0.08 ± 4.71 | −0.02 ± 2.85 | 0.246 | |
| Tc (ms) | 3.50 ± 25.16 | −0.10 ± 34.01 | 0.466 | −4.17 ± 15.56 | −4.10 ± 31.02 | 0.699 | |
| Dm (mm) | −0.53 ± 2.95 | −0.69 ± 2.59 | 0.687 | −0.97 ± 2.62 | −0.96 ± 2.47 | 0.982 * | |
| MCS (NPRS 0–10) | 0.11 ± 0.88 | 0.03 ± 0.70 | 0.553 | 0.05 ± 1.06 | −0.00 ± 0.91 | 0.880 | |
| Biceps Femoris | Tone (Hz) | −0.39 ± 1.04 | −0.00 ± 1.02 | 0.009 | −0.15 ± 1.04 | −0.24 ± 1.01 | 0.960 |
| Stiffness (N/m) | −13.91 ± 18.21 | −7.48 ± 31.04 | 0.019 | −6.50 ± 20.67 | −7.45 ± 23.57 | 0.909 | |
| Relaxation (m/s) | 0.78 ± 1.32 | 0.39 ± 1.93 | 0.053 | 0.34 ± 1.23 | −0.30 ± 1.54 | 0.045 | |
| Tc (ms) | −1.87 ± 12.90 | −2.21 ± 14.83 | 0.546 | −1.97 ± 14.10 | −0.65 ± 17.88 | 0.891 | |
| Dm (mm) | −0.10 ±2.38 | −0.67 ± 2.58 | 0.120 | −0.24 ± 2.22 | −0.60 ± 1.85 | 0.134 | |
| MCS (NPRS 0–10) | −0.18 ± 0.86 | −0.14 ± 0.72 | 0.365 | −0.17 ± 0.94 | −0.00 ± 0.94 | 0.534 | |
| Semitendinosus | Tone (Hz) | −0.17 ± 1.11 | −0.18 ± 1.24 | 0.803 | −0.02 ± 1.19 | −0.03 ± 1.34 | 0.766 |
| Stiffness (N/m) | −9.72 ± 30.42 | −7.41 ± 36.31 | 0.325 | −10.40 ± 33.47 | −6.58 ± 35.70 | 0.263 | |
| Relaxation (m/s) | 0.74 ± 2.65 | 0.24 ± 2.56 | 0.344 | 0.50 ± 3.09 | −0.02 ± 2.83 | 0.114 | |
| Tc (ms) | −1.37 ± 10.45 | 0.38 ± 12.29 | 0.502 | 0.65 ± 10.19 | 1.45 ± 12.95 | 0.771 | |
| Dm (mm) | −0.27 ± 1.77 | −0.23 ± 2.43 | 0.690 | −0.34 ± 2.04 | −0.45 ± 2.74 | 0.074 * | |
| MCS (NPRS 0–10) | −0.06 ± 1.09 | −0.03 ± 0.93 | 0.742 | −0.08 ± 1.22 | 0.02 ± 0.97 | 0.487 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cadellans-Arróniz, A.; López-de-Celis, C.; Pérez-Bellmunt, A.; Rodríguez-Sanz, J.; Llurda-Almuzara, L.; González-Rueda, V.; Rodríguez-Rubio, P.R. Effects of Diacutaneous Fibrolysis on Passive Neuromuscular Response and Mechanosensitivity in Athletes with Hamstring Shortening: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 6554. https://doi.org/10.3390/ijerph18126554
Cadellans-Arróniz A, López-de-Celis C, Pérez-Bellmunt A, Rodríguez-Sanz J, Llurda-Almuzara L, González-Rueda V, Rodríguez-Rubio PR. Effects of Diacutaneous Fibrolysis on Passive Neuromuscular Response and Mechanosensitivity in Athletes with Hamstring Shortening: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2021; 18(12):6554. https://doi.org/10.3390/ijerph18126554
Chicago/Turabian StyleCadellans-Arróniz, Aida, Carlos López-de-Celis, Albert Pérez-Bellmunt, Jacobo Rodríguez-Sanz, Luis Llurda-Almuzara, Vanessa González-Rueda, and Pere Ramón Rodríguez-Rubio. 2021. "Effects of Diacutaneous Fibrolysis on Passive Neuromuscular Response and Mechanosensitivity in Athletes with Hamstring Shortening: A Randomized Controlled Trial" International Journal of Environmental Research and Public Health 18, no. 12: 6554. https://doi.org/10.3390/ijerph18126554
APA StyleCadellans-Arróniz, A., López-de-Celis, C., Pérez-Bellmunt, A., Rodríguez-Sanz, J., Llurda-Almuzara, L., González-Rueda, V., & Rodríguez-Rubio, P. R. (2021). Effects of Diacutaneous Fibrolysis on Passive Neuromuscular Response and Mechanosensitivity in Athletes with Hamstring Shortening: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 18(12), 6554. https://doi.org/10.3390/ijerph18126554









