Trends and Age-Period-Cohort Effects on the Prevalence, Incidence and Mortality of Hepatocellular Carcinoma from 2008 to 2017 in Tianjin, China
Abstract
1. Introduction
2. Method
2.1. Data Source
2.2. Target Population and Case Identification
2.3. Statistical Analysis
2.3.1. Joinpoint Regression Analysis
2.3.2. Age-Period-Cohort Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.F.B.; International Agency for Research on Cancer. Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.fr/today (accessed on 14 January 2021).
- Liu, Z.; Jiang, Y.; Yuan, H.; Fang, Q.; Cai, N.; Suo, C.; Jin, L.; Zhang, T.; Chen, X. The trends in incidence of primary liver cancer caused by specific etiologies: Results from the Global Burden of Disease Study 2016 and implications for liver cancer prevention. J. Hepatol. 2019, 70, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sun, H.C.; Wang, Z.; Cong, W.M.; Wang, J.H.; Zeng, M.S.; Yang, J.M.; Bie, P.; Liu, L.X.; Wen, T.F.; et al. Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition). Liver Cancer 2018, 7, 235–260. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.B.; Chang, P.E.; Tan, C.K. Changing epidemiology of hepatocellular carcinoma in Asia. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Yeesoonsang, S.; McNeil, E.; Virani, S.; Bilheem, S.; Pittayawonganon, C.; Jiraphongsa, C.; Sriplung, H. Trends in Incidence of Two Major Subtypes of Liver and Bile Duct Cancer: Hepatocellular Carcinoma and Cholangiocarcinoma in Songkhla, Southern Thailand, 1989–2030. J. Cancer Epidemiol. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Wallace, M.C.; Preen, D.; Jeffrey, G.P.; Adams, L.A. The evolving epidemiology of hepatocellular carcinoma: A global perspective. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 765–779. [Google Scholar] [CrossRef]
- Wallace, M.C.; Preen, D.B.; Short, M.W.; Adams, L.A.; Jeffrey, G.P. Hepatocellular carcinoma in Australia 1982–2014: Increasing incidence and improving survival. Liver Int. Off. J. Int. Assoc. Study Liver 2019, 39, 522–530. [Google Scholar] [CrossRef]
- Jepsen, P.; Andersen, M.W.; Villadsen, G.E.; Ott, P.; Vilstrup, H. Time-trends in incidence and prognosis of hepatocellular carcinoma in Denmark: A nationwide register-based cohort study. Liver Int. Off. J. Int. Assoc. Study Liver 2017, 37, 871–878. [Google Scholar] [CrossRef]
- White, D.L.; Thrift, A.P.; Kanwal, F.; Davila, J.; El-Serag, H.B. Incidence of Hepatocellular Carcinoma in All 50 United States, From 2000 Through 2012. Gastroenterology 2017, 152, 812–820.e815. [Google Scholar] [CrossRef]
- Bertuccio, P.; Turati, F.; Carioli, G.; Rodriguez, T.; La Vecchia, C.; Malvezzi, M.; Negri, E. Global trends and predictions in hepatocellular carcinoma mortality. J. Hepatol. 2017, 67, 302–309. [Google Scholar] [CrossRef]
- Bai, L.; Liu, Z.; Fang, Q.; Yan, Q.; Shi, O.; Bao, P.; Mu, L.; Chen, X.; Zhang, T. The trends and projections in the incidence and mortality of liver cancer in urban Shanghai: A population-based study from 1973 to 2020. Clin. Epidemiol. 2018, 10, 277–288. [Google Scholar] [CrossRef]
- Ling, L.; Hong, X.; Yueping, M.; Bo, C. Trend and quantitative analysis of liver cancer mortality in Nantong City from 2005 to 2014. Occup. Health 2017, 33, 2224–2227. (In Chinese) [Google Scholar]
- Zheng, W.; Hui, L. The mortality rate of liver cancer from 2011 to 2018 in Fuzhou. J. Chronic Med. 2019, 19, 40–43. (In Chinese) [Google Scholar]
- Liang, Z.; Wen, L. The trend in mortality of liver cancer among urban residents from 2007 to 2015 in Tieling. Chin. Health Stat. 2017, 34, 467–468. (In Chinese) [Google Scholar]
- Wenbin, H.; Ting, Z.; Wei, Q.; Yong, S.; Lan, T. Incidence and Mortality Trend of Liver Cancer in Kunshan, Jiangsu Province, 2006–2013. China Cancer 2015, 24, 824–829. (In Chinese) [Google Scholar]
- Ji, X.W.; Jiang, Y.; Wu, H.; Zhou, P.; Tan, Y.T.; Li, H.L.; Zhang, L.; Zhao, W.S.; Xia, Q.H.; Bray, F.; et al. Long-term liver cancer incidence and mortality trends in the Changning District of Shanghai, China. J. Dig. Dis. 2020, 21, 230–236. [Google Scholar] [CrossRef]
- Lin, L.; Haibin, Z.; Qinggang, S.; Weigeng, L. Epidemiologic and incidence trends and characters of liver cancer in Shenzhen. Chin. J. Prev. Control Chronic Dis. 2019, 5, 340–343. (In Chinese) [Google Scholar]
- Li, K.; Lin, G.; Li, Y.; Xu, H. Epidemiologic trends and patterns of primary liver cancer in Guangzhou, 2004–2013. Chin. J. Cancer Prev. Treat. 2016, 23, 1527–1530. (In Chinese) [Google Scholar]
- Wenjie, L.; Liangyou, W.; Dongju, Q.; Xiangyang, Y. The trend of incidence and mortality of liver cancer among residents in Taizhou from 2010 to 2016. Prev. Med. 2019, 31, 933–935. (In Chinese) [Google Scholar]
- Liu, W.; Liu, Q.; Huang, Q.; Lu, Y.; Xie, S.; Lin, A.; Cao, S. Time trend analysis of primary liver cancer incidence in Sihui county of Guangdong Province, China (1987–2011). BMC Cancer 2016, 16, 796. [Google Scholar] [CrossRef]
- Xianbin, D.; Xiaoyan, L.; Wenge, T.; Deqiang, M.; Yan, J. Trend changes of incidence of liver cancer in Chongqing Municipality, 2006–2015. Pract. Prev. Med. 2017, 24, 1435–1439. (In Chinese) [Google Scholar]
- Shih, W.L.; Chang, H.C.; Liaw, Y.F.; Lin, S.M.; Lee, S.D.; Chen, P.J.; Liu, C.J.; Lin, C.L.; Yu, M.W. Influences of tobacco and alcohol use on hepatocellular carcinoma survival. Int. J. Cancer 2012, 131, 2612–2621. [Google Scholar] [CrossRef] [PubMed]
- National Bureau of Statistics. The People’s Republic of China Statistical Bulletin on National Economic and Social Development in 2019. Available online: http://www.stats.gov.cn/tjsj/zxfb/202002/t20200228_1728913.html (accessed on 24 September 2020).
- Fan, G.; Deng, Z.; Wu, X.; Wang, Y. Medical insurance and health equity in health service utilization among the middle-aged and older adults in China: A quantile regression approach. BMC Health Serv. Res. 2020, 20, 553. [Google Scholar] [CrossRef]
- Tianjin Municipal People’s Government. Tianjin Statistical Bulletin on National Economic and Social Development in 2020. Available online: http://stats.tj.gov.cn/tjsj_52032/tjgb/202103/t20210317_5386752.html (accessed on 17 March 2021).
- Doll, R.; Smith, P.G. Comparison between registries: Age-standardized rates. Vol. IV. IARC Sci Publ No. 42.. Cancer incidence in fivecontinents. Lyon IARC 1982, 671–675. [Google Scholar]
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef]
- Fu, W.J.; Land, K.C. A Methodological Comparison of Age-Period-Cohort Models: The Intrinsic Estimator and Conventional Generalized Linear Models. Sociol. Methodol. 2004, 34, 75–110. [Google Scholar]
- Hajizadeh, N.; Baghestani, A.R.; Pourhoseingholi, M.A.; Ashtari, S.; Fazeli, Z.; Vahedi, M.; Zali, M.R. Trend of hepatocellular carcinoma incidence after Bayesian correction for misclassified data in Iranian provinces. World J. Hepatol. 2017, 9, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Fu, X.; Zhou, Y.; Liu, X.; Wu, J.; Huang, C.; Deng, M.; Li, Y.; Li, L.; Yang, S. Disease burden of liver cancer in China from 1997 to 2016: An observational study based on the Global Burden of Diseases. BMJ Open 2019, 9, e025613. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Qu, C.; Zhang, S.; Zeng, H.; Sun, K.; Gu, X.; Xia, C.; Yang, Z.; Li, H.; Wei, W.; et al. Liver cancer incidence and mortality in China: Temporal trends and projections to 2030. Chin. J. Cancer Res. Chung-Kuo Yen Cheng Yen Chiu 2018, 30, 571–579. [Google Scholar] [CrossRef]
- Peto, R.; Parish, S.E.; Gray, R.G. There is no such thing as ageing, and cancer is not related to it. IARC Sci. Publ. 1985, 58, 43–53. [Google Scholar]
- Liu, P.; Xie, S.H.; Hu, S.; Cheng, X.; Gao, T.; Zhang, C.; Song, Z. Age-specific sex difference in the incidence of hepatocellular carcinoma in the United States. Oncotarget 2017, 8, 68131–68137. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Mubarik, S.; Zhang, Y.; Wang, L.; Wang, Y.; Yu, C.; Li, H. Long-Term Trends of Liver Cancer Incidence and Mortality in China 1990–2017: A Joinpoint and Age-Period-Cohort Analysis. Int. J. Environ. Res. Public Health 2019, 16, 2878. [Google Scholar] [CrossRef] [PubMed]

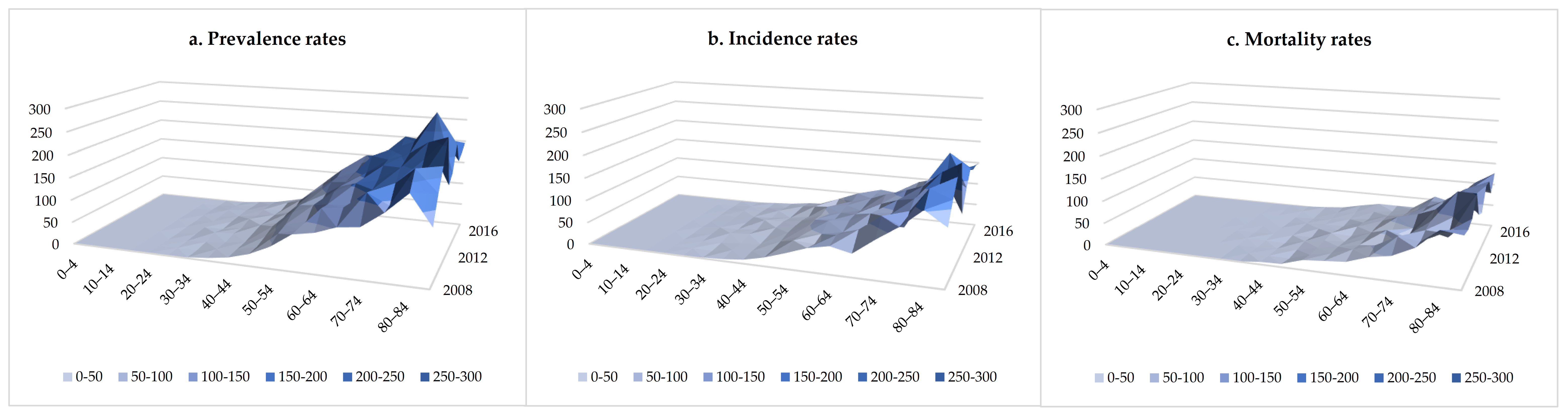

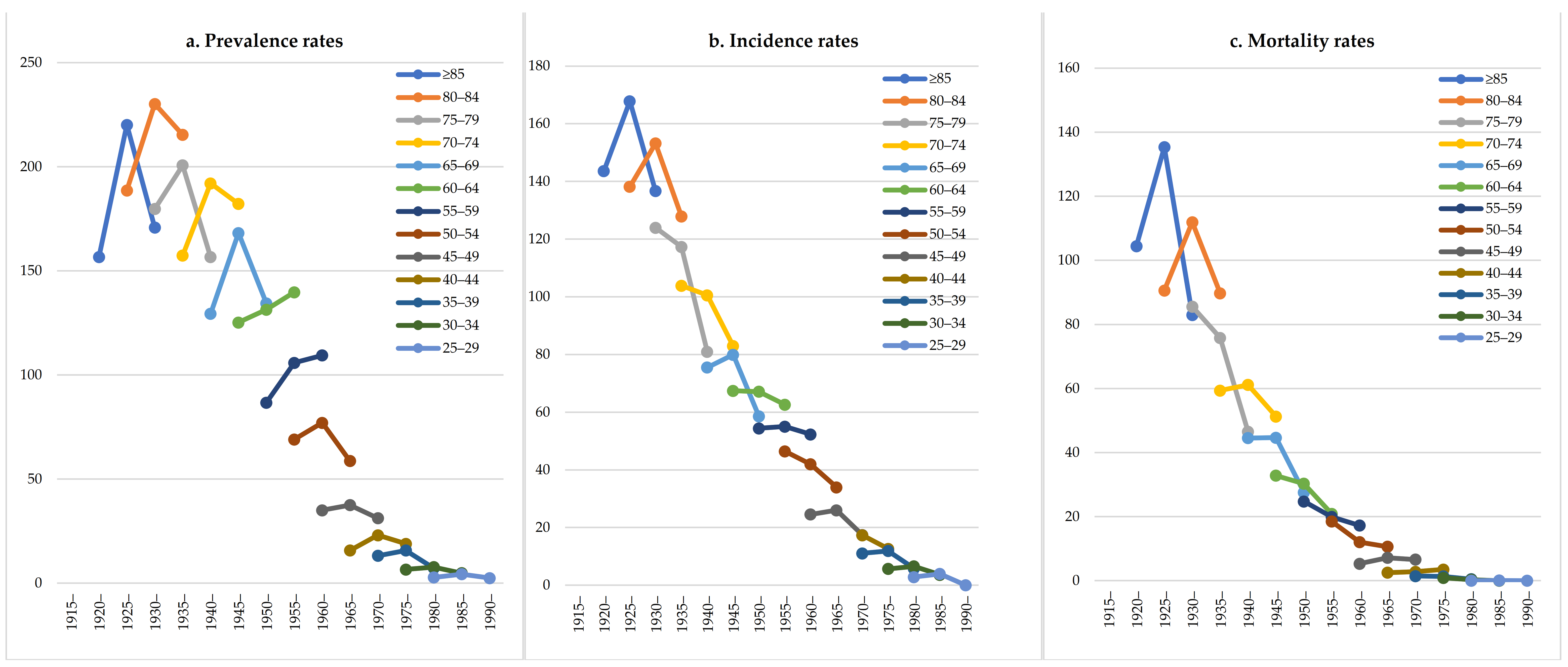

| Year | Overall | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Crude Rate (1/105) | ASR † (1/105) | Cases | Crude Rate (1/105) | ASR † (1/105) | Cases | Crude Rate (1/105) | ASR † (1/105) | |
| Prevalence | |||||||||
| 2008 | 477 | 38.07 | 20.12 | 386 | 60.22 | 30.97 | 91 | 14.87 | 8.34 |
| 2009 | 649 | 46.22 | 24.85 | 463 | 62.81 | 33.01 | 186 | 27.88 | 15.80 |
| 2010 | 715 | 46.86 | 25.73 | 519 | 63.56 | 34.67 | 196 | 27.64 | 15.60 |
| 2011 | 874 | 60.36 | 31.19 | 586 | 76.65 | 39.78 | 288 | 42.13 | 21.85 |
| 2012 | 894 | 58.68 | 30.24 | 632 | 78.84 | 40.98 | 262 | 36.30 | 18.66 |
| 2013 | 982 | 61.40 | 31.41 | 684 | 81.19 | 41.82 | 298 | 39.37 | 20.23 |
| 2014 | 1075 | 70.33 | 33.76 | 743 | 93.16 | 45.31 | 332 | 45.42 | 21.65 |
| 2015 | 1102 | 65.45 | 32.28 | 763 | 86.11 | 43.41 | 339 | 42.51 | 20.42 |
| 2016 | 1176 | 72.00 | 32.88 | 827 | 96.80 | 45.64 | 349 | 44.80 | 19.40 |
| 2017 | 1141 | 71.37 | 30.49 | 809 | 97.30 | 42.50 | 332 | 43.27 | 17.89 |
| Incidence | |||||||||
| 2008 | 334 | 26.65 | 13.91 | 264 | 41.19 | 20.86 | 70 | 11.43 | 6.33 |
| 2009 | 443 | 31.55 | 16.93 | 305 | 41.38 | 21.78 | 138 | 20.69 | 11.48 |
| 2010 | 445 | 29.17 | 15.91 | 310 | 37.96 | 20.49 | 135 | 19.04 | 10.67 |
| 2011 | 557 | 38.47 | 19.98 | 346 | 45.26 | 23.66 | 211 | 30.87 | 16.06 |
| 2012 | 557 | 36.56 | 18.87 | 375 | 46.78 | 24.37 | 182 | 25.21 | 12.96 |
| 2013 | 592 | 37.01 | 19.24 | 379 | 44.99 | 23.55 | 213 | 28.14 | 14.65 |
| 2014 | 612 | 40.04 | 19.50 | 393 | 49.28 | 24.28 | 219 | 29.96 | 14.43 |
| 2015 | 611 | 36.29 | 18.36 | 410 | 46.27 | 23.94 | 201 | 25.20 | 12.32 |
| 2016 | 630 | 38.57 | 17.88 | 430 | 50.33 | 24.16 | 200 | 25.67 | 11.27 |
| 2017 | 524 | 32.78 | 14.09 | 351 | 42.21 | 18.43 | 173 | 22.55 | 9.56 |
| Mortality ‡ | |||||||||
| 2008 | 196 | 15.64 | 8.18 | 171 | 26.68 | 13.40 | 25 | 4.08 | 2.28 |
| 2009 | 226 | 16.09 | 8.63 | 172 | 23.33 | 12.00 | 54 | 8.09 | 4.75 |
| 2010 | 213 | 13.96 | 7.47 | 165 | 20.21 | 10.65 | 48 | 6.77 | 3.94 |
| 2011 | 235 | 16.23 | 8.28 | 173 | 22.63 | 11.42 | 62 | 9.07 | 4.77 |
| 2012 | 240 | 15.75 | 7.67 | 175 | 21.83 | 10.60 | 65 | 9.00 | 4.43 |
| 2013 | 272 | 17.01 | 8.52 | 202 | 23.98 | 12.12 | 70 | 9.25 | 4.63 |
| 2014 | 253 | 16.55 | 7.79 | 188 | 23.57 | 11.19 | 65 | 8.89 | 4.12 |
| 2015 | 246 | 14.61 | 6.86 | 179 | 20.20 | 9.49 | 67 | 8.40 | 4.04 |
| 2016 | 261 | 15.98 | 7.02 | 194 | 22.71 | 10.29 | 67 | 8.60 | 3.58 |
| 2017 | 281 | 17.58 | 7.34 | 216 | 25.98 | 11.14 | 65 | 8.47 | 3.37 |
| Group | Joinpoint | Years | APC (95%CI) | p-Value | AAPC (95%CI) | p-Value |
|---|---|---|---|---|---|---|
| Prevalence | ||||||
| Overall | 1 | 2008–2011 | 14.97 (1.83, 29.81) | 0.032 * | 5.13 (1.56, 8.83) | 0.005 * |
| 2011–2017 | 0.53 (−2.52, 3.68) | 0.678 | ||||
| Male | 1 | 2008–2014 | 6.38 (3.69, 9.14) | 0.002 * | 3.62 (1.54, 5.76) | 0.001 * |
| 2014–2017 | −1.68 (−7.55, 4.57) | 0.512 | ||||
| Female | 1 | 2008–2011 | 27.65 (−5.29, 72.05) | 0.090 | 7.01 (−1.52, 16.27) | 0.110 |
| 2011–2017 | −2.03 (−8.32, 4.70) | 0.463 | ||||
| Incidence | ||||||
| Overall | 1 | 2008–2014 | 5.05 (−0.47, 10.88) | 0.066 | −0.31 (−4.77, 4.35) | 0.893 |
| 2014–2017 | −10.24 (−22.27, 3.66) | 0.112 | ||||
| Male | 1 | 2008–2015 | 2.71 (0.48, 4.98) | 0.026 * | −0.82 (−3.62, 2.07) | 0.576 |
| 2015–2017 | −12.22 (−24.51, 2.07) | 0.077 | ||||
| Female | 1 | 2008–2011 | 29.10 (−8.30, 81.75) | 0.113 | 3.98 (−5.60, 14.53) | 0.429 |
| 2011–2017 | −6.69 (−14.13, 1.41) | 0.085 | ||||
| Mortality | ||||||
| Overall | 0 | 2008–2017 | −1.80 (−3.37, −0.20) | 0.032 * | −1.80 (−3.37, −0.20) | 0.032 * |
| Male | 0 | 2008–2017 | −0.10 (−5.85, 5.99) | 0.969 | −0.10 (−5.85, 5.99) | 0.969 |
| Female | 0 | 2008–2017 | −12.19 (−22.32, −0.75) | 0.040 * | −12.19 (−22.32, −0.75) | 0.040 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Wu, J.; Chang, Z. Trends and Age-Period-Cohort Effects on the Prevalence, Incidence and Mortality of Hepatocellular Carcinoma from 2008 to 2017 in Tianjin, China. Int. J. Environ. Res. Public Health 2021, 18, 6034. https://doi.org/10.3390/ijerph18116034
Liu C, Wu J, Chang Z. Trends and Age-Period-Cohort Effects on the Prevalence, Incidence and Mortality of Hepatocellular Carcinoma from 2008 to 2017 in Tianjin, China. International Journal of Environmental Research and Public Health. 2021; 18(11):6034. https://doi.org/10.3390/ijerph18116034
Chicago/Turabian StyleLiu, Chengyu, Jing Wu, and Zheng Chang. 2021. "Trends and Age-Period-Cohort Effects on the Prevalence, Incidence and Mortality of Hepatocellular Carcinoma from 2008 to 2017 in Tianjin, China" International Journal of Environmental Research and Public Health 18, no. 11: 6034. https://doi.org/10.3390/ijerph18116034
APA StyleLiu, C., Wu, J., & Chang, Z. (2021). Trends and Age-Period-Cohort Effects on the Prevalence, Incidence and Mortality of Hepatocellular Carcinoma from 2008 to 2017 in Tianjin, China. International Journal of Environmental Research and Public Health, 18(11), 6034. https://doi.org/10.3390/ijerph18116034







