Recovery-Stress Response of Blood-Based Biomarkers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Outcome Measures
2.3. Blood Sampling and Analyses
2.4. Statistical Analyses
3. Results
3.1. Analyses of Blood-Based Biomarkers across Disciplines
3.2. Analyses of Blood-Based Biomarkers within Disciplines
3.2.1. Cytokines
3.2.2. Enzymes
3.2.3. Chemokines, Growth Factors, Hormones, Other Inflammatory Signaling Molecules, and Proteinases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kellmann, M.; Bertollo, M.; Bosquet, L.; Brink, M.; Coutts, A.J.; Duffield, R.; Erlacher, D.; Halson, S.L.; Hecksteden, A.; Heidari, J.; et al. Recovery and Performance in Sport: Consensus Statement. Int. J. Sports Physiol. Perform. 2018, 13, 240–245. [Google Scholar] [CrossRef]
- Eckard, T.G.; Padua, D.A.; Hearn, D.W.; Pexa, B.S.; Frank, B.S. The Relationship Between Training Load and Injury in Athletes: A Systematic Review. Sports Med. 2018, 48, 1929–1961. [Google Scholar] [CrossRef]
- Greenham, G.; Buckley, J.D.; Garrett, J.; Eston, R.; Norton, K. Biomarkers of Physiological Responses to Periods of Intensified, Non-Resistance-Based Exercise Training in Well-Trained Male Athletes: A Systematic Review and Meta-Analysis. Sports Med. 2018, 48, 2517–2548. [Google Scholar] [CrossRef]
- Thorpe, R.T.; Atkinson, G.; Drust, B.; Gregson, W. Monitoring Fatigue Status in Elite Team-Sport Athletes: Implications for Practice. Int. J. Sports Physiol. Perform. 2017, 12, S227–S234. [Google Scholar] [CrossRef]
- Halson, S.L. Monitoring Training Load to Understand Fatigue in Athletes. Sports Med. 2014, 44, 139–147. [Google Scholar] [CrossRef]
- Kellmann, M. Preventing overtraining in athletes in high-intensity sports and stress/recovery monitoring: Preventing overtraining. Scand. J. Med. Sci. Sports 2010, 20, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Proschinger, S.; Freese, J. Neuroimmunological and neuroenergetic aspects in exercise-induced fatigue. Exerc. Immunol. Rev. 2019, 25, 8–19. [Google Scholar] [PubMed]
- Lee, E.C.; Fragala, M.S.; Kavouras, S.A.; Queen, R.M.; Pryor, J.L.; Casa, D.J. Biomarkers in Sports and Exercise: Tracking Health, Performance, and Recovery in Athletes. J. Strength Cond. Res. 2017, 31, 2920–2937. [Google Scholar] [CrossRef] [PubMed]
- Hecksteden, A.; Skorski, S.; Schwindling, S.; Hammes, D.; Pfeiffer, M.; Kellmann, M.; Ferrauti, A.; Meyer, T. Blood-Borne Markers of Fatigue in Competitive Athletes—Results from Simulated Training Camps. PLoS ONE 2016, 11, e0148810. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Griffiths, P.C.; Mellalieu, S.D. Training Load and Fatigue Marker Associations with Injury and Illness: A Systematic Review of Longitudinal Studies. Sports Med. Auckl. NZ 2017, 47, 943–974. [Google Scholar] [CrossRef] [PubMed]
- Hecksteden, A.; Pitsch, W.; Julian, R.; Pfeiffer, M.; Kellmann, M.; Ferrauti, A.; Meyer, T. A New Method to Individualize Monitoring of Muscle Recovery in Athletes. Int. J. Sports Physiol. Perform. 2017, 12, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Barth, V.; Käsbauer, H.; Ferrauti, A.; Kellmann, M.; Pfeiffer, M.; Hecksteden, A.; Meyer, T. Individualized Monitoring of Muscle Recovery in Elite Badminton. Front. Physiol. 2019, 10, 778. [Google Scholar] [CrossRef] [PubMed]
- Krüger, K.; Reichel, T.; Zeilinger, C. Role of heat shock proteins 70/90 in exercise physiology and exercise immunology and their diagnostic potential in sports. J. Appl. Physiol. Bethesda Md 1985 2019, 126, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Pedlar, C.R.; Newell, J.; Lewis, N.A. Blood Biomarker Profiling and Monitoring for High-Performance Physiology and Nutrition: Current Perspectives, Limitations and Recommendations. Sports Med. 2019, 49, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Reichel, T.; Boßlau, T.K.; Palmowski, J.; Eder, K.; Ringseis, R.; Mooren, F.C.; Walscheid, R.; Bothur, E.; Samel, S.; Frech, T.; et al. Reliability and suitability of physiological exercise response and recovery markers. Sci. Rep. 2020, 10, 11924. [Google Scholar] [CrossRef]
- Finsterer, J.; Drory, V.E. Wet, volatile, and dry biomarkers of exercise-induced muscle fatigue. BMC Musculoskelet. Disord. 2016, 17, 40. [Google Scholar] [CrossRef]
- Peake, J.M.; Della Gatta, P.; Suzuki, K.; Nieman, D.C. Cytokine expression and secretion by skeletal muscle cells: Regulatory mechanisms and exercise effects. Exerc. Immunol. Rev. 2015, 21, 8–25. [Google Scholar]
- Schild, M.; Eichner, G.; Beiter, T.; Zügel, M.; Krumholz-Wagner, I.; Hudemann, J.; Pilat, C.; Krüger, K.; Niess, A.M.; Steinacker, J.M.; et al. Effects of Acute Endurance Exercise on Plasma Protein Profiles of Endurance-Trained and Untrained Individuals over Time. Mediat. Inflamm. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Schneider, C.; Wiewelhove, T.; McLaren, S.J.; Röleke, L.; Käsbauer, H.; Hecksteden, A.; Kellmann, M.; Pfeiffer, M.; Ferrauti, A. Monitoring training and recovery responses with heart rate measures during standardized warm-up in elite badminton players. PLoS ONE 2020, 15, e0244412. [Google Scholar] [CrossRef]
- Kellmann, M.; Kölling, S.; Hitzschke, B. Das Akutmaß und die Kurzskala zur Erfassung von Erholung und Beanspruchung im Sport: Manual. 1; Sportverlag Strauß: Hellenthal, Germany, 2016; ISBN 978-3-86884-538-9. [Google Scholar]
- Brancaccio, P.; Maffulli, N.; Limongelli, F.M. Creatine kinase monitoring in sport medicine. Br. Med. Bull. 2007, 81–82, 209–230. [Google Scholar] [CrossRef]
- Nieman, D.; Groen, A.; Pugachev, A.; Vacca, G. Detection of Functional Overreaching in Endurance Athletes Using Proteomics. Proteomes 2018, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Ferris, L.T.; Williams, J.S.; Shen, C.-L. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med. Sci. Sports Exerc. 2007, 39, 728–734. [Google Scholar] [CrossRef]
- Deminice, R.; Sicchieri, T.; Payão, P.O.; Jordão, A.A. Blood and Salivary Oxidative Stress Biomarkers Following an Acute Session of Resistance Exercise in Humans. Int. J. Sports Med. 2010, 31, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Kabasakalis, A.; Nikolaidis, S.; Tsalis, G.; Mougios, V. Response of Blood Biomarkers to Sprint Interval Swimming. Int. J. Sports Physiol. Perform. 2020, 15, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.M.W.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef]
- Sugama, K.; Suzuki, K.; Yoshitani, K.; Shiraishi, K.; Kometani, T. IL-17, neutrophil activation and muscle damage following endurance exercise. Exerc. Immunol. Rev. 2012, 18, 116–127. [Google Scholar]
- Kakanis, M.W.; Peake, J.; Brenu, E.W.; Simmonds, M.; Gray, B.; Marshall-Gradisnik, S.M. T helper cell cytokine profiles after endurance exercise. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2014, 34, 699–706. [Google Scholar] [CrossRef]
- Kostrzewa-Nowak, D.; Nowak, R. T helper cell-related changes in peripheral blood induced by progressive effort among soccer players. PLoS ONE 2020, 15, e0227993. [Google Scholar] [CrossRef]
- Maddur, M.S.; Miossec, P.; Kaveri, S.V.; Bayry, J. Th17 cells: Biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am. J. Pathol. 2012, 181, 8–18. [Google Scholar] [CrossRef]
- Ispirlidis, I.; Fatouros, I.G.; Jamurtas, A.Z.; Nikolaidis, M.G.; Michailidis, I.; Douroudos, I.; Margonis, K.; Chatzinikolaou, A.; Kalistratos, E.; Katrabasas, I.; et al. Time-course of changes in inflammatory and performance responses following a soccer game. Clin. J. Sport Med. Off. J. Can. Acad. Sport Med. 2008, 18, 423–431. [Google Scholar] [CrossRef]
- Mooren, F.C.; Lechtermann, A.; Fobker, M.; Brandt, B.; Sorg, C.; Völker, K.; Nacken, W. The response of the novel pro-inflammatory molecules S100A8/A9 to exercise. Int. J. Sports Med. 2006, 27, 751–758. [Google Scholar] [CrossRef]
- Souglis, A.; Bogdanis, G.C.; Giannopoulou, I.; Papadopoulos, C.; Apostolidis, N. Comparison of inflammatory responses and muscle damage indices following a soccer, basketball, volleyball and handball game at an elite competitive level. Res. Sports Med. Print 2015, 23, 59–72. [Google Scholar] [CrossRef]
- Faude, O.; Meyer, T.; Rosenberger, F.; Fries, M.; Huber, G.; Kindermann, W. Physiological characteristics of badminton match play. Eur. J. Appl. Physiol. 2007, 100, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, Y.; Coetzee, B.; van den Berg, L. Relationships Between Results of an Internal and External Match Load Determining Method in Male, Singles Badminton Players. J. Strength Cond. Res. 2019, 33, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.F.; Faude, O.; aus der Fünten, K. Sportmedizin im Fußball: Erkenntnisse aus dem Profifußball für alle Leistungsklassen; Meyer & Meyer: Aachen, Germany, 2014; ISBN 978-3-89899-791-1. [Google Scholar]
- Hoekstra, S.P.; Westerman, M.N.; Beke, F.; Bishop, N.C.; Leicht, C.A. Modality-specific training adaptations—Do they lead to a dampened acute inflammatory response to exercise? Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2019, 44, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Wiewelhove, T.; Raeder, C.; Meyer, T.; Kellmann, M.; Pfeiffer, M.; Ferrauti, A. Markers for Routine Assessment of Fatigue and Recovery in Male and Female Team Sport Athletes during High-Intensity Interval Training. PLoS ONE 2015, 10, e0139801. [Google Scholar] [CrossRef]
- Niemelä, M.; Kangastupa, P.; Niemelä, O.; Bloigu, R.; Juvonen, T. Acute Changes in Inflammatory Biomarker Levels in Recreational Runners Participating in a Marathon or Half-Marathon. Sports Med. Open 2016, 2, 21. [Google Scholar] [CrossRef]
- Peake, J.; Peiffer, J.J.; Abbiss, C.R.; Nosaka, K.; Okutsu, M.; Laursen, P.B.; Suzuki, K. Body temperature and its effect on leukocyte mobilization, cytokines and markers of neutrophil activation during and after exercise. Eur. J. Appl. Physiol. 2008, 102, 391–401. [Google Scholar] [CrossRef]
- Peake, J.; Peiffer, J.J.; Abbiss, C.R.; Nosaka, K.; Laursen, P.B.; Suzuki, K. Carbohydrate gel ingestion and immunoendocrine responses to cycling in temperate and hot conditions. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 229–246. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Pedersen, M.; Krabbe, K.S.; Bruunsgaard, H.; Matthews, V.B.; Febbraio, M.A. Role of exercise-induced brain-derived neurotrophic factor production in the regulation of energy homeostasis in mammals: Brain-derived neurotrophic factor in metabolism. Exp. Physiol. 2009, 94, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pinilla, F.; Vaynman, S.; Ying, Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur. J. Neurosci. 2008, 28, 2278–2287. [Google Scholar] [CrossRef]
- Nofuji, Y.; Suwa, M.; Sasaki, H.; Ichimiya, A.; Nishichi, R.; Kumagai, S. Different circulating brain-derived neurotrophic factor responses to acute exercise between physically active and sedentary subjects. J. Sports Sci. Med. 2012, 11, 83–88. [Google Scholar]
- Tonoli, C.; Heyman, E.; Buyse, L.; Roelands, B.; Piacentini, M.F.; Bailey, S.; Pattyn, N.; Berthoin, S.; Meeusen, R. Neurotrophins and cognitive functions in T1D compared with healthy controls: Effects of a high-intensity exercise. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2015, 40, 20–27. [Google Scholar] [CrossRef]
- Zoladz, J.A.; Pilc, A.; Majerczak, J.; Grandys, M.; Zapart-Bukowska, J.; Duda, K. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2008, 59 (Suppl. 7), 119–132. [Google Scholar]
- Dinoff, A.; Herrmann, N.; Swardfager, W.; Liu, C.S.; Sherman, C.; Chan, S.; Lanctôt, K.L. The Effect of Exercise Training on Resting Concentrations of Peripheral Brain-Derived Neurotrophic Factor (BDNF): A Meta-Analysis. PLoS ONE 2016, 11, e0163037. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.; Herbsleb, M.; de la Cruz, F.; Schumann, A.; Brünner, F.; Schachtzabel, C.; Gussew, A.; Puta, C.; Smesny, S.; Gabriel, H.W.; et al. Hippocampal structure, metabolism, and inflammatory response after a 6-week intense aerobic exercise in healthy young adults: A controlled trial. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2015, 35, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Currie, J.; Ramsbottom, R.; Ludlow, H.; Nevill, A.; Gilder, M. Cardio-respiratory fitness, habitual physical activity and serum brain derived neurotrophic factor (BDNF) in men and women. Neurosci. Lett. 2009, 451, 152–155. [Google Scholar] [CrossRef]
- Beier, M.; Bombardier, C.H.; Hartoonian, N.; Motl, R.W.; Kraft, G.H. Improved physical fitness correlates with improved cognition in multiple sclerosis. Arch. Phys. Med. Rehabil. 2014, 95, 1328–1334. [Google Scholar] [CrossRef]
- Petersen, A.M.W.; Pedersen, B.K. The role of IL-6 in mediating the anti-inflammatory effects of exercise. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2006, 57 (Suppl. 10), 43–51. [Google Scholar]
- Sugama, K.; Suzuki, K.; Yoshitani, K.; Shiraishi, K.; Kometani, T. Urinary excretion of cytokines versus their plasma levels after endurance exercise. Exerc. Immunol. Rev. 2013, 19, 29–48. [Google Scholar]
- Barros, E.S.; Nascimento, D.C.; Prestes, J.; Nóbrega, O.T.; Córdova, C.; Sousa, F.; Boullosa, D.A. Acute and Chronic Effects of Endurance Running on Inflammatory Markers: A Systematic Review. Front. Physiol. 2017, 8, 779. [Google Scholar] [CrossRef] [PubMed]


| Badminton (n = 13) | Soccer (n = 22) | |
|---|---|---|
| Age, years | 25.6 ± 2.4 | 28.4 ± 3.4 |
| Height, cm | 183 ± 7 | 180 ± 7 |
| Weight, kg | 78 ± 10 | 79 ± 6 |
| Body fat, % | 11.9 ± 3.0 | 12.4 ± 1.8 |
| Category | Biomarker |
|---|---|
| Chemokines | CC-chemokine ligand (CCL)2, CCL4 |
| Cytokines | Interferon-gamma (IFN-γ), interleukin (IL-)10, IL-12p40, IL-17A, IL-1ß, IL-1RA, IL-6, IL-8 |
| Enzymes | Creatine kinase (CK), lactate dehydrogenase (LDH), myeloperoxidase (MPO) |
| Growth factors | Brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF) |
| Hormones | Growth hormone (GH), sex hormone-binding globulin (SHBG) |
| Other inflammatory signaling molecules | Cluster of differentiation 163 (CD163), S100 calcium-binding protein (S100)A8, S100A12 |
| Proteinases | Matrix metalloproteinases (MMP-)2, MMP-3, MMP-9 |
| Recovered | Non-Recovered | Mean Difference, 95% CI | p Value | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Chemokines | ||||||
| CCL2 | 737.7 | 229.8 | 744.3 | 211.9 | 6.6 [−47.3, 60.5] | 0.804 |
| CCL4 | 1329.3 | 163.2 | 1345.4 | 157.4 | 30.1 [−32.3, 77.9] W | 0.377 |
| Cytokines | ||||||
| IFN-γ | 173.2 | 123.6 | 190.7 | 151.2 | 17.4 [−23.5, 58.4] | 0.464 |
| IL-10 | 12.4 | 5.2 | 12.9 | 4.1 | 0.5 [−0.8, 1.7] | 0.444 |
| IL-12p40 | 2792.8 | 714.1 | 2796.5 | 851.7 | 18.9 [−371.8, 441.1] W | 0.896 |
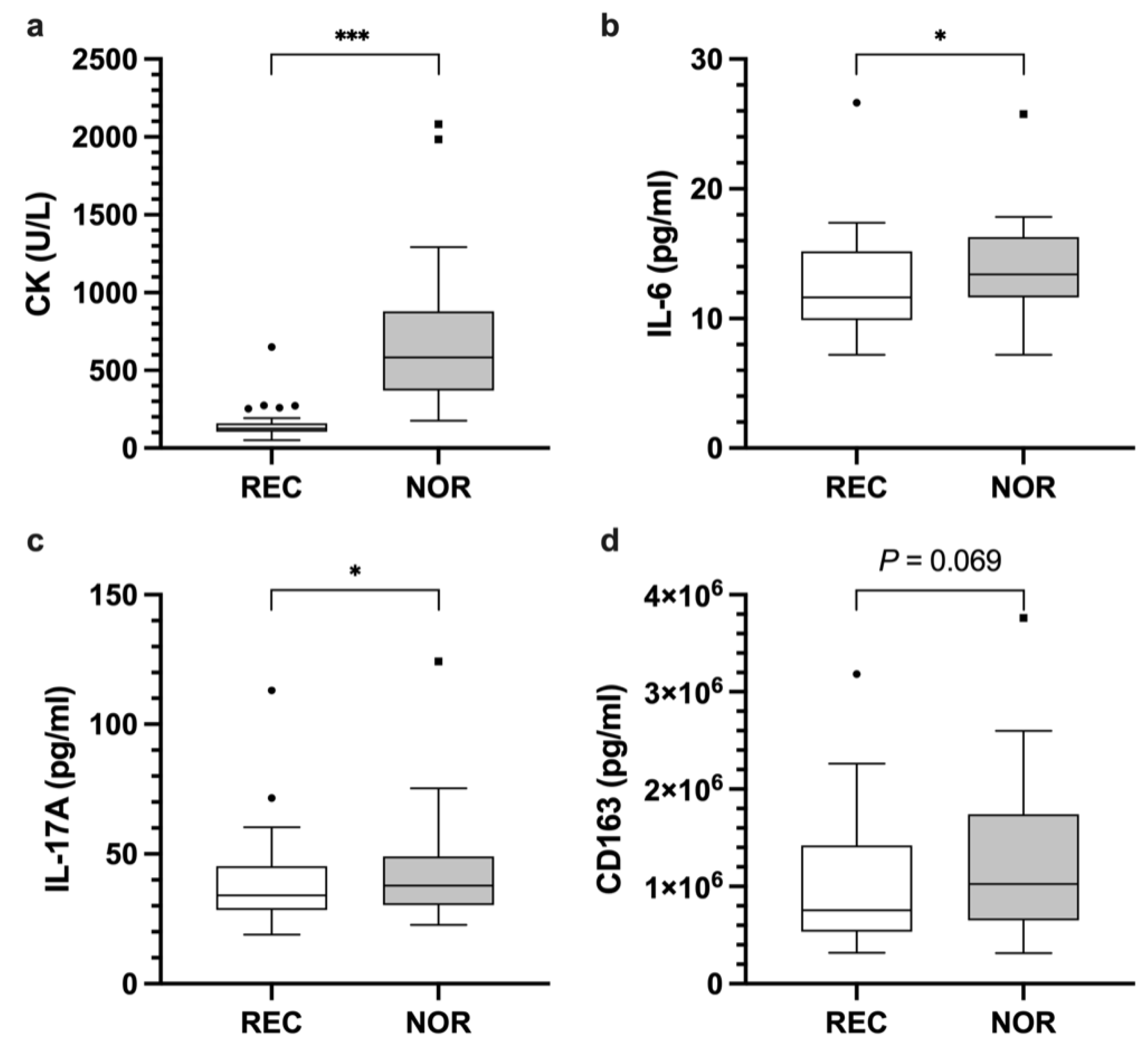
| IL-17A | 38.8 | 17.7 | 42.8 | 19.6 | 4.7 [0.9, 8.5] W | 0.033 * |
| IL-1ß | 56.7 | 14.3 | 63.7 | 25.5 | 1.7 [−4.5, 11.7] W | 0.561 |
| IL-1RA | 1130.9 | 374.7 | 1196.1 | 606.5 | 65.2 [−151.3, 281.7] | 0.663 |
| IL-6 | 12.5 | 3.8 | 13.7 | 3.7 | 1.3 [0.2, 2.4] | 0.024 * |
| IL-8 | 42.7 | 14.5 | 47.5 | 17.3 | 4.7 [−1.7, 11.2] | 0.145 |
| Enzymes | ||||||
| CK | 152.6 | 102.4 | 693.1 | 435.5 | 540.6 [399.2, 681.9] | <0.001 * |
| LDH | 435 | 387.1 | 434.6 | 376.5 | −0.4 [−31.4, 30.5] | 0.701 |
| MPO | 109,757.2 | 44,962.7 | 118,082.6 | 40,440.4 | 8325.5 [−8074.9, 24,725.8] | 0.268 |
| Growth factors | ||||||
| BDNF | 31,282.7 | 6456.2 | 30,294.2 | 6565.3 | −988.5 [−2298.5, 321.4] | 0.134 |
| GDNF | 29.5 | 8 | 30.4 | 9.7 | 0.9 [−1.8, 3.6] | 0.645 |
| Hormones | ||||||
| GH | 2504.4 | 4223.1 | 1891 | 2502 | 120 [−207, 367.1] W | 0.199 |
| SHBG | 1.5 × 107 | 3.2 × 106 | 1.5 × 107 | 3.4 × 106 | −434,178.6 [−994,313.3, 125,956.1] | 0.124 |
| Other inflammatory signaling molecules | ||||||
| CD163 | 1.0 × 106 | 650,929.7 | 1.2 × 106 | 794,388.1 | 174,283.6 [11,188, 337,379.3] | 0.069 |
| S100A8 | 213.4 | 66.7 | 204.3 | 58.7 | −5.7 [−27.3, 16] | 0.600 |
| S100A12 | 47,342.8 | 17,408.7 | 46,630.2 | 16,819 | −712.6 [−6942.6, 5517.3] | 0.818 |
| Proteinases | ||||||
| MMP-2 | 292,144.4 | 37,363.4 | 297,876 | 36,001.2 | 5731.6 [−3788.8, 15,252] | 0.230 |
| MMP-3 | 50,011.2 | 20,945.4 | 50,960.5 | 16,505.7 | 949.3 [−4812.8, 6711.4] | 0.422 |
| MMP-9 | 114,122.4 | 84,183.6 | 115,426.2 | 89,562.2 | 1303.8 [−22,467.9, 25,075.6] | 0.912 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hacker, S.; Reichel, T.; Hecksteden, A.; Weyh, C.; Gebhardt, K.; Pfeiffer, M.; Ferrauti, A.; Kellmann, M.; Meyer, T.; Krüger, K. Recovery-Stress Response of Blood-Based Biomarkers. Int. J. Environ. Res. Public Health 2021, 18, 5776. https://doi.org/10.3390/ijerph18115776
Hacker S, Reichel T, Hecksteden A, Weyh C, Gebhardt K, Pfeiffer M, Ferrauti A, Kellmann M, Meyer T, Krüger K. Recovery-Stress Response of Blood-Based Biomarkers. International Journal of Environmental Research and Public Health. 2021; 18(11):5776. https://doi.org/10.3390/ijerph18115776
Chicago/Turabian StyleHacker, Sebastian, Thomas Reichel, Anne Hecksteden, Christopher Weyh, Kristina Gebhardt, Mark Pfeiffer, Alexander Ferrauti, Michael Kellmann, Tim Meyer, and Karsten Krüger. 2021. "Recovery-Stress Response of Blood-Based Biomarkers" International Journal of Environmental Research and Public Health 18, no. 11: 5776. https://doi.org/10.3390/ijerph18115776
APA StyleHacker, S., Reichel, T., Hecksteden, A., Weyh, C., Gebhardt, K., Pfeiffer, M., Ferrauti, A., Kellmann, M., Meyer, T., & Krüger, K. (2021). Recovery-Stress Response of Blood-Based Biomarkers. International Journal of Environmental Research and Public Health, 18(11), 5776. https://doi.org/10.3390/ijerph18115776






