Burden of COVID-19 on Italian Internal Medicine Wards: Delphi, SWOT, and Performance Analysis after Two Pandemic Waves in the Local Health Authority “Roma 6” Hospital Structures
Abstract
1. Introduction
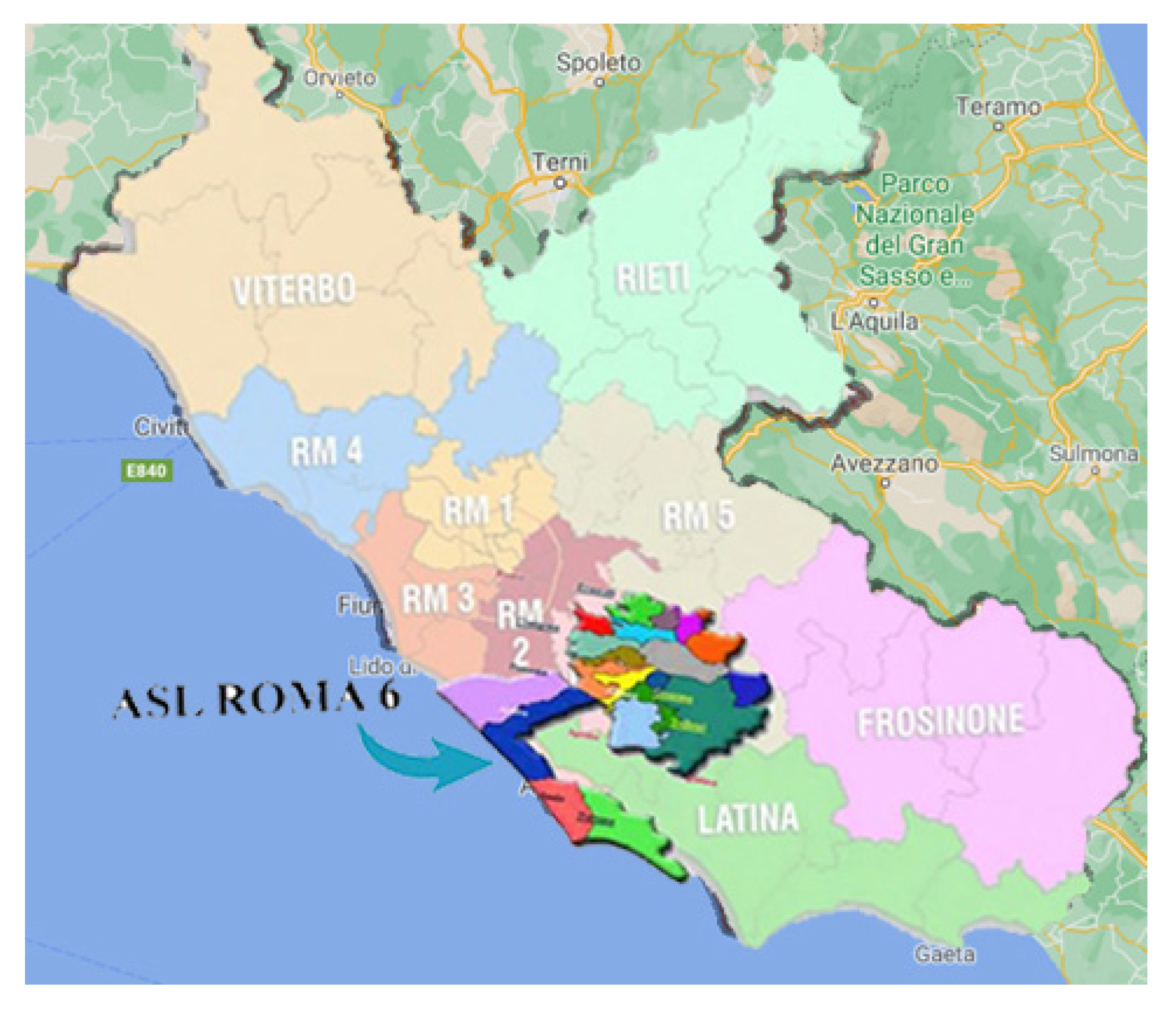
1.1. The Local Health Authority “Azienda ASL Roma 6”
2. Materials and Methods
2.1. Study Design
- Phase 1: Delphi Method application [21].
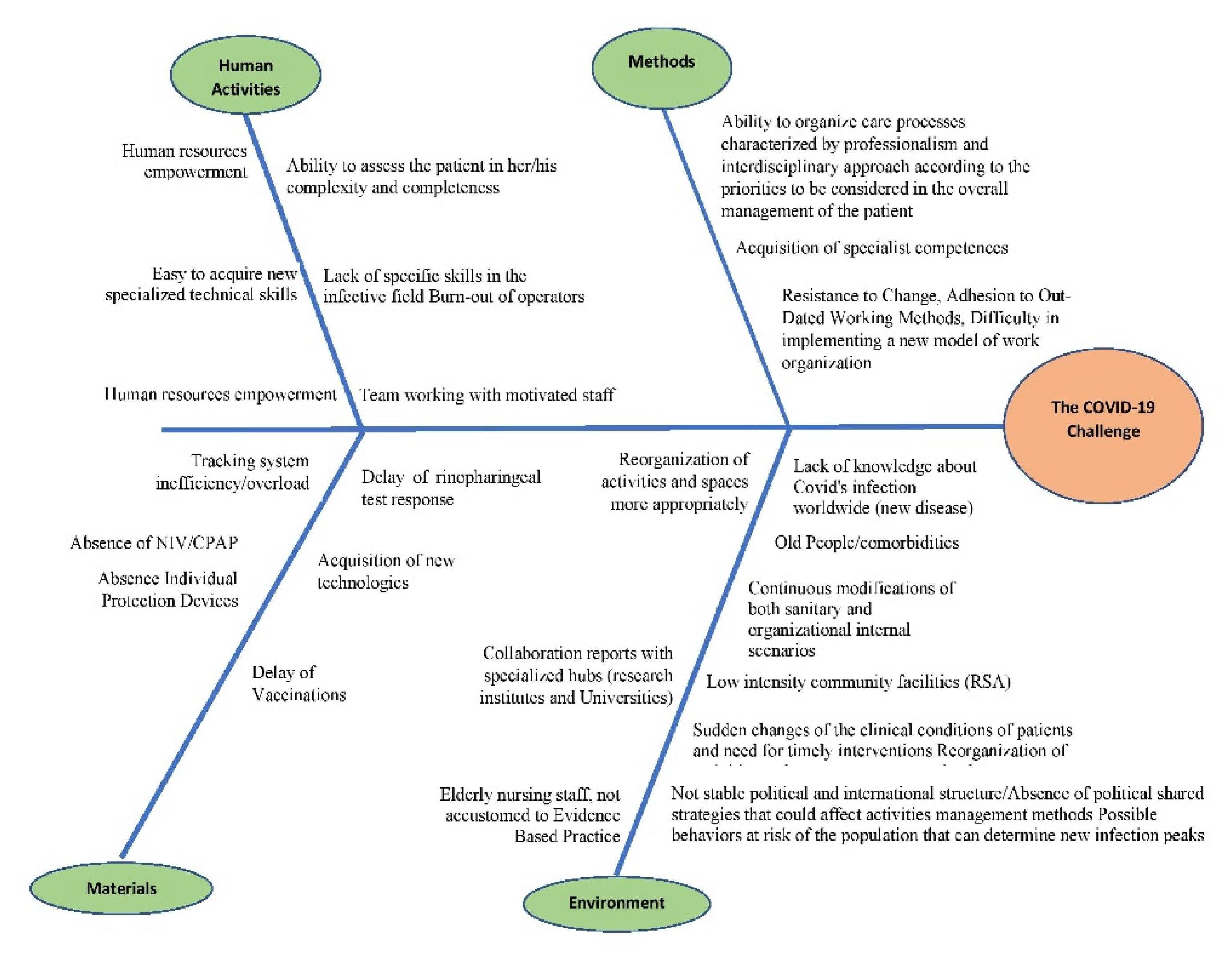
- The Delphi method is a forecasting process framework based on the results of multiple rounds of questionnaires sent to a panel of experts. Its application is quick and easy and can lead to a consensus among a group of experts. In this case, the personnel involved in the MC and MCF wards took turns to answer the question: “What is the reason behind bad clinical outcome in some COVID patients?”
- For any topic retrieved during the previous phase, a panel of experts (10 people, with clinical, public health, health economics, and statistics expertise) assigned a score relating to:
- −
- perceived value resulting from the balance between strengths and weaknesses;
- −
- potential risk resulting from the balance between threats and opportunities.
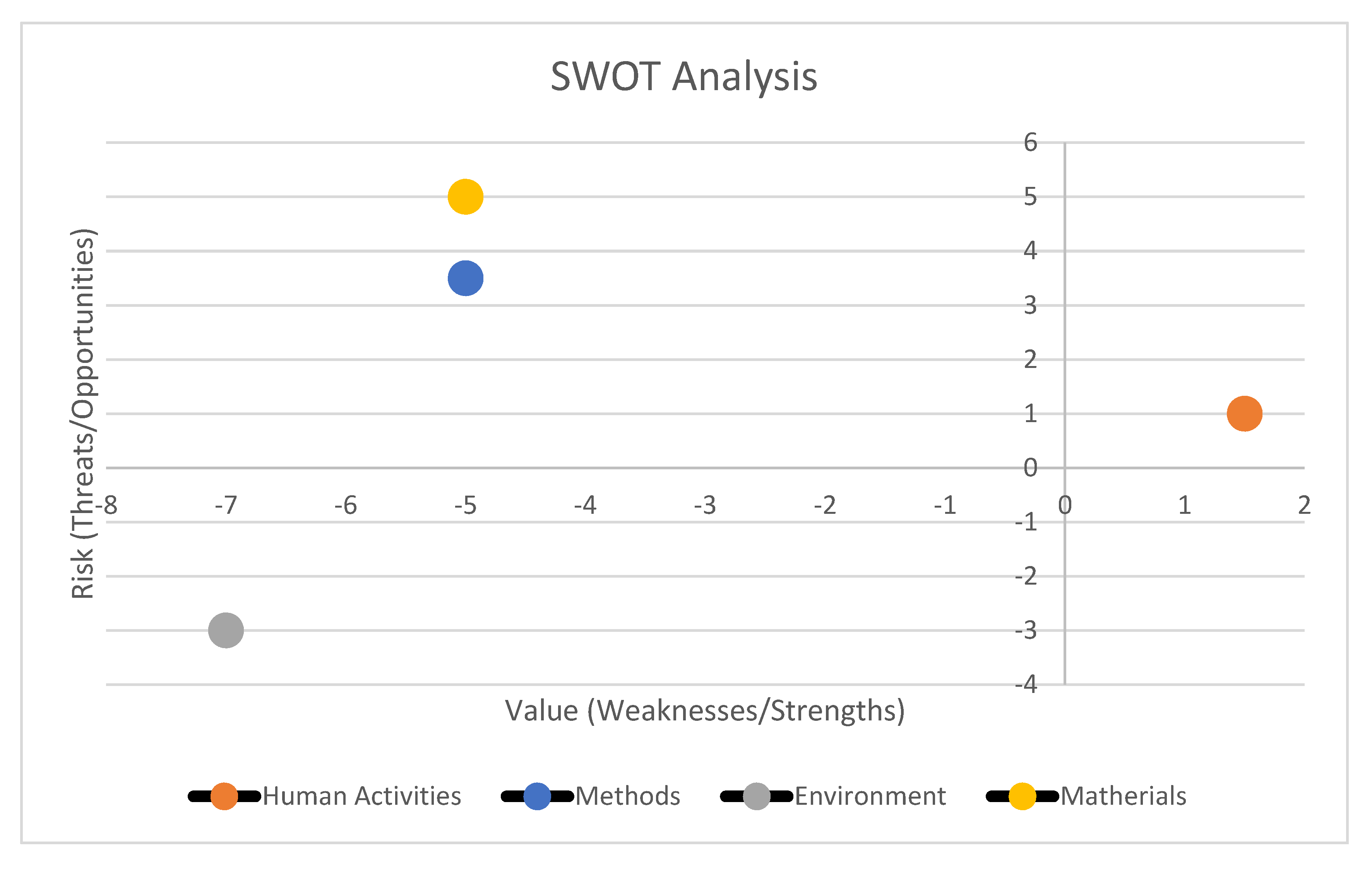
- These scores ranged on a Likert scale from -10 (minimum added value or minimum risk) to +10 (which corresponded to the maximum added value or maximum risk) [9]. Then, a scatterplot graph was drawn. Overall weights relating to the perceived value were plotted on the x-axis and the associated potential risk was plotted on the y-axis. The risk/value ratio could fall into any of the following 4 areas, as identified by the graph: a low risk and a high value area (Comfort Zone), a high risk and low value area (Danger Zone), a high risk and high value area (Challenge Area), and a low risk and low value area (Improvement Area).
- Phase 3: Retrospective Ecological Performance Analysis (with data retrieved from existing sources).
- During this phase, data from the hospital discharge forms (SDO—Scheda Dimissione Ospedaliera) of the patients were retrieved and analyzed.
- Descriptive population statistics were calculated (age, death rate, number of comorbidities, and provenience). Likewise, for every ward unit involved in the study, all of the elements necessary for drawing a Barber’s nomogram were either retrieved (total patient numbers, total admission days, total admission numbers, total discharges/deaths, start/end of activity dates, and available bed numbers) or calculated (average bed availability per month (B/M), bed occupancy rate (BOR), length of stay (LOS), turnover index (TI), bed rotation index (BRI), and admitted patients’ average age (AV.AG)).
- Barber’s nomogram is an index of bed utilization and is defined on the basis of the following: LOS [24], average LOS [25], BOR [26], TI [27], and BRI. A nomogram that takes into account the aforementioned variables, and allows the setup of a range of values in order to assess the performance in the delivery of care, is then derived. In our study, the following was considered acceptable: a LOS within 12 days, a BOR of 75%, and a maximum TI between 1 and 3 days [28].
2.2. Population, Variables, and Data Sources
3. Results
3.1. Phase 1
- Human activities (i.e., mostly reasons associated with staff empowerment, motivation, and well-being)
- Methods (i.e., mostly related to workflow, work mentality, resistance to change)
- Materials (i.e., situational causes due to material shortages or procedural delays)
- Environment (i.e., situational causes due to either the emergent pandemic itself, or the structural situation of healthcare in the region, whose manpower and resources have been depleted after years of public sector neglect, healthcare budget cuts, and turnover lock).
3.2. Phase 2
3.3. Phase 3
4. Discussion
5. Conclusions
Implications for Professional Practice and Policy Makers
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Characterizes COVID-19 as a Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen (accessed on 25 May 2021).
- Stawicki, S.P.; Jeanmonod, R.; Miller, A.; Paladino, L.; Gaieski, D.F.; Yaffee, A.Q.; De Wulf, A.; Grover, J.; Papadimos, T.J.; Bloem, C.; et al. The 2019–2020 Novel Coronavirus (Severe Acute Respiratory Syndrome Coronavirus 2) Pandemic: A Joint American College of Academic International Medicine-World Academic Council of Emergency Medicine Multidisciplinary COVID-19 Working Group Consensus Paper. J. Glob. Infect. Dis. 2020, 12, 47–93. [Google Scholar] [CrossRef]
- Jin, Y.-H.; Evidence-Based Medicine Chapter of China International Exchange and Promotive Association for Medical and Health Care (CPAM); Zhan, Q.-Y.; Peng, Z.-Y.; Ren, X.-Q.; Yin, X.-T.; Cai, L.; Yuan, Y.-F.; Yue, J.-R.; Zhang, X.-C.; et al. Chemoprophylaxis, diagnosis, treatments, and discharge management of COVID-19: An evidence-based clinical practice guideline (updated version). Mil. Med. Res. 2020, 4, 41. [Google Scholar] [CrossRef]
- Gualano, M.R.; Lo Moro, G.; Voglino, G.; Bert, F.; Siliquini, R. Effects of Covid-19 Lockdown on Mental Health and Sleep Disturbances in Italy. Int. J. Environ. Res. Public Health 2020, 2, 17. [Google Scholar]
- Sartor, G.; Del Riccio, M.; Dal Poz, I.; Bonanni, P.; Bonaccorsi, G. COVID-19 in Italy: Considerations on official data. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020, 98, 188–190. [Google Scholar] [CrossRef] [PubMed]
- IHME/COVID-19 Projections. Institute for Health Metrics and Evaluation. Available online: https://covid19.healthdata.org/ (accessed on 25 May 2021).
- Laskar, P.; Yallapu, M.M.; Chauhan, S.C. ‘Tomorrow Never Dies’: Recent Advances in Diagnosis, Treatment, and Prevention Modalities against Coronavirus (COVID-19) amid Controversies. Diseases 2020, 6, 30. [Google Scholar] [CrossRef]
- Giamello, J.D.; Abram, S.; Bernardi, S.; Lauria, G. The emergency department in the COVID-19 era. Who are we missing? Eur. J. Emerg. Med. Off. J. Eur. Soc. Emerg. Med. 2020, 27, 305–306. [Google Scholar] [CrossRef]
- Ruggeri, M.; Cadeddu, C.; Roazzi, P.; Mandolini, D.; Grigioni, M.; Marchetti, M. Multi-Criteria-Decision-Analysis (MCDA) for the Horizon Scanning of Health Innovations an Application to COVID 19 Emergency. Int. J. Environ. Res. Public Health 2020, 26, 17. [Google Scholar]
- Italian National Institute for Health. Task force COVID-19 del Dipartimento Malattie Infettive e Servizio di Informatica, Istituto Superiore di Sanità. Epidemia COVID-19, Aggiornamento Nazionale: 2 Dicembre 2020. Available online: https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_2-dicembre-2020.pdf (accessed on 25 May 2021).
- Rosiello, F.; Desideri, E.; Vinci, A.; Zelinotti, L. Adequacy of hospitals in Rome to an unconventional event (CBRNe): TTX simulation and HTA. Eur. J. Public Health 2020. [Google Scholar] [CrossRef]
- Armocida, B.; Formenti, B.; Ussai, S.; Palestra, F.; Missoni, E. The Italian health system and the COVID-19 challenge. Lancet Public Health 2020, 5, e253. [Google Scholar] [CrossRef]
- Guglielmetti, L.; Chiesi, S. COVID-19 in Italy: Passing through bitter waters. Eur. Respir. J. 2020, 56. [Google Scholar] [CrossRef] [PubMed]
- Spandonaro, F.; D’Angela, D.; Polistena, B. Sanità: “Oltre l’emergenza: Verso una nuova ‘vision’ del nostro SSN”. Osservatorio Malattie Rare. Available online: https://www.osservatoriomalattierare.it/news/attualita/16942-sanita-oltre-l-emergenza-verso-una-nuova-vision-del-nostro-ssn (accessed on 25 May 2021).
- Cicchetti, A.; Bidino, R.D. Interim Analysis Sull’impatto Economico per l’Ssn del Covid-19 (DRG Ospedalieri e Costo Terapie Intensive):14. Available online: https://www.iss.it/documents/20126/0/Rapporto+ISS+COVID-19++49_2020.pdf/817f28da-128f-e2d6-6f53-a9ee3cf8e3d7?t=1592561756983 (accessed on 1 June 2021).
- Coronavirus Disease (COVID-19): Vaccines. Available online: https://www.who.int/news-room/q-a-detail/coronavirus-disease-(covid-19)-vaccines (accessed on 25 May 2021).
- Italian Ministry of Health. Piano Vaccinale Marzo. Available online: https://www.governo.it/sites/governo.it/files/210313_Piano_Vaccinale_marzo_2021_1.pdf (accessed on 25 May 2021).
- Pianificazione e Territorio—ASL Roma 6. Available online: https://www.aslroma6.it/amministrazione-trasparente/pianificazione-e-territorio (accessed on 25 May 2021).
- ASL Roma 6. Atto di Autonomia Aziendale. Available online: https://www.aslroma6.it/documents/20143/1082106/ATTO+Aziendale+2020+-+rettifiche+regione+23+giugno.pdf/969a667a-3ccd-9ecd-73a7-0c972b93a079 (accessed on 25 May 2021).
- Horton, R. Offline: COVID-19 is not a pandemic. Lancet Lond. Engl. 2020, 26, 874. [Google Scholar] [CrossRef]
- Niederberger, M.; Spranger, J. Delphi Technique in Health Sciences: A Map. Front. Public Health 2020. Available online: https://www.frontiersin.org/articles/10.3389/fpubh.2020.00457/full (accessed on 25 May 2021).
- Topor, D.R.; Dickey, C.; Stonestreet, L.; Wendt, J.; Woolley, A.; Budson, A. Interprofessional Health Care Education at Academic Medical Centers: Using a SWOT Analysis to Develop and Implement Programming. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6342375/ (accessed on 25 May 2021).
- Von Kodolitsch, Y.; Bernhardt, A.M.; Robinson, P.N.; Kölbel, T.; Reichenspurner, H.; Debus, S.; Detter, C. Analysis of Strengths, Weaknesses, Opportunities, and Threats as a Tool for Translating Evidence into Individualized Medical Strategies (I-SWOT). Aorta Stamford Conn. 2015, 3, 98–107. [Google Scholar] [CrossRef]
- Schweikhart, S.A.; Dembe, A.E. The applicability of Lean and Six Sigma techniques to clinical and translational research. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2009, 57, 748–755. [Google Scholar] [CrossRef]
- Simpson, J.E. Length of stay. Lancet Lond. Engl. 1977, 1, 531–533. [Google Scholar] [CrossRef]
- Casale, C.; Labbrozzi, D.; Nicolucci, A.; Carinci, F.; Avanzi, C.; Dell’Aquila, R.; Forcella, M.; Montemurno, C.; Procaccini, D.A.; Ruscitto, F.; et al. Evaluation of the efficiency of a nephrology department by the DRGs and Barber’s Nomogram. The role of comorbidity. Contrib. Nephrol. 1994, 109, 84–89. [Google Scholar]
- Kahn, K.L.; Rubenstein, L.V.; Draper, D.; Kosecoff, J.; Rogers, W.H.; Keeler, E.B.; Brook, R.H. The Effects of the DRG-Based Prospective Payment System on Quality of Care for Hospitalized Medicare Patients: An Introduction to the Series. JAMA 1990, 264, 1953–1955. [Google Scholar] [CrossRef] [PubMed]
- Draper, D.; Kahn, K.L.; Reinisch, E.J.; Sherwood, M.J.; Carney, M.F.; Kosecoff, J.; Keeler, E.B.; Rogers, W.H.; Savitt, H.; Allen, H. Studying the effects of the DRG-based prospective payment system on quality of care. Design, sampling, and fieldwork. JAMA 1990, 264, 1956–1961. [Google Scholar] [CrossRef]
- Mistichelli, J. Diagnosis Related Groups (DRGs) and the Prospective Payment System:10. Available online: https://repository.library.georgetown.edu/bitstream/handle/10822/556896/sn4.pdf?sequence=1&isAllowed=y (accessed on 1 June 2021).
- Vinci, A.; Ingravalle, F.; Mancinelli, S.; D’Ercole, M.; Lucaroni, F.; Palombi, L. Impact of Asbestos-Related Toxicity on Italian Working Population: Preliminary Incidence Data from the Last 5 Years across the Whole Country. In Toxic Chemical and Biological Agents; Sindona, G., Banoub, J.H., Di Gioia, M.L., Eds.; Springer: Dordrecht, The Netherlands, 2020; pp. 271–275. Available online: http://link.springer.com/10.1007/978-94-024-2041-8_29 (accessed on 25 May 2021).
- Ziller, E.; Milkowski, C. A Century Later: Rural Public Health’s Enduring Challenges and Opportunities. Am. J. Public Health 2020, 110, 1678–1686. [Google Scholar] [CrossRef]
- Cocoros, N.M.; Ording, A.G.; Horváth-Puhó, E.; Henderson, V.W.; Sørensen, H.T. In utero exposure to the 1918 pandemic influenza in Denmark and risk of dementia. Influenza Other Respir. Viruses 2018, 12, 314–318. [Google Scholar] [CrossRef]
- Coovadia, H.; Jewkes, R.; Barron, P.; Sanders, D.; McIntyre, D. The health and health system of South Africa: Historical roots of current public health challenges. Lancet Lond. Engl. 2009, 374, 817–834. [Google Scholar] [CrossRef]
- Coovadia, H.; Jewkes, R.; Barron, P.; Sanders, D.; McIntyre, D. How the COVID-19 infection tsunami revolutionized the work of respiratory physiotherapists: An experience from Northern Italy. Monaldi Arch. Chest Dis. Arch. Monaldi Mal. Torace 2020, 90. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Scotti, E. Internal medicine network: A new way of thinking hospital-territory integration and public-private partnership. Ital. J. Med. 2017, 11, 85–94. [Google Scholar] [CrossRef]
- Ambrosi, E.; Canzan, F.; Di Giulio, P.; Mortari, L.; Palese, A.; Tognoni, G.; Saiani, L.; di, A.C. The covid-19 emergency in the words of the nurses. Assist. Inferm. E Ric. AIR 2020, 39, 66–108. [Google Scholar]
- Fumagalli, C.; Rozzini, R.; Vannini, M.; Coccia, F.; Cesaroni, G.; Mazzeo, F.; Cola, M.; Bartoloni, A.; Fontanari, P.; Lavorini, F.; et al. Clinical risk score to predict in-hospital mortality in COVID-19 patients: A retrospective cohort study. BMJ Open 2020, 10, e040729. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Ventura, F.; Molinelli, A.; Barranco, R. COVID-19-related deaths in residential care homes for elderly: The situation in Italy. J. Forensic Leg Med. 2021, 80, 102179. [Google Scholar] [CrossRef] [PubMed]
- Joint Rapid Responce Forces (JRRFs). Joint Integrating Concept (JIC 003). Italian Ministry of Defence. Available online: https://www.difesa.it/SMD_/Staff/Reparti/III/Trasformazione/Pagine/Joint_Rapid_Responce_Forces.aspx (accessed on 25 May 2021).
- Sun, H.; Edziah, B.K.; Kporsu, A.K.; Sarkodie, S.A.; Taghizadeh-Hesary, F. Energy efficiency: The role of technological innovation and knowledge spillover. Technol. Forecast. Soc. Chang. 2021, 167, 120659. [Google Scholar] [CrossRef]
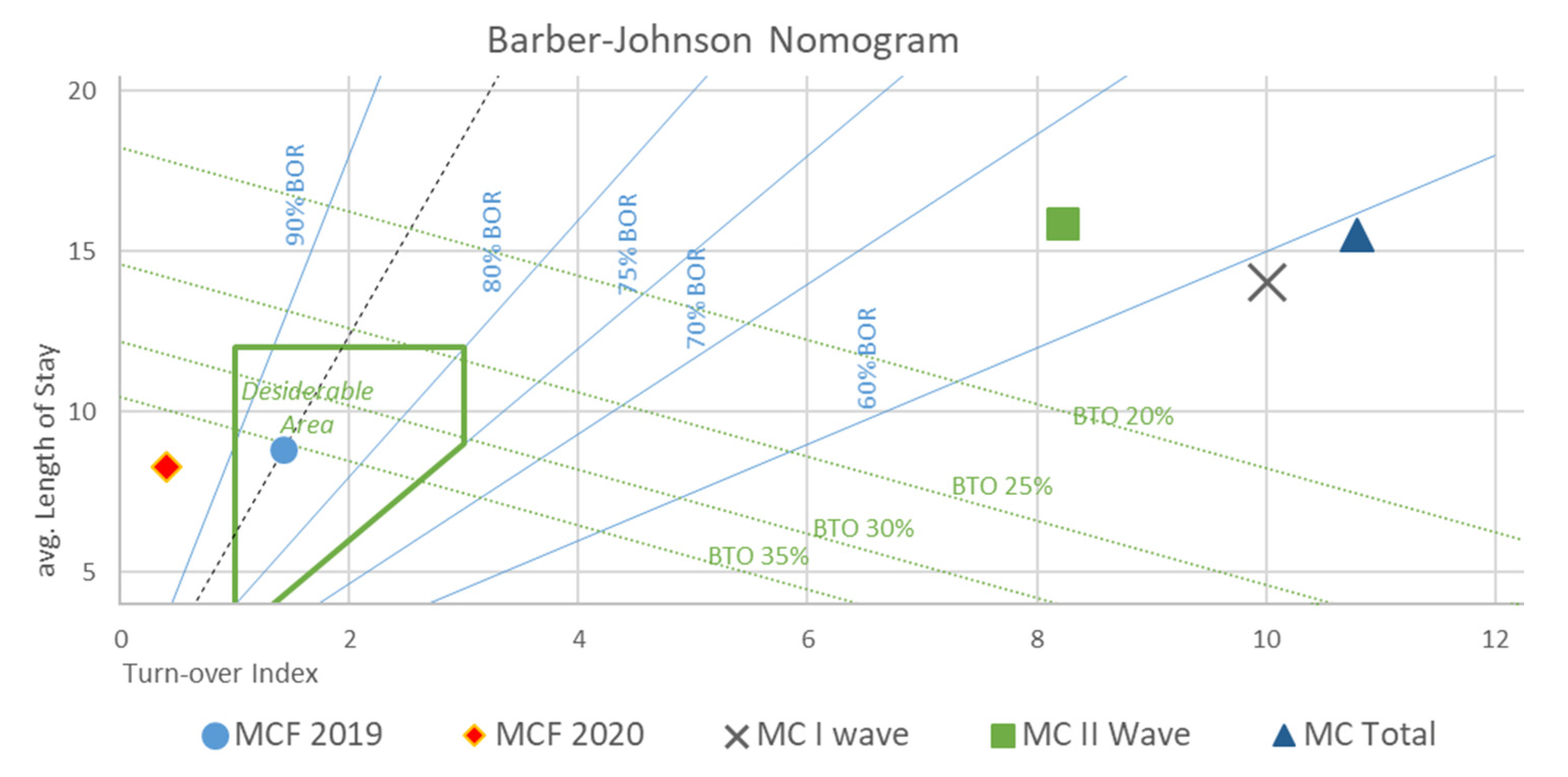
| B/M (BEDS) | BOR | LOS (DAYS) | TI (DAYS) | BRI | TOT (M/F) (PATIENTS) | AV.AG (M/F) (YEARS) | AD (DAYS) | HDD (DAYS) | |
|---|---|---|---|---|---|---|---|---|---|
| MCF 2019 | 29.25 | 86.11% | 8.8 | 1.42 | 36.5% | 1038 (496/542) | 79.03 (78.18/79.81) | 365 | 9168 |
| MCF 2020 | 26.5 | 98.98% | 8.26 | 0.09 | 29.81% | 791 (394/397) | 77.73 (75.42/80.02) | 249 | 6531 |
| MC I WAVE | 30.5 | 58.34% | 14.01 | 10.01 | 2.62% | 85 (36/49) | 77.68 (73.68/80.8) | 63 | 1121 |
| MC II WAVE | 86 | 65.90% | 15.88 | 8.22 | 3.06% | 264 (163/101) | 70.22 (68.74/72.95) | 74 | 4194 |
| MC TOTAL | 63.8 | 58.97% | 15.52 | 10.8 | 5.47% | 350 (199/151) | 72.03 (69.39/75.54) | 144 | 5418 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrantonio, F.; Rosiello, F.; Alessi, E.; Pascucci, M.; Rainone, M.; Cipriano, E.; Di Berardino, A.; Vinci, A.; Ruggeri, M.; Ricci, S. Burden of COVID-19 on Italian Internal Medicine Wards: Delphi, SWOT, and Performance Analysis after Two Pandemic Waves in the Local Health Authority “Roma 6” Hospital Structures. Int. J. Environ. Res. Public Health 2021, 18, 5999. https://doi.org/10.3390/ijerph18115999
Pietrantonio F, Rosiello F, Alessi E, Pascucci M, Rainone M, Cipriano E, Di Berardino A, Vinci A, Ruggeri M, Ricci S. Burden of COVID-19 on Italian Internal Medicine Wards: Delphi, SWOT, and Performance Analysis after Two Pandemic Waves in the Local Health Authority “Roma 6” Hospital Structures. International Journal of Environmental Research and Public Health. 2021; 18(11):5999. https://doi.org/10.3390/ijerph18115999
Chicago/Turabian StylePietrantonio, Filomena, Francesco Rosiello, Elena Alessi, Matteo Pascucci, Marianna Rainone, Enrica Cipriano, Alessandra Di Berardino, Antonio Vinci, Matteo Ruggeri, and Serafino Ricci. 2021. "Burden of COVID-19 on Italian Internal Medicine Wards: Delphi, SWOT, and Performance Analysis after Two Pandemic Waves in the Local Health Authority “Roma 6” Hospital Structures" International Journal of Environmental Research and Public Health 18, no. 11: 5999. https://doi.org/10.3390/ijerph18115999
APA StylePietrantonio, F., Rosiello, F., Alessi, E., Pascucci, M., Rainone, M., Cipriano, E., Di Berardino, A., Vinci, A., Ruggeri, M., & Ricci, S. (2021). Burden of COVID-19 on Italian Internal Medicine Wards: Delphi, SWOT, and Performance Analysis after Two Pandemic Waves in the Local Health Authority “Roma 6” Hospital Structures. International Journal of Environmental Research and Public Health, 18(11), 5999. https://doi.org/10.3390/ijerph18115999







