COVID-19 Impact on Musculoskeletal Regenerative Medicine Research: Maintaining Lab Continuity
Abstract
1. Introduction
2. Materials and Methods
2.1. Working Group
2.2. Literature and Regulation Review
2.3. Risk Assessment
2.3.1. Context
2.3.2. Hazard
2.3.3. Risk
2.3.4. Existing vs. Additional Control Measures
3. Results
3.1. Working Group
3.2. Literature and Regulation Review
3.3. Risk Assessment
3.3.1. Hazard
3.3.2. Risk
3.3.3. Existing vs. Additional Control Measures
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harapan, H.; Itoh, N.; Yufika, A.; Winardi, W.; Keam, S.; Te, H.; Megawati, D.; Wagner, A.L.; Mudatsir, M. Coronavirus disease 2019 (COVID-19): A literature review. J. Infect. Public Health 2020, 13, 667–673. [Google Scholar] [CrossRef]
- Rajendran, D.K.; Rajagopal, V.; Alagumanian, S.; Kumar, T.S.; Prabhakaran, S.P.S.; Kasilingam, D. Systematic literature review on novel corona virus SARS-CoV-2: A threat to human era. Virusdisease 2020, 31, 61–173. [Google Scholar] [CrossRef]
- Tatara, A.M. Role of Tissue Engineering in COVID-19 and Future Viral Outbreaks. Tissue Eng. Part A 2020, 26, 468–474. [Google Scholar] [CrossRef]
- Shafiee, A.; Moradi, L.; Maysary, L.; Brown, J. Coronavirus disease 2019: A tissue engineering and regenerative medicine perspective. Stem Cells Transl. Med. 2021, 10, 27–38. [Google Scholar] [CrossRef]
- Vannini, F.; Mazzotti, A.; Stefanini, N.; Faldini, C. Coronavirus disease 2019 pandemic: Should we delay cartilage regenerative procedures and accept the consequences, or can we find a new normality? Int. Orthop. 2020, 44, 2189–2190. [Google Scholar] [CrossRef]
- Parvizi, J.; Gehrke, T.; Krueger, C.A.; Chisari, E.; Citak, M.; Van Onsem, S.; Walter, W.L. Resuming Elective Orthopaedic Surgery During the COVID-19 Pandemic: Guidelines Developed by the International Consensus Group (ICM). J. Bone Joint Surg. Am. 2020, 102, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Vermeşan, D.; Todor, A.; Andrei, D.; Niculescu, M.; Tudorache, E.; Haragus, H. Effect of COVID-19 Pandemic on Orthopedic Surgery in Three Centers from Romania. Int. J. Environ. Res. Public Health 2021, 18, 2196. [Google Scholar] [CrossRef] [PubMed]
- Bayrak, E.; Huri, P.Y. Engineering Musculoskeletal Tissue Interfaces. Front. Mater. 2018, 5, 24. [Google Scholar] [CrossRef]
- Kloppenburg, M.; Berenbaum, F. Osteoarthritis year in review 2019: Epidemiology and therapy. Osteoarthr. Cartil. 2020, 28, 242–248. [Google Scholar] [CrossRef]
- Disser, N.P.; De Micheli, A.J.; Schonk, M.M.; Konnaris, M.A.; Piacentini, A.N.; Edon, D.L.; Toresdahl, B.G.; Rodeo, S.A.; Casey, E.K.; Mendias, C.L. Musculoskeletal Consequences of COVID-19. JBJS 2020, 102, 1197–1204. [Google Scholar] [CrossRef]
- D’Andréa Greve, J.M.; Brech, G.; Lopes Quintana, M.S.; de Seixas Soares, A.L.; Alonso, A.C. Impacts of covid-19 on the immune, neuromuscular, and musculoskeletal systems and rehabilitation. Rev. Bras. Med. Esporte 2020, 26, 285–288. [Google Scholar] [CrossRef]
- Abdullahi, A.; Candan, S.A.; Abba, M.A.; Bello, A.H.; Alshehri, M.A.; Afamefuna, V.E.; Umar, N.A.; Kundakci, B. Neurological and Musculoskeletal Features of COVID-19: A Systematic Review and Meta-Analysis. Front. Neurol. 2020, 11, 687. [Google Scholar] [CrossRef] [PubMed]
- Prudêncio, M.; Costa, J.C. Research funding after COVID-19. Nat. Microbiol. 2020, 5, 986. [Google Scholar] [CrossRef]
- OECD. Science, Technology and Innovation Outlook 2021: Times of Crisis and Opportunity; OECD Publishing: Paris, France, 2021. [Google Scholar] [CrossRef]
- Edgeworth, J.D.; Batra, R.; Nebbia, G.; Bisnauthsing, K.; MacMahon, E.; Sudhanva, M.; Douthwaite, S.; Goldenberg, S.; O’Hara, G.; Shankar-Hari, M.; et al. Translational Research in the Time of COVID-19-Dissolving Boundaries. PLoS Pathog. 2020, 16, e1008898. [Google Scholar] [CrossRef]
- Magan, A.A.; Plastow, R.; Haddad, F.S. Impact of COVID-19 on research. Bone Joint Res. 2020, 9, 531–533. [Google Scholar] [CrossRef]
- Marinaccio, A.; Guerra, R.; Iavicoli, S. Work a key determinant in COVID-19 risk. Lancet 2020, 8, e1368. [Google Scholar] [CrossRef]
- WHO. Risk Assessment and Management of Health-Care Workers in the Context of COVID-19; World Health Organization (WHO): Geneva, Switzerland, 2020. [Google Scholar]
- OSHA. Guidance on Preparing Workplaces for COVID-19, Occupational Safety and Health Administration; U.S. Department of Labor, Occupational Safety and Health Administration (OSHA): Washington, DC, USA, 2020.
- Cockburn, W. Covid-19: Back to the Workplace. Adapting Workplaces and Protecting Workers; European Agency for Safety and Health at Work: Bilbao, Spain, 2020. [Google Scholar]
- Rial-González, E.; Copsey, S.; Paoli, P.; Schneider, E. Working Environment Information; Working Paper, Priorities for Occupational Safety and Health Research in the EU-25; European Agency for Safety and Health at Work: Bilbao, Spain, 2005. [Google Scholar]
- U.S. Department of Labor. Data DictionaryO*NET® 24.2 Database; U.S. Department of Labor, Employment and Training Administration: Washington, DC, USA, 2020.
- Iavicoli, S.; Boccuni, F.; Buresti, G.; Gagliardi, D.; Persechino, B.; Rondinone, B.M.; Valenti, A. Documento Tecnico Sulla Possibile Rimodulazione Delle Misure di Contenimento del Contagio da SARS-CoV-2 nei Luoghi di Lavoro e Strategie di Prevenzione; Istituto Nazionale Assicurazione contro gli Infortuni sul Lavoro, (INAIL): Rome, Italy, 2020. [Google Scholar]
- IOSH. Covid-19 Risk Assessment Guidance; Institution of Occupational Safety and Health (IOSH): Wigston, UK, 2020. [Google Scholar]
- Brodeur, A.; Gray, D.M.; Islam, A.; Bhuiyan, S.J. A Literature Review of the Economics of COVID-19, I.ZA; Institute of Labor Economics: Bonn, Germany, 2020. [Google Scholar]
- H M Government. Working Safely during COVID-19 in Labs and Research Facilities. COVID-19 Secure Guidance for Employers, Employees and the Self-Employed; H M Government: London, UK, 2020.
- Dyson, M.C.; Carpenter, C.B.; Colby, L.A. Institutional Oversight of Occupational Health and Safety for Research Programs Involving Biohazards. Comp. Med. 2017, 67, 192–202. [Google Scholar]
- Guidelines for Research Lab Use During the COVID-19 Pandemic; Mc Master University: Hamilton, ON, Canada, 2021.
- Research and Sponsored Programs. Available online: https://www.utoledo.edu/research/rsp/coronavirus/ (accessed on 3 May 2021).
- CDER. Good Manufacturing Practice Considerations for Responding to COVID-19 Infection in Employees in Drug and Biological Products Manufacturing- Guidance for Industry; U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research: Silver Spring, MD, USA, 2020.
- Radecki, J.; Schonfeld, R.C. The Impacts of COVID-19 on the Research Enterprise: A Landscape Review; Ithaka S+R: New York, NY, USA, 2020. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Chams, N.; Chams, S.; Badran, R.; Shams, A.; Araji, A.; Raad, M.; Mukhopadhyay, S.; Stroberg, E.; Duval, E.J.; Barton, L.M.; et al. COVID 19: A Multidisciplinary Review. Front. Public Health 2020, 8, 383. [Google Scholar] [CrossRef]
- Meyerowitz, E.A.; Richterman, A.; Gandhi, R.T.; Sax, P.E. Transmission of SARS-CoV-2: A Review of Viral, Host, and Environmental Factors. Ann. Intern. Med. 2021, 174, 69–79. [Google Scholar] [CrossRef]
- Manigandan, S.; Wu, M.-T.; Ponnusamy, V.K.; Raghavendr, V.B.; Pugazhendhi, A.; Brindhadevi, K. A systematic review on recent trends in transmission, diagnosis, prevention and imaging features of COVID-19. Process Biochem. 2020, 98, 233–240. [Google Scholar] [CrossRef] [PubMed]
- OECD. The Territorial Impact of COVID-19: Managing the Crisis across Levels of Government; The Organisation for Economic Co-operation and Development (OECD): Paris, France, 2020. [Google Scholar]
- Forouzandeh, P.; O’Dowd, K.; Pillai, S.C. Face masks and respirators in the fight against the COVID-19 pandemic: An overview of the standards and testing methods. Saf. Sci. 2021, 133, 104995. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.Y.; Thone, M.N.; Kwon, Y.J. COVID-19 vaccines: The status and perspectives in delivery points of view. Adv. Drug Deliv. Rev. 2021, 170, 1–25. [Google Scholar] [CrossRef]
- de Bruin, Y.B.; Lequarre, A.-S.; McCourt, J.; Clevestig, P.; Pigazzani, F.; Jeddi, M.Z.; Colosio, C.; Goulart, M. Initial impacts of global risk mitigation measures taken during the combating of the COVID-19 pandemic. Saf. Sci. 2020, 128, 104773. [Google Scholar] [CrossRef] [PubMed]

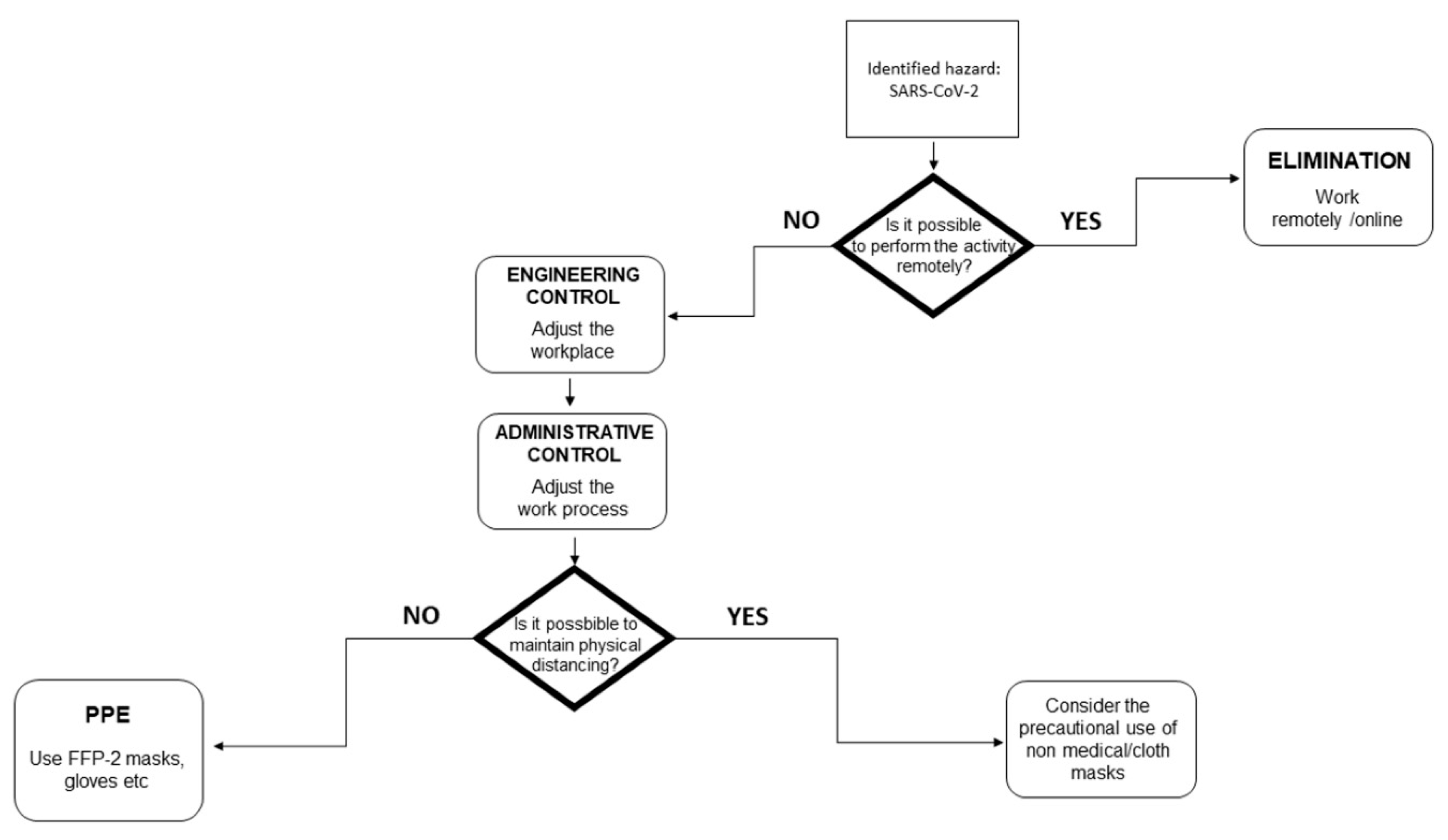
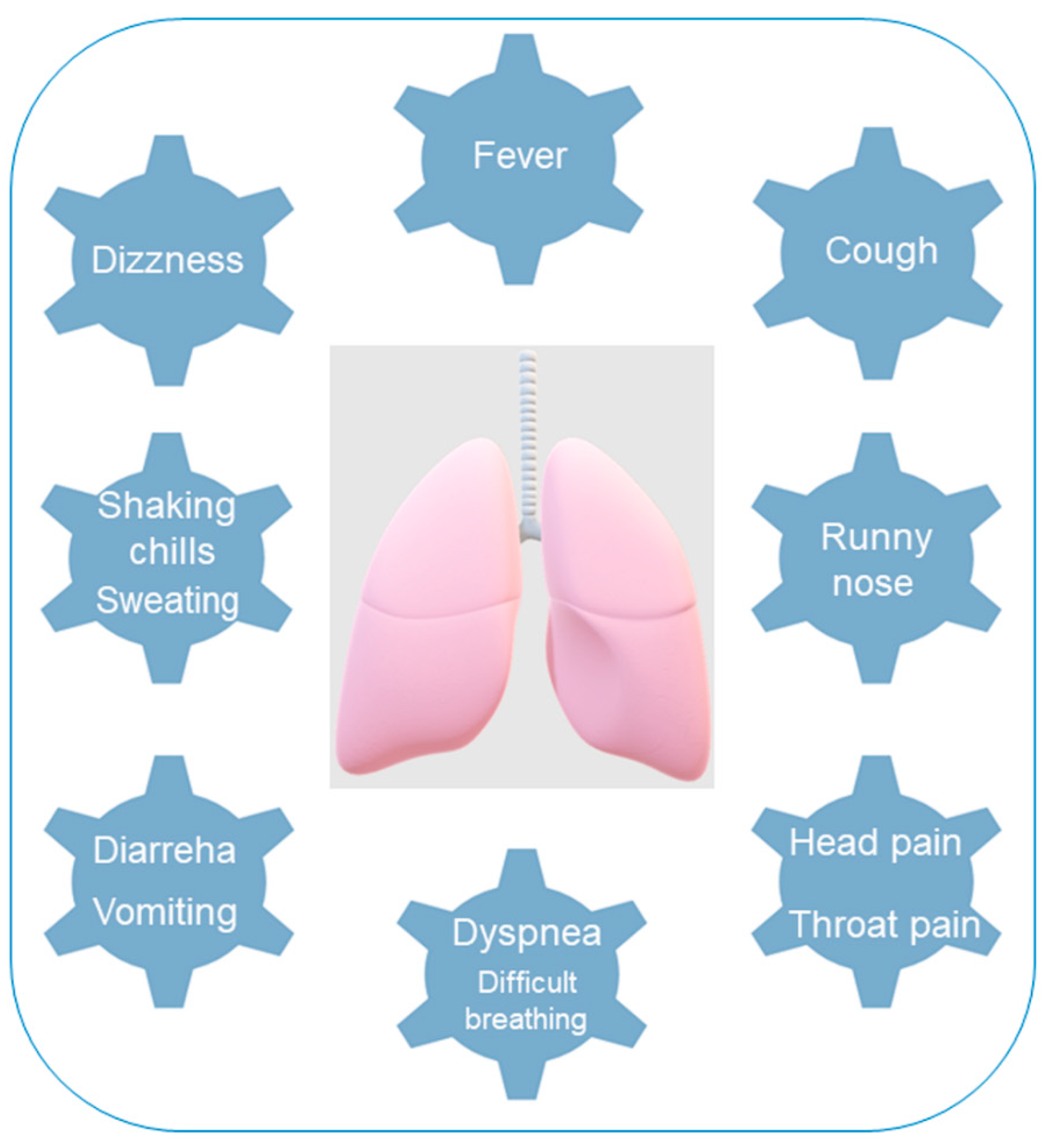
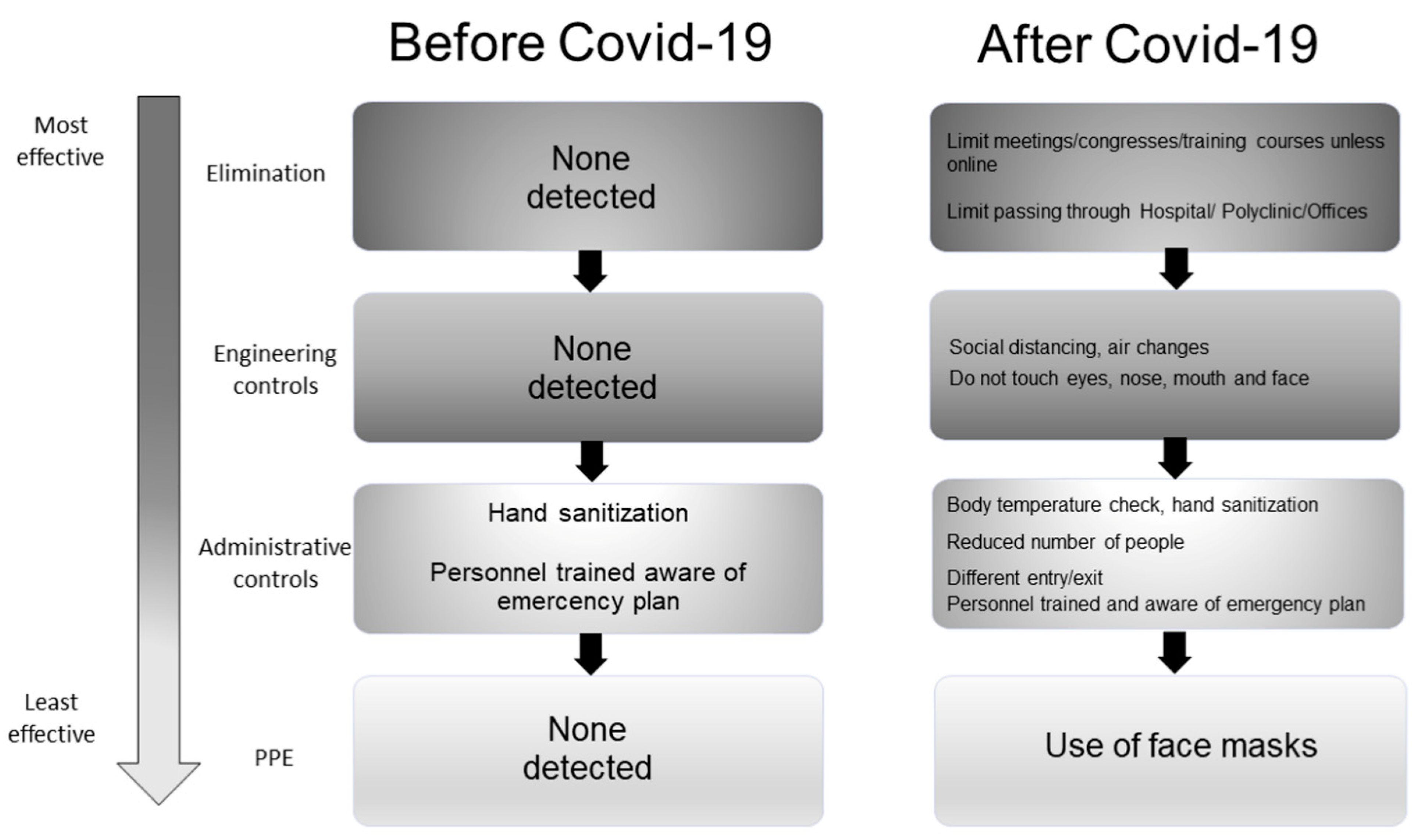

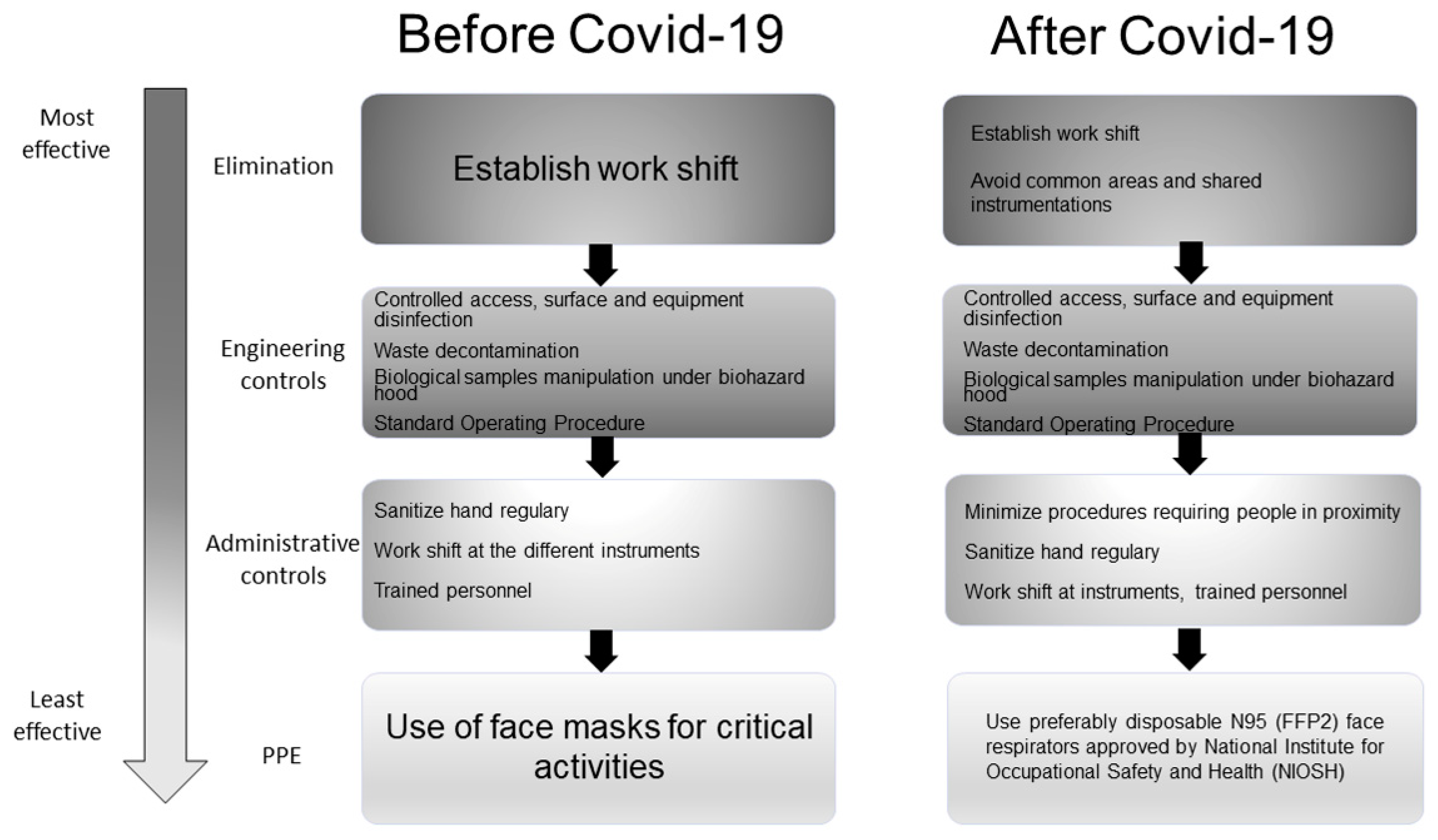
| Variable | Definition |
|---|---|
| Exposure | The probability of contagion during work activities |
| Proximity | Physical distancing during working periods |
| Aggregation | Contact with other subjects in addition to colleagues |
| Level of Risk Assessment | Sub-Context | Operator | Activity |
|---|---|---|---|
| General | Hospital, polyclinic | Orthopedics, rheumatologists, radiologists, oncologists, other specialists, nurses, physiotherapists, pharmacists, teachers, social workers, psychologists, researchers | Clinical and surgical activities carried out on patients |
| Management and administrative offices | Technical and administrative staff | Administrative and managing activities | |
| Extra-Lab | Staff, meeting, training rooms | Researchers (biologists, biotechnologists, technicians, bioengineers, physicians, chemists) | Project/manuscript design, writing and submission bibliographic research, meetings, teaching |
| Warehouse | Warehouse workers | Materials handling | |
| Toilets | Cleaners | Cleaning | |
| Lab | Lab rooms: cell culture, histochemistry immunohistochemistry, molecular biology, 3D printing | Researchers (biologists, biotechnologists, technicians, bioengineers, physicians, chemists) | Cell culturing, immunohistochemistry, molecular biology, 3D printing |
| Animal facility | Veterinarians | Animal caring and surgery |
| Control Measure | Definition |
|---|---|
| Elimination | Physically remove the hazard |
| Substitution | Replace the hazard |
| Engineering controls | Isolate people from the hazard |
| Administrative controls | Change the way people work |
| PPE | Protect the worker with PPE |
| Type of Document | Characteristic |
|---|---|
| Institutional guidelines for research facilities [25,26,27] | Legitimate at state level |
| Guidelines for research facilities [28] | Legitimate at local level |
| University guidelines for research facilities [29] | Tailored for each University |
| Enterprise guidelines for R&D facilities [30,31] | Specific for R&D environment |
| Risk | Transmission |
|---|---|
| Contact | Direct (through secretions), indirect |
| Respiratory droplet (>5–10 μm) | Proximity with an infected person coughing, sneezing, talking, or singing |
| Airborne | Aerosols (<5 μm droplets) that remain infectious when suspended in air over long times and distances |
| Fomite | Touching of fomites (surfaces or objects contaminated with secretions or droplets from an infected person), followed by touching the mouth, nose, or eyes |
| Animal-to-human | SARS-CoV-2 is most closely related to beta coronaviruses in bats; the role of intermediate host in facilitating transmission in humans remains unclear |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roseti, L.; Grigolo, B. COVID-19 Impact on Musculoskeletal Regenerative Medicine Research: Maintaining Lab Continuity. Int. J. Environ. Res. Public Health 2021, 18, 6110. https://doi.org/10.3390/ijerph18116110
Roseti L, Grigolo B. COVID-19 Impact on Musculoskeletal Regenerative Medicine Research: Maintaining Lab Continuity. International Journal of Environmental Research and Public Health. 2021; 18(11):6110. https://doi.org/10.3390/ijerph18116110
Chicago/Turabian StyleRoseti, Livia, and Brunella Grigolo. 2021. "COVID-19 Impact on Musculoskeletal Regenerative Medicine Research: Maintaining Lab Continuity" International Journal of Environmental Research and Public Health 18, no. 11: 6110. https://doi.org/10.3390/ijerph18116110
APA StyleRoseti, L., & Grigolo, B. (2021). COVID-19 Impact on Musculoskeletal Regenerative Medicine Research: Maintaining Lab Continuity. International Journal of Environmental Research and Public Health, 18(11), 6110. https://doi.org/10.3390/ijerph18116110







