Abstract
Dichlorvos (O,O-dimethyl O-(2,2-dichlorovinyl)phosphate, DDVP) is a widely acknowledged broad-spectrum organophosphorus insecticide and acaracide. This pesticide has been used for more than four decades and is still in strong demand in many developing countries. Extensive application of DDVP in agriculture has caused severe hazardous impacts on living systems. The International Agency for Research on Cancer of the World Health Organization considered DDVP among the list of 2B carcinogens, which means a certain extent of cancer risk. Hence, removing DDVP from the environment has attracted worldwide attention. Many studies have tested the removal of DDVP using different kinds of physicochemical methods including gas phase surface discharge plasma, physical adsorption, hydrodynamic cavitation, and nanoparticles. Compared to physicochemical methods, microbial degradation is regarded as an environmentally friendly approach to solve several environmental issues caused by pesticides. Till now, several DDVP-degrading microbes have been isolated and reported, including but not limited to Cunninghamella, Fusarium, Talaromyces, Aspergillus, Penicillium, Ochrobium, Pseudomonas, Bacillus, and Trichoderma. Moreover, the possible degradation pathways of DDVP and the transformation of several metabolites have been fully explored. In addition, there are a few studies on DDVP-degrading enzymes and the corresponding genes in microorganisms. However, further research relevant to molecular biology and genetics are still needed to explore the bioremediation of DDVP. This review summarizes the latest development in DDVP degradation and provides reasonable and scientific advice for pesticide removal in contaminated environments.
1. Introduction
The overly common use of organophosphorus pesticides (OPs) has led to a high risk of exposure to acute toxic compounds for various kinds of creatures, including humans [1]. As a representative organophosphorus pesticide, dichlorvos (O,O-dimethyl O-(2,2-dichlorovinyl)phosphate, DDVP) has been commonly used in developing countries and many other regions for more than 40 years [2]. DDVP has the molecular formula C4H7Cl2O4P, with a molecular weight of 220.98, vapor pressure of 1.2 × 10–2 mmHg at 20 °C, and density of 1.415 g/mL at 25 °C. It is classified by the World Health Organization (WHO) as class 2B: possible carcinogens [3]. In addition, the United States Environmental Protection Agency (EPA) has also classified it as a Class Ⅰ pollutant (highly toxic) [4].
Since DDVP came into commercial use in 1961, it could be seen in many countries due to its significant advantages in terms of controlling internal and external parasites in crops and livestock and its ability to eliminate several pests in houses and farmlands [1]. The yearly sales in 2019 around the world were about USD 88 million [5]. In many developing countries, the excessive use or misuse of DDVP in agricultural production leads to serious environmental problems and hazardous conditions. This situation usually has an impact on the soil biome and becomes people’s environmental concern because of its residue toxicity in the ecological system. This issue has caused an organophosphorus pesticide contamination problem.
The massive application of DDVP can affect non-target organisms profoundly through various kinds of pathways (Figure 1). Some reports have shown that exposure to DDVP in childhood is related to an increased risk of diabetes and may lead to the increasing risk of breast cancer in adulthood [6]. Scientific research has demonstrated certain effects of chronic exposure to DDVP on mouse. Those animals exposed to DDVP showed nigrostriatal neuron degeneration and remarkable behavioral impairment. Such animals have representative symptoms called catalepsy which is similar to those of Parkinson’s disease in humans [7]. However, the current situation shows that OPs, including DDVP, are still widely used in China, India, Brazil, and many other developing countries. Exposure is inevitable for people in those countries. Therefore, there is an extremely urgent need to deal with DDVP residues in the environment and to protect people from further physiological damage.
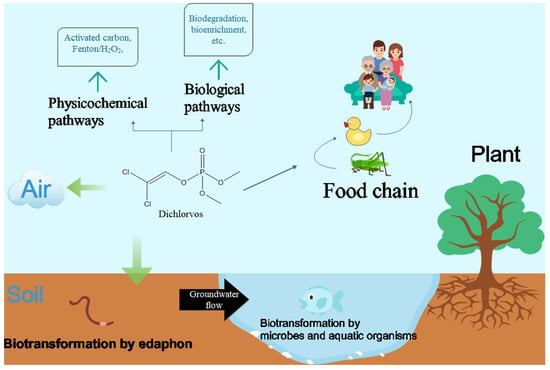
Figure 1.
Contamination and removal of dichlorvos from natural environment.
Many studies have tested the degradation of DDVP using different kinds of physicochemical methods, including gas phase surface discharge plasma, physical adsorption, hydrodynamic cavitation, and nanoparticles [8]. These approaches are not cost-effective and hard to apply to large contaminated areas. Therefore, microbial degradation of DDVP has become a powerful and attractive method to solve the exposure problem of this hazardous pesticide. Several microbes, including Cunninghamell aelegans, Fusarium solani, Talaromyces atroroseus, Aspergillus oryzae, Ochrobactrum intermedium, Pseudomonas aeruginosa, and Penicillium sp., have been isolated and play vital roles in DDVP degradation. It has been reported that the organic pollutants can be used by edaphon including soil bacteria and soil fungi as a sole carbon source [9,10,11,12,13]. These studies showed that microbial degradation seems to be a more environmentally friendly and convenient treatment method to reduce hazardous effects of toxic pollutants or contaminants [14,15,16,17,18,19]. In addition, there are a few studies on degrading enzymes with correlative genes in microbes. However, most of the studies have paid little attention to the mechanisms and degradation pathways of DDVP. This review summarizes different kinds of solutions to the DDVP contamination problem and describes the promising application prospect of microbial degradation. Moreover, it also discusses the mechanisms and degradation pathways of DDVP.
2. Toxicological Effects of DDVP
DDVP has the tendency to remain in solution due to its solubleness, with a limited tendency to absorb sediment. DDVP is subject to both abiotic and biological degradation in solution [20]. In addition, DDVP has the ability to regulate the neurotransmitter acetylcholine which leads to irreversible inhibition of acetylcholinesterase. Thus, it has harmful effect on nontarget invertebrates and vertebrates [21]. Based on the laboratorial research, DDVP can be hydrolyzed to dichloroacetaldehyde, dichloroethanol, dichloroacetic acid, dimethylphosphate, and dimethylphosphoric acid [22]. The process of DDVP degradation in moist soil is similar to that in aqueous solution. There are two main routes of exposure: inhalation and skin contact. People who are brought into contact with toxic waste containing DDVP or use domestic pesticides can potentially be exposed to them by inhalation. Due to its long half-life and current situation of usage, the toxic effects caused by DDVP residue should not be ignored. The toxicological effects of DDVP are presented in Table 1.

Table 1.
Toxicological effects of dichlorvos in humans and animals.
Toxic DDVP exposure in zebrafish was reported by Nguyen et al. [23], who illustrated various kinds of procedures in energy utilization and stress response in liver. Three concentrations of DDVP show that the effect on liver energy metabolism is rigorously controlled. Toxic exposure may lead to a certain amount of neuromuscular impairment in exposed zebrafish. Moreover, a study on tilapia demonstrated that acetylcholinesterase (AChE) suppression in brain and liver is caused by DDVP which exerts cholinergic action by blocking cholinesterase in the central and peripheral nervous system [24]. Tilapia lived under a sublethal concentration (0.5 mg/L) of DDVP, and all sizes of fish showed a significant inhibition of brain and liver AChE activities. AChE activity was regarded as an indication of the extent of pollution of the aquatic environment by organic chemicals and was correlated with water contamination.
Harmful impact on rats according to DDVP have also been investigated as representatives of damage to land mammals. Okamura et al. [25] reported a study in which Wistar rats were injected with four different dosages of DDVP dissolved in saline on their neck. Sperm motility is deteriorated by DDVP exposure at different doses, which means humans may suffer from testicular dysfunction. Another study focused on the biochemical and behavioral sequelae of chronic DDVP exposure in rats [26]. This study illustrated that all components of spontaneous locomotor activity in rats exposed to DDVP have reduced remarkably. DDVP administration also led to evident damage on rats’ muscle strength and coordination. According to a cellular-level study, exposure to DDVP may result in neuronal cell death in primary rats [27]. This study observed significant upregulation of pro-inflammatory molecules like nitric oxide, tumor necrosis factor alpha (TNF-α), and interleukin 1 beta (IL-1β) when microglia were treated with DDVP (10 μM). The study concluded that DDVP can induce microglial activation and then cause cell apoptosis. Another study described the influence of butyrylcholinesterase (BuChE) activity in rats with continued exposure to DDVP [28]. Different types of doses of DDVP (8.0 mg/kg of body weight) were given to both sexes of rats, with two-day intervals between administrations. This study clearly showed that exposure to DDVP significantly decreased the BuChE activity in both male and female rats.
There are several reports about the hazardous effects of DDVP on the human body indicating that a higher concentration of DDVP can cause death. A woman died a day after ingesting DDVP and an infant died after ingesting a cake-like bait that contained DDVP [29]. Although most of the studies showed little proof that exposure to DDVP is related to any cancer risk, Eroğlu et al. [2] and Koutros et al. [4] indicated the toxic effects of DDVP on human peripheral blood lymphocytes. As a result, DDVP-induced micronuclei decreased the mitotic and replication indices. This kind of genotoxic product causes chromosomal damage and cell death (decreased mitotic and replication indices). It has been classified by the United States Environmental Protection Agency as a toxic chemical in the Toxics Release Inventory (TRI) [30].
Some studies have illuminated the molecular mechanism of DDVP neurotoxicity. The dominating mechanism of action of DDVP is the inhibition of AChE, which causes an increase in the level of acetylcholine in the synaptic cleft and produces nicotine and muscarinic signs, which are also accompanied by symptoms of poisoning in the central nervous system [31]. However, a certain amount of acetylcholinesterase inhibition can be tolerant to nervous system without any toxic effects. In all kinds of mammals, toxic signs were discovered until acetylcholinesterase was inhibited by at least 20% [1].
3. Physicochemical Transformation of DDVP
Several physicochemical methods have been applied to control residual DDVP pollutant (Table 2). On the whole, these methods are efficient to a certain extent, but too expensive for developing countries that are suffering from DDVP contamination. Several researches have reported hazardous effects of DDVP exposure on aquatic animals, land mammals, and humans. Thus, removal of DDVP residue from contaminated environments is extremely urgent.

Table 2.
Physical and chemical methods used to degrade dichlorvos from environments.
The main technique for solving pesticide pollutants is chemical degradation [43]. Other common solutions for solving pesticide pollutants include chlorination, hydrodynamic cavitation, active carbon, O2 plasma, metal catalysts, and H2O2 and O3 adsorption [14]. Bustos et al. [44] highlighted the urgent need and intricacy of photo-induced oxygen-mediated reactions of DDVP. DDVP is photoionized by electron transfer to dissolved oxygen, followed by superoxide radicals, and finally the HO yield. It might be the main mechanism of degradation taking place during photolysis. In addition, it has been investigated that the hydrodynamic cavitation reactor can be applied to degrade an aqueous solution of DDVP. As shown in another study, a chlorinated organophosphate compound can be effectively degraded using treatment strategies based on hydrodynamic cavitation in a large-scale operation [45]. According to this report, active carbon is an efficient substituent that absorbs DDVP residual, because powder-activated carbon shows excellent adsorption of aromatic compounds, including pesticides, herbicides, surface activators, natural pigments, and phenols [46].
Advanced oxidation processes (AOPs) containing various kinds of oxidants have been applied to remove hazardous pollutants from soil and water environments successfully. Bai et al. [47] noted that the O2 plasma treatment worked well in the DDVP remediation process, and the usefulness of degradation is mainly dependent on the related operating parameters and chemical structures of pesticides. Hydroxyl radicals have the ability to break the double bond in the DDVP molecule, and DDVP is further oxidized to 1,1-dichloro ethoxy dimethyl phosphate, 1,1,1-trichloro-2-hydroxyl-ethyl dimethyl phosphate, dimethyl phosphite, dimethyl phosphate, trimethyl phosphate, methyl phosphate, dichloro acetaldehyde, oxalic acid, CH2Cl2, CHCl3 (parts of which are mineralized to phosphoric acid), CO2, H2O, and chloridion [48]. It took only 90 min to push the elimination ration up to 98% under acidic and saturated dissolved oxygen conditions [49]. Through the known products, the reaction mechanism of DDVP oxidized by H2O2 was discussed, and the conclusion was made that the main decontamination mechanism is radical chain reaction [50].
Comparatively, ozone and hydroxyl radicals are vital for DDVP abatement. The abiotic hydrolysis degradation pathway is presented in Figure 2. However, we still need more detailed studies on aqueous solutions and lower concentrations of contaminants in order to properly assess the process performance.
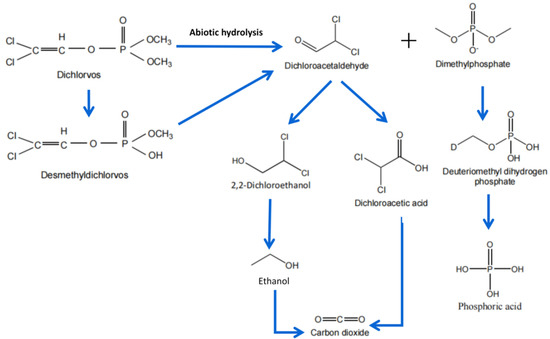
Figure 2.
Proposed physical degradation pathways for dichlorvos decomposition in water and soil system.
Iron-modified ZSM-11 zeolites were applied as heterogeneous catalysts in the degradation process of DDVP water solutions. ZSM-11 zeolite matrices were synthesized by the hydrothermal method and iron was incorporated by the wet impregnation method in four concentrations. From this report, Fe/ZSM-11 with 6 wt% of incorporated iron showed the best catalytic behavior based on DDVP [51].
Removing DDVP pollutants from water is a real challenge due to the presence of the direct carbon-to-phosphorous covalent bond, which reveals its stability under chemical and thermal degradation. From recent studies, nanomaterials seem to be a possible solution for degradation. Mehrotra et al. [52] reported an efficient way for catalytic degradation of DDVP using protein-capped zero-valent iron nanoparticles, which removed the pesticide in 1 h. Moreover, the degradation mechanisms of DDVP during oxygen plasma treatment have been successfully detailed [53], so several active materials (high-energy electrons and free radicals) in oxygen plasma can thoroughly degrade DDVP within a short exposure time.
4. Microbial Degradation of DDVP
Microbial degradation is regarded as a cost-effective and promising method with a huge potential for the removal of pesticides, compared to physicochemical approaches [9,13,19]. Soil bacteria and fungi have been documented as being able to mineralize various organic pollutants as a sole carbon source [56,57,58,59,60]. Based on the existing research, the effective soil microorganisms for solving the DDVP residual have been isolated and studied (Table 3).

Table 3.
Microbial degradation of dichlorvos.
Five kinds of strains were selected and studied for the plant-fungi-spent mushroom compost (SMC) interaction, which has the potential to speed up the DDVP degradation rate [56]. According to this research, fungal strains identified as Cunninghamella elegans, Fusarium solani, Talaromycesatro roseus, Aspergillus oryzae, and Penicillium sp. were isolated from pesticide-polluted soil. Their rhizosphere interaction with plants (Panicum maximum) was shown in this study. The plant-fungi-SMC interaction synergistically sped up the DDVP degradation rate in a shorter time period, and an appreciable loss of DDVP of 72.23% and 82.70% degradation efficiency was observed in 30% and 40% of treatments, respectively, as compared to controls 1 and 2, with 62.20% and 62.33% degradation efficiency, respectively.
As a representative organophosphorus pesticide, DDVP has been applied in biodegradation studies. In a study by Jiang et al. [61], they found a bacterium that can degrade DDVP rapidly, Ochrobactrum intermedium DV-B31. This bacterium degraded 96.38% of DDVP samples in 8 days, which proved its potential for bioremediation. Interestingly, some bacteria, such as Pseudomonas aeruginosa and Bacillus amyloliquefaciens YP6, can degrade DDVP and other pesticides [62]. Nonetheless, the bioremediation ability of the bacterial cultures can be affected by different factors, including the type of inoculum and its density, pH, temperature, and toxic compounds present in the system [63].
Recently, there has been increasing interest in the biodegradation pathway of DDPV. A study of Trichoderma atroviride strain T23 presented two possible ways of degrading DDVP [60]. According to the results of this study, the first pathway is related to the breakage of the P-O bond. DDVP was converted to dimethyl phosphate (DMP) and dichloroacetaldehyde, and these intermediates can be rapidly tautomerized to dichloroacetic acid (DCAA) and dichloroethane (DCE). Some of the DCE is then transformed into trichloroethane (TCE) and the rest is dechlorinated into ethanol. Potentially, through the esterification of DCAA to ethyl dichloroacetate (EDCA), DMP is eventually converted into phosphate ions by strain T23. Moreover, the stochiometric amount of metabolites is lower than the consumption of DDVP, which leads to the second possible pathway. The second pathway involves the de-chlorination of DDVP to the isomers, (Z)-2-chlorovinyl dimethylphosphate and (E)-2-chlorovinyl dimethyl phosphate, while these isomers hardly undergo further de-chlorination to phosphoric acid trimethyl ester. Thus, they are unlikely the main by-products and are not easy to detect using normal techniques.
Sun et al. [64] noted that Trichoderma atroviride mutant AMT-28 is one of the most effective fungal bacteria and can completely remove DDVP pollution in 7 days. The DDVP removal is related to biomineralization process which attributed to fungal biodegradation. Parte et al. [65] demonstrated another biodegradation pathway in Pseudomonas stutzeri strain smk. This study elucidated the aerobic degradation pathway of DDVP: two dichlorination steps producing 2-chlorovinyl dimethyl phosphate and vinyl dimethyl phosphate. The vinyl dimethyl phosphate was then devinylated to produce dimethyl phosphate, which, upon two sequential demethylation steps was separated into 2-methyl moieties and a free phosphate to serve as the sole carbon and phosphate source to support growth. These various kinds of degradation pathways indicate that microbial degradation seems to be more adaptable to current agriculture and living conditions.
In some cases, a single type of a bacterial strain is not applicable due to the current degradation requirements. Based on this situation, Ning et al. [66] reported that degradation ability can be mutually promoted by a bacterial community. It seems that more kinds of bacteria have higher active constituents. A consortium of Pseudomonas, Xanthomonas, Sphingomonas, Acidovorax, Agrobacterium, and Chryseobacterium was reported, which extends the range of pesticide degradation by phyllosphere microbial communities and consequently provides a brand-new idea for the biodegradation of DDVP with pure microbial cultures from the plant phyllosphere.
5. Molecular Mechanism of DDVP Biodegradation
The proposed DDVP microbial degradation pathways are presented in Figure 3. The biodegradation mechanisms of many other organophosphorus pesticides have been deeply studied, especially those pesticides whose degradation genes and enzymes were cloned and purified [67,68,69,70,71]. According to previous research, most of the microbial degradation of DDVP is closely related to a functional gene that encodes for the enzymes, which is crucial in the degradation process [64].
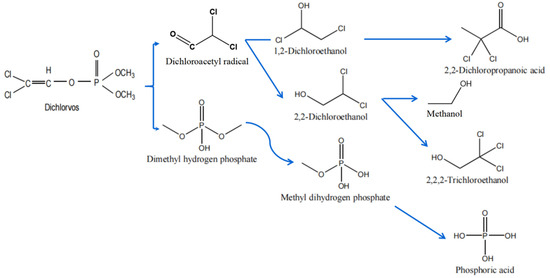
Figure 3.
Proposed microbial degradation pathways of dichlorvos.
Parte et al. [65] revealed the correlation between pesticide concentration and biodegradation ability. It seems that a lower concentration of DDVP supports bacterial growth, while higher concentration harms the bacteria. The reason may be that the cells and enzyme systems are hampered by increasing concentrations of pesticide, leading to lower biodegradation efficiency. Sun et al. [64] also found that the enzyme produced by TaPon1-like had a low Km for DDVP (0.23 mM) and a high Kcat (204.3 s–1). The enzyme was able to hydrolyze broad substrates with stable activity in a wide range of pH and temperature values. TaPon1-like hydrolase plays an important role in the first step of DDVP degradation by strain T23 and contributes to a comprehensive understanding of the mechanism of organophosphate pesticide degradation. The deletion of TaPon1-like weakened the efficiency of the DDVP degradation, but it did not abolish the hydrolysis ability, which indicates that TaPon1-like is one of the key enzymes of strain T23 that is responsible for the hydrolysis of the P-O bond in DDVP.
Moreover, a study demonstrated that AMT-28 could produce inducible intracellular-degrading enzyme of DDVP, causing immobilized cells to display ever-increasing DDVP degradation ability in reusability determinations. To thoroughly investigate the mechanism of DDVP bioremediation, research on the isolation and purification of inducible intracellular degrading enzyme are ongoing [72]. Although the degradation mechanism has not been clearly explained, some kinds of fungi can produce novel OPs degrading enzyme [73,74,75].
The elimination of DDVP from saline solutions has been attributed to its ability to penetrate into the cytoplasm via a principle, called “organic-osmolyte.” Moreover, the PON1 gene, which exists widely in mammals, has been found to have a powerful influence on the detoxification of organophosphate compounds. This led to the result that PON1 has the ability to prevent oxidative damage to tissues, which seems to be reasonable [76]. Therefore, PON1 may prevent tissue damage due to organophosphate toxicity, especially in the central nervous system [76]. This study has shown that PON1 could effectively reduce the blood concentration and decrease the peak concentration of DDVP, and lessen the amount that enters the blood. This study also compared the hydrolytic effect of PON1 with atropine + PAM, the most widely used clinical therapy. It showed that atropine + PAM did not affect the metabolism of DDVP, which was consistent with a recent research [76]. Co-treatment does not alter the impact of PON1 on DDVP concentration, which implies that there is no interaction between PON1 and atropine + PAM-CI; therefore, it is supposed that co-treatment may be feasible in the clinical treatment of human organophosphate-related toxicity [77,78,79].
Based on these studies, it seems that using microorganisms merely is not the most effective method. Unfortunately, current knowledge of the DDVP biodegradation mechanism is still very limited; more research should be focused on identifying the novel genes and enzymes to explore the degradation pathway.
6. Conclusions and Future Perspectives
Different physicochemical methods have been developed for the removal of DDVP from contaminated environments, and microbial degradation is regarded as a promising method to solve several harmful residuals caused by DDVP. The biodegradation mechanism of many OPs has been studied deeply, especially for the methyl parathion, whose degradation genes and enzymes were cloned and purified. There is a need to select more useful strains, since only a few bacteria have been studied thoroughly in relation to the functional enzymes and genes. Moreover, the large number of different DDVP biotransformation metabolites should be detected to avoid secondary pollution.
Under the current situation of DDVP usage distribution, developing countries are more liable to suffer from exposure toxicity, but they are not allowed to use several physicochemical methods to solve the problem due to their economic capability. As a result, there is an urgent need for further study of biodegradation, in order to provide cloned strains to reduce the threat of DDVP exposure at lower cost. In the future, advanced scientific technologies such as gene editing and DNA isotope probes could be used to search for and evaluate more adaptable microorganisms for pesticide degradation. Moreover, next-generation sequencing analysis of the complete genome could explore the bioremediation potential of indigenous microbial strains in detail. The potential strains can be applied for large-scale treatment of DDVP and other pesticides.
Author Contributions
Conceptualization: S.C. and D.Z.; data analysis: Y.Z.; writing—original draft preparation: Y.Z.; writing—review and editing: W.Z., J.L., S.P., S.M., P.B., D.Z., and S.C.; supervision, funding acquisition, and project administration: S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key-Area Research and Development Program of Guangdong Province (2018B020206001, 2020B0202090001), the Natural Science Foundation of Guangdong Province (2021A1515010889), and the Guangdong Special Branch Plan for Young Talent with Scientific and Technological Innovation, China (2017TQ04N026).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| DDVP | O,O-Dimethyl O-(2,2-dichlorovinyl)phosphate |
| OPs | Organophosphorus pesticides |
| WHO | World Health Organization |
| AChE | Acetylcholinesterase |
| BuCh | Butyrylcholinesterase |
| TRI | Toxics Release Inventory |
| AOPs | Advanced oxidation processes |
| SMC | Spent mushroom compost |
| DMP | Dimethyl phosphate |
| DCAA | Dichloroacetic acid |
| DCE | Dichloroethane |
| TCE | Trichloroethane |
| EDCA | Ethyl dichloroacetate |
References
- Okoroiwu, H.U.; Iwara, I.A. Dichlorvos toxicity: A public health perspective. Interdiscip. Toxicol. 2018, 11, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Eroğlu, H. Toxic nuclear effects of the organophosphorus insecticide dichlorvos (DDVP) in human peripheral blood lympho-cytes. Acta Biol. Hung. 2009, 60, 409–416. [Google Scholar] [CrossRef]
- Cancer IAFR. Agents Classified by the IARC Monographs; Cancer IAFR: Lyon, France, 2021. [Google Scholar]
- Koutros, S.; Mahajan, R.; Zheng, T.; Hoppin, J.A.; Ma, X.; Lynch, C.F.; Blair, A.; Alavanja, M.C.R. Dichlorvos exposure and human cancer risk: Results from the Agricultural Health Study. Cancer Causes Control. 2008, 19, 59–65. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. Public Health Service Agency for Toxic Substances and Disease Registry. 1994. Available online: https://www.hhs.gov/ (accessed on 15 January 2021).
- Montgomery, M.P.; Kamel, F.; Saldana, T.M.; Alavanja, M.C.R.; Sandler, D.P. Incident diabetes and pesticide exposure among licensed pesticide applicators: Agricultural health study, 1993-2003. Am. J. Epidemiol. 2008, 167, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Binukumar, B.K.; Bal, A.; Kandimalla, R.J.; Gill, K.D. Nigrostriatal neuronal death following chronic dichlorvos exposure: Crosstalk between mitochondrial impairments, alpha synuclein aggregation, oxidative damage and behavioral changes. Mol. Brain. 2010, 3, 35. [Google Scholar] [PubMed]
- Cao, J.; Wang, M.; She, Y.; Abd El-Aty, A.M.; Hacımüftüoğlu, A.; Wang, J.; Yan, M.; Hong, S.; Lao, S.; Wang, Y. Rapid colorimetric determination of the pesticides carbofuran and dichlorvos by exploiting their inhibitory effect on the aggregation of peroxi-dase-mimicking platinum nanoparticles. Microchim. Acta 2019, 186, 390. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Lin, Z.; Zhang, Y.; Zhang, W.; Alansary, N.; Mishra, S.; Bhatt, P.; Chen, S. Insights into the toxicity and degradation mechanisms of imidacloprid via physicochemical and microbial approaches. Toxics 2020, 8, 65. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, Z.; Zhang, W.; Pang, S.; Bhatt, P.; Rene, E.; Kumar, A.; Chen, S. New insights into the microbial degradation of D-cyphenothrin in contaminated water/soil environments. Microorganisms 2020, 8, 473. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Huang, Y.; Zhan, H.; Bhatt, P.; Chen, S. An overview of strobilurin fungicide degradation: Current status and future perspective. Front. Microbiol. 2020, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- Birolli, W.G.; Vacondio, B.; Alvarenga, N.; Seleghim, M.; Porto, A. Enantioselective biodegradation of the pyrethroid (+/−)-lambda-cyhalothrin by marine-derived fungi. Chemosphere 2018, 197, 651–660. [Google Scholar] [CrossRef]
- Chen, S.; Dong, Y.H.; Chang, C.; Deng, Y.; Zhang, X.F.; Zhong, G.; Song, H.; Hu, M.; Zhang, L.H. Characterization of a novel cyfluthrin-degrading bacterial strain Brevibacterium aureum and its biochemical degradation pathway. Bioresour. Technol. 2013, 132, 16–23. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a strategy for the remediation of pesticide-polluted soil: A review. Chemosphere 2017, 172, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.K.; Srivastava, A.; Garg, S.K.; Singh, V.P. Recent advances in degradation of chloronitrophenols. Bioresour. Technol. 2018, 250, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Birolli, W.G.; Lima, R.N.; Porto, A. Applications of marine-derived microorganisms and their enzymes in biocatalysis and biotransformation, the underexplored potentials. Front. Microbiol. 2019, 10, 1453. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Rene, E.R.; Kumar, A.J.; Zhang, W.; Chen, S. Binding interaction of allethrin with esterase: Bioremediation potential and mechanism. Bioresour. Technol. 2020, 315, 123845. [Google Scholar] [CrossRef]
- Mishra, S.; Zhang, W.; Lin, Z.; Pang, S.; Huang, Y.; Bhatt, P.; Chen, S. Carbofuran toxicity and its microbial degradation in contaminated environments. Chemosphere 2020, 259, 127419. [Google Scholar] [CrossRef]
- Zhang, W.; Pang, S.; Lin, Z.; Mishra, S.; Bhatt, P.; Chen, S. Biotransformation of perfluoroalkyl acid precursors from various environmental systems: Advances and perspectives. Environ. Pollut. 2021, 268, 115908. [Google Scholar] [CrossRef]
- Pooja, T.; Priyanga, M.; Parag, R. Improvement in biological oxidation process for the removal of dichlorvos from aqueous solutions using pretreatment based on hydrodynamic cavitation. J. Water Process. Eng. 2018, 23, 20–26. [Google Scholar]
- Ben Salem, I.; Boussabbeh, M.; Bacha, H.; Abid, S. Dichlorvos-induced toxicity in HCT116 cells: Involvement of oxidative stress and apoptosis. Pestic. Biochem. Physiol. 2015, 119, 62–66. [Google Scholar] [CrossRef]
- WHO. Dichlorvos: Environmental Health Criteria; WHO: Geneva, Switzerland, 1989. [Google Scholar]
- Bui-Nguyen, T.M.; Baer, C.E.; Lewis, J.A.; Yang, D.; Lein, P.J.; Jackson, D.A. Dichlorvos exposure results in large scale disruption of energy metabolism in the liver of the zebrafish, Danio rerio. BMC Genom. 2015, 16, 853. [Google Scholar] [CrossRef]
- Rath, S.; Misra, B.N. Toxicological effects of dichlorvos (DDVP) on brain and liver acetylcholinesterase (AChE) activity of Tilapia mossambi-ca, peters. Toxicology 1981, 19, 239–245. [Google Scholar] [CrossRef]
- Okamura, A.; Kamijima, E.; Shibata, K.; Ohtani, K.; Takagi, J.; Ueyama, Y.; Watanabe, M.; Omura, H.; Wang, G.; Ichihara, T.; et al. Comprehensive evaluation of the testicular toxicity of dichlorvos in Wistar rats. Toxicology 2005, 213, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Imam, A.; Ogunniyi, A.; Ibrahim, A.; Abdulmajeed, W.I.; Aboyeji, L.O.; Lawan, A.H.; Sulaimon, F.A.; Adana, M.Y.; Ajao, S.M. Dichlorvos induced oxidative and neuronal responses in rats: Mitigative efficacy of Nigella sativa (Black Cumin). Niger. J. Physiol. Sci. 2018, 33, 83–88. [Google Scholar] [PubMed]
- Sunkaria, A.; Wani, W.Y.; Sharma, D.R.; Gill, K.D. Dichlorvos exposure results in activation induced apoptotic cell death in primary rat microglia. Chem. Res. Toxicol. 2012, 25, 1762–1770. [Google Scholar] [CrossRef]
- Lucić, A.; Bradamante, V.; Radić, B.; Peraica, M.; Domijan, A.M.; Fuchs, R.; Stavljenić-Rukavina, A. The effect of dichlorvos treatment on butyrylcholinesterase activity and lipid metabolism in rats. Arch. Ind. Hyg. Toxicol. 2002, 53, 275–282. [Google Scholar]
- Hayes, W.J. Pesticides Studied in Man; Williams & Wilkins: Baltimore, MA, USA, 1982. [Google Scholar]
- U.E.P. Agency. Dichlorvos: Initiation of special review. Fed. Regist. 1988, 53, 5549–6642. [Google Scholar]
- Binukumar, B.K.; Gill, K.D. Cellular and molecular mechanisms of dichlorvos neurotoxicity: Cholinergic, nonchlolinergic, cell signaling, gene expression and therapeutic aspects. Indian J. Exp. Boil. 2010, 48, 697–709. [Google Scholar]
- Zhang, Y.; You, J.Z.; Zhou, Y.; Zhang, P.W.; Qin, D.Q.; Zhang, Z.X. The effect of dichlorvos on control of drosophila and its safety evaluation under different application methods. Environ. Sci. Pollut. Res. 2017, 24, 22940–22947. [Google Scholar] [CrossRef]
- Hoang, T.C.; Rand, G.M. Acute toxicity and risk assessment of permethrin, naled, and dichlorvos to larval butterflies via in-gestion of contaminated foliage. Chemosphere 2015, 120, 714–721. [Google Scholar] [CrossRef]
- Kunwar, P.S.; Parajuli, K.; Badu, S.; Sapkota, B.; Sinha, A.K.; De Boeck, G.; Sapkota, K. Mixed toxicity of chlorpyrifos and di-chlorvos show antagonistic effects in the endangered fish species golden mahseer (Tor putitora). Comp. Biochem. Physiol. 2021, 240, 108–923. [Google Scholar]
- Nan, P.; Yan, S.; Du, Q.; Li, L.; Chen, J.; Chang, Z. Toxicity effect of dichlorvos on loach (Misgurnus anguillicaudatus) assessed by micronucleus test, hepatase activity analysis and comet assay. Toxicol. Ind. Health 2013, 31, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Al-Zubaidy, M.; Mousa, Y.; Hasan, M.; Mohamma, F.K. Acute toxicity of veterinary and agricultural formulations of organophosphates dichlorvos and diazinon in chicks. Arh. Hig. Rada. Toksiko. 2011, 62, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.F.; Xu, X.R.; Duan, S.S.; Wang, Y.S.; Cheng, H.; Zhang, Z.W.; Zhou, G.J.; Hong, Y.G. Ecotoxicity of two organophosphate pesticides chlorpyrifos and dichlorvos on non-targeting cyanobacteria Microcystis wesenbergii. Ecotoxicology 2015, 24, 1498–1507. [Google Scholar] [CrossRef]
- Guvenç, D.; Aksoy, A.; Kursad, Y.; Atmaca, E.; Yavuz, O. 3-Nitrotyrosine levels in dichlorvos-induced neurotoxicity. Arch. Ind. Hyg. Toxicol. 2014, 65, 109–112. [Google Scholar] [CrossRef]
- Gaspari, R.J.; Dunn, C. Dichlorvos exposure to the Kölliker-fuse nuclei is sufficient but not necessary for OP induced apnea. Neurotoxicology 2013, 39, 132–137. [Google Scholar] [CrossRef]
- Chaudhary, B.; Bist, R. Protective manifestation of bacoside A and bromelain in terms of cholinesterases, gamma-amino butyric acid, serotonin level and stress proteins in the brain of dichlorvos-intoxicated mice. Cell Stress Chaperon. 2017, 22, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Nwankwo, R.C.; Ibegbu, M.D.; Onyekwelu, K.C.; Ejezie, C.S.; Ikekpeazu, J.E.; Ejezie, F.E. Biochemical and histopathological effects of sub-acute exposure of albino rats to fumigants–dichlorvos and cypermethrin. Interdiscip. Toxicol. 2019, 12, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J. Arsenic and dichlorvos: Possible interaction between two environmental contaminants. J. Trace Elements Med. Biol. 2016, 35, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Pang, S.; Zhang, W.; Lin, Z.; Bhatt, P.; Chen, S. Insights into the microbial degradation and biochemical mechanisms of carbamates. Chemosphere 2021, 279, 130500. [Google Scholar] [CrossRef]
- Bustos, N.; Cruz-Alcalde, A.; Iriel, A.; Cirelli, A.F.; Sans, C. Sunlight and UVC-254 irradiation induced photodegradation of organophosphorus pesticide dichlorvos in aqueous matrices. Sci. Total. Environ. 2019, 649, 592–600. [Google Scholar] [CrossRef]
- Joshi, R.K.; Gogate, P.R. Degradation of dichlorvos using hydrodynamic cavitation based treatment strategies. Ultrason. Sonochem. 2012, 19, 532–539. [Google Scholar] [CrossRef]
- Yan, X.J.; Yu, S.L.; Fu, S.T.; Zhao, F.B.; An, Y.T. Pollution removal efficiency of powdered activated carbon and microfiltration integrated process. Huan Jing Ke Xue 2008, 29, 87–91. [Google Scholar] [PubMed]
- Bai, Y.; Chen, J.; Mu, H.; Zhang, C.; Li, B. Reduction of dichlorvos and omethoate residues by O2 plasma treatment. J. Agric. Food Chem. 2009, 57, 6238–6245. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.G.; Wang, W.Q.; Xi, H.L.; Zhao, H.J. Decontamination of dichlorvos by hydrogen peroxide. Adv. Mater. Res. 2013, 781–784, 59–62. [Google Scholar] [CrossRef]
- Lu, M.C.; Chen, J.N.; Chang, C.P. Oxidation of dichlorvos with hydrogen peroxide using ferrous ion as catalyst. J. Hazard. Mater. 1999, 65, 277–288. [Google Scholar] [CrossRef]
- Cruz-Alcalde, A.; Sans, C.; Esplugas, S. Priority pesticides abatement by advanced water technologies: The case of acetamiprid removal by ozonation. Sci. Total. Environ. 2017, 599–600, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Gomez, S.; Lerici, L.; Saux, C.; Perez, A.L.; Brondino, C.D.; Pierella, L.; Pizzio, L. Fe/ZSM-11 as a novel and efficient photocatalyst to degrade dichlorvos on water solutions. Appl. Catal. B Environ. 2017, 202, 580–586. [Google Scholar] [CrossRef]
- Mehrotra, N.; Tripathi, R.M.; Zafar, F.; Singh, M.P. Catalytic degradation of dichlorvos using biosynthesized zero valent iron nanoparticles. IEEE Trans. Nanobiosci. 2017, 16, 280–286. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, J.; Yang, Y.; Guo, L.; Zhang, C. Degradation of organophosphorus pesticide induced by oxygen plasma: Effects of operating parameters and reaction mechanisms. Chemosphere 2010, 81, 408–414. [Google Scholar] [CrossRef]
- Hu, Y.; Bai, Y.; Yu, H.; Zhang, C.; Chen, J. Degradation of selected organophosphate pesticides in wastewater by dielectric barrier discharge plasma. Bull. Environ. Contam. Toxicol. 2013, 91, 314–319. [Google Scholar] [CrossRef]
- Von Der Wellen, J.; Bierwisch, A.; Worek, F.; Thiermann, H.; Wille, T. Kinetics of pesticide degradation by human fresh frozen plasma (FFP) in vitro. Toxicol. Lett. 2016, 244, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Asemoloye, M.D.; Jonathan, S.G.; Ahmad, R. Degradation of 2,2-dichlorovinyl dimethyl phosphate (dichlorvos) through the rhizosphere interaction between Panicum maximum Jacq and some selected fungi. Chemosphere 2019, 221, 403–411. [Google Scholar] [CrossRef]
- Bhatt, P.; Gangola, S.; Bhandari, G.; Zhang, W.; Maithani, D.; Mishra, S.; Chen, S. New insights into the degradation of synthetic pollutants in contaminated environments. Chemosphere 2021, 268, 128827. [Google Scholar] [CrossRef]
- Gaonkar, O.; Nambi, I.M.; Suresh Kumar, G. Biodegradation kinetics of dichlorvos and chlorpyrifos by enriched bacterial cultures from an agricultural soil. Bioremediat. J. 2019, 23, 259–276. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, W.; Pang, S.; Lin, Z.; Zhang, Y.; Huang, Y.; Pankaj, B.; Chen, S. Kinetics and new mechanism of azoxystrobin biodegradation by an Ochrobactrum anthropi strain SH14. Microorganisms 2020, 8, 625. [Google Scholar] [CrossRef]
- Sun, J.; Yuan, X.; Li, Y.; Wang, X.; Chen, J. The pathway of 2,2-dichlorovinyl dimethyl phosphate (DDVP) degradation by Trichoderma atroviride strain T23 and characterization of a paraoxonase-like enzyme. Appl. Microbiol. Biotechnol. 2019, 103, 8947–8962. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, N.; Xing, Y.; Lian, L.N.; Chen, Y.; Zhang, D.; Li, G.; Sun, G.; Song, Y. Microbial degradation of organophosphorus pesticides: Novel degraders, kinetics, functional genes, and genotoxicity assessment. Environ. Sci. Pollut. Res. Int. 2019, 5, 21668–21681. [Google Scholar] [CrossRef]
- Meng, D.; Jiang, W.; Huang, L.; Zhai, L.; Zhang, L.; Guan, Z.; Cai, Y.; Liao, X. An alkaline phosphatase from Bacillus amyloliquefaciens YP6 of new application in biodegradation of five broad-spectrum organophosphorus pesticides. J. Environ. Sci. Health B 2019, 3, 336–343. [Google Scholar] [CrossRef]
- Mulla, S.I.; Hu, A.; Sun, Q.; Li, J.; Suanon, F.; Ashfaq, M.; Yu, C.P. Biodegradation of sulfamethoxazole in bacteria from three different origins. J. Environ. Manag. 2018, 206, 93–102. [Google Scholar] [CrossRef]
- Sun, W.; Chen, Y.; Liu, L.; Tang, J.; Chen, J.; Liu, P. Conidia immobilization of T-DNA inserted Trichoderma atroviride mutant AMT-28 with dichlorvos degradation ability and exploration of biodegradation mechanism. Bioresour. Technol. 2010, 101, 9197–9203. [Google Scholar] [CrossRef]
- Parte, S.G.; Mohekar, A.D.; Kharat, A.S. Aerobic dichlorvos degradation by Pseudomonas stutzeri smk: Complete pathway and implications for toxicity in Mus musculus. Iran. J. Microbiol. 2020, 12, 138–147. [Google Scholar] [CrossRef]
- Ning, J.; Bail, Z.; Gang, G.; Jiang, D.; Hu, Q.; He, J.; Zhang, H.; Zhuang, G. Functional assembly of bacterial communities with activity for the biodegradation of an organophosphorus pesticide in the rape phyllosphere. FEMS Microbiol. Lett. 2010, 306, 135–143. [Google Scholar] [CrossRef]
- Singh, B.K.; Walker, A. Microbial degradation of organophosphorus compounds. FEMS Microbiol. Rev. 2006, 30, 428–471. [Google Scholar] [CrossRef]
- Bhatt, P.; Joshi, T.; Bhatt, K.; Zhang, W.; Huang, Y.; Chen, S. Binding interaction of glyphosate with glyphosate oxidoreductase and C–P lyase: Molecular docking and molecular dynamics simulation studies. J. Hazard. Mater. 2021, 409, 124927. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, W.; Pang, S.; Chen, J.; Bhatt, P.; Mishra, S.; Chen, S. Insights into the microbial degradation and catalytic mechanisms of chlorpyrifos. Environ. Res. 2021, 194, 110660. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Liu, C.L.; Peng, C.Y.; Liu, H.M.; Hu, M.Y.; Zhong, G.H. Biodegradation of chlorpyrifos and its hydrolysis product 3,5,6-trichloro-2-pyridinol by a new fungal strain Cladosporium cladosporioides Hu-01. PLoS ONE 2012, 7, e47205. [Google Scholar] [CrossRef]
- Zhan, H.; Feng, Y.; Fan, X.; Chen, S. Recent advances in glyphosate biodegradation. Appl. Microbiol. Biotechnol. 2018, 102, 5033–5043. [Google Scholar] [CrossRef] [PubMed]
- Spina, F.; Cecchi, G.; Torres, A.Y.L.; Pecoraro, L.; Russo, F.; Wu, B.; Cai, L.; Liu, X.Z.; Tosi, S.; Varese, G.C.; et al. Fungi as a toolbox for sustainable bioremediation of pesticides in soil and water. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2018, 152, 474–488. [Google Scholar] [CrossRef]
- Bhatt, P.; Verma, A.; Gangola, S.; Bhandari, G.; Chen, S. Microbial glycoconjugates in organic pollutant bioremediation: Recent advances and applications. Microb. Cell Fact. 2021, 20, 72. [Google Scholar] [CrossRef] [PubMed]
- Maya, K.; Upadhyay, S.; Singh, R.; Dubey, S.K. Degradation kinetics of chlorpyrifos and 3,5,6-trichloro-2-pyridinol (TCP) by fungal communities. Bioresour. Technol. 2012, 126, 216–223. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, S.H.; Hu, M.Y.; Hu, Q.B.; Luo, J.J.; Li, Y.N. Purification and characterization of a novel chlorpyrifos hydrolase from Cladosporium cladosporioides Hu-01. PLoS ONE 2012, 7, e38137. [Google Scholar] [CrossRef]
- Wang, N.N.; Yuan, L.; Dai, H.; Han, Z.K.; Zhao, M. Effect of PON1 on dichlorvos toxico kinetics. Emerg. Med. J. 2010, 28, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Oncescu, T.; Oancea, P.; Enache, M.; Popescu, G.; Dumitru, L.; Kamekura, M. Halophilic bacteria are able to decontaminate dichlorvos, a pesticide, from saline environments. Cent. Eur. J. Biol. 2007, 2, 563–573. [Google Scholar] [CrossRef]
- Bhatt, P.; Zhou, X.; Huang, Y.; Zhang, W.; Chen, S. Characterization of the role of esterases in the biodegradation of organophosphate, carbamate, and pyrethroid pesticides. J. Hazard. Mater. 2021, 411, 125026. [Google Scholar] [CrossRef]
- Bhatt, P.; Bhatt, K.; Sharma, A.; Zhang, W.; Mishra, S.; Chen, S. Biotechnological basis of microbial consortia for the removal of pesticides from the environment. Crit. Rev. Biotechnol. 2021, 41, 317–338. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).