Abstract
Research into the potential impact of the food environment on liver cancer incidence has been limited, though there is evidence showing that specific foods and nutrients may be potential risk or preventive factors. Data on hepatocellular carcinoma (HCC) cases were obtained from the Surveillance, Epidemiology, and End Results (SEER) cancer registries. The county-level food environment was assessed using the Modified Retail Food Environment Index (mRFEI), a continuous score that measures the number of healthy and less healthy food retailers within counties. Poisson regression with robust variance estimation was used to calculate incidence rate ratios (IRRs) and 95% confidence intervals (CIs) for the association between mRFEI scores and HCC risk, adjusting for individual- and county-level factors. The county-level food environment was not associated with HCC risk after adjustment for individual-level age at diagnosis, sex, race/ethnicity, year, and SEER registry and county-level measures for health conditions, lifestyle factors, and socioeconomic status (adjusted IRR: 0.99, 95% CI: 0.96, 1.01). The county-level food environment, measured using mRFEI scores, was not associated with HCC risk.
1. Introduction
Hepatocellular carcinoma (HCC) comprises 85–90% of primary liver cancer cases [1,2]. HCC incidence and mortality have generally increased over time [1]. Furthermore, geographic variation in incidence and mortality suggests that environmental exposures may play a role in etiology [3]. HCC risk factors include chronic hepatitis B virus (HBV) infection, chronic hepatitis C virus (HCV) infection, aflatoxin, alcohol consumption, obesity, diabetes, metabolic syndrome, and tobacco [4]. Some research suggests that dietary factors may be associated with HCC risk. There is evidence suggesting that a lower vegetable intake [5], low selenium levels [6] and a higher intake of red meat and saturated fats [7,8] are associated with an increased risk of HCC, while inverse associations have been observed for the higher intake of plant-based fats and protein [9,10], whole grains and fiber [11], tree nuts [12], milk and yogurt, white meats, eggs, fruit, and tofu [13,14,15]. There is also evidence of dietary patterns impacting HCC development, where improved diet quality (measured by the Alternative Healthy Eating Index or AHEI) may reduce the risk of HCC [16].
However, the role of food environments in liver cancer has not been well explored. Several studies have shown that food environments (i.e., the spatial distribution of food retailers) providing more opportunities to purchase nutritious options may be important in preventing and managing chronic diseases such as obesity and cardiovascular disease, as it relates to a healthy diet [17,18,19]. Two studies showed that unhealthy food availability was positively associated with colorectal cancer incidence [20,21]. While some research has examined the association between factors related to the food environment, such as geographic variability in the dietary intake of nitrate, nitrite, and nitrosodimethylamine, and liver cancer risk [22], these studies did not specifically assess the physical food environment. To our knowledge, no epidemiologic studies have investigated the association between the food environment and HCC risk. Healthful food availability, which can be measured based on the geographic density of healthy food retailers, may impact patterns of HCC incidence [23].
To address geographic variability in HCC incidence and the limited research on the potential role of the food environment, we examined data from population-based cancer registries across the U.S., combining individual- and county-level data on case characteristics, health conditions, lifestyle factors, and socioeconomic status (SES). The objective of this study was to prospectively examine the association between the county-level food environment using the Modified Retail Food Environment Index (mRFEI) and HCC incidence in the U.S.
2. Materials and Methods
The Surveillance, Epidemiology, and End Results (SEER) database is a National Cancer Institute program, collecting individual-level information on cancer incidence from population-based cancer registries covering 34.6% of the U.S. population [24,25,26]. HCC cases identified from the following 19 registries were included in the analysis: Atlanta (metropolitan); Greater California; Connecticut; Detroit (metropolitan); Greater Georgia; Idaho; Iowa; Kentucky; Los Angeles; Louisiana (excluding July–December 2005 cases due to Hurricanes Katrina and Rita); Massachusetts; New Jersey; New Mexico; New York; Rural Georgia; San Francisco-Oakland; San Jose-Monterey; Seattle (Puget Sound); and Utah. A total of 727 counties were located in the catchment areas captured by these 19 SEER registries; these registries did not restrict coverage to specific populations and were located in the contiguous U.S., with available air pollution and ultraviolet radiation covariate data as potential confounders (a priori factors determined to be considered in modeling) [27,28]. To protect patient confidentiality, the SEER database does not include personal identifiers. This study was exempt from an Institutional Review Board review.
The inclusion criteria for cases in this study were the following: individuals residing in the area captured by the 19 SEER registries, and diagnosed with incident-confirmed HCC. HCC cases were defined using the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) topography code C22.0 for primary liver cancer and ICD-O-3 histology codes 8170 to 8175 [29]; diagnostic confirmation (e.g., positive histology) excluding clinical diagnosis only; a sequence number of one primary only; diagnosis between 2000 and 2016; and not reported via autopsy or death certificate only. As conducted in previous SEER-based epidemiologic studies, the counts of HCC cases were stratified by age at diagnosis, sex, race/ethnicity, year of diagnosis, and SEER registry [30]. Each county was associated with one SEER registry.
The food environment was estimated for each county in the study area using the Modified Retail Food Environment Index (mRFEI) [31]. The mRFEI is a continuous score that measures the number of healthy and less healthy food retailers within counties, defined by the typical food offerings in specific types of retail stores (e.g., supermarkets, convenience stores, and fast-food restaurants). The number of food retailers was obtained from the U.S. Department of Agriculture (USDA) Food Environment Atlas for 2009; sensitivity analyses were performed for the number of retailers in 2014 [32]. The standard classification of healthy and less healthy food retailers was used, with grocery stores, supercenters, club stores, and farmers’ markets considered to be healthy, and fast-food restaurants, full-service restaurants, and convenience stores considered to be less healthy. The mRFEI was calculated for each county using the following formula:
mRFEI scores ranged from 0 to 100, with higher values suggesting healthier food environments. The calculated mRFEI values were linked to each county via U.S. Federal Information Processing Standard (FIPS) codes, using the county of residence at diagnosis that was available for each case from the SEER registries. Spatial analyses were conducted in ArcGIS version 10.8 (Esri, Redlands, CA, USA).
Individual- and county-level information on established or suspected HCC risk factors and/or variables known to be associated with the exposure were evaluated as potential confounders. From the SEER database, we acquired individual-level data on age at diagnosis (years), sex (male, female), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, non-Hispanic Asian or Pacific Islander, non-Hispanic American Indian or Alaska Native), year of diagnosis (2000–2016), and SEER registry. From the 2000 U.S. Census Bureau summary files available through SEER, we obtained county-level data on median household income (per $10,000), poverty (percentage below the poverty level), percentage unemployed, educational attainment (percentage with a bachelor’s degree or higher), and the percentage of foreign-born individuals. Percentage of foreign-born data were used as a proxy for HBV prevalence, which is endemic in parts of Asia and Africa [33]. We also acquired information on county-level urbanicity (rural, urban) using USDA Rural-Urban Continuum Codes, which was used as a proxy for HCV prevalence, as differences in HCV have been observed in rural vs. urban areas [34,35].
Additional county-level data were acquired from the Institute for Health Metrics and Evaluation, created using information from the U.S. Behavioral Risk Factor Surveillance System (BRFSS) and the U.S. National Health and Nutrition Examination Survey (NHANES). Information on the sex-specific age-adjusted prevalence of alcohol consumption in 2005 (average > 1 drink per day for women, or >2 drinks per day for men in the past 30 days) [36], current smoking in 2000 (currently smoking cigarettes daily or non-daily) [37], diabetes in 2000 (percentage of adults ≥ 20 years who reported a previous diabetes diagnosis and/or have a fasting plasma glucose of ≥126 mg/dL and/or hemoglobin A1c ≥ 6.5%) [38,39], and obesity in 2001 (body mass index (BMI) ≥ 30 kg/m2) [40] was ascertained. Ambient particulate matter < 2.5 microns in diameter (PM2.5) exposure (μg/m3) was estimated for each county using a spatial PM2.5 exposure model [27]. Additionally, ambient ultraviolet radiation (UV) exposure (mW/m2), a proxy for circulating levels of vitamin D, was estimated using a spatiotemporal geostatistical exposure model [28]. The region of residence was defined as counties located in the U.S. Census Bureau-defined regions: Northeast, Midwest, South, and West. All county-level data were compiled using unique U.S. FIPS codes.
Poisson regression with robust variance estimation was used to calculate basic and fully adjusted incidence rate ratios (IRRs) and 95% confidence intervals (CIs) for the association between the county-level food environment (using the mRFEI) and HCC risk. mRFEI scores were examined continuously per interquartile range (IQR) increase (IQR: 9.3) and categorically using quintiles estimated across all counties in the study area. All models were a priori-determined to include individual-level age at diagnosis, sex, race/ethnicity, year of diagnosis, SEER registry, and the following county-level variables: urbanicity, median household income, percentage with a bachelor’s degree or higher, percentage unemployed, percentage of individuals below the poverty level, percentage of foreign-born individuals, and the prevalence of alcohol consumption, smoking, obesity, and diabetes. We evaluated potential confounding by ambient PM2.5 and UV exposure, which have been shown to have positive and inverse associations with HCC risk, respectively [27,28]. After adjustment for these variables, the IRR for mRFEI and HCC risk did not substantially change and were thus not included in the final fully adjusted model. The natural logarithm of the county population was used as the offset in all models. County-level population data were acquired from the National Center for Health Statistics Bridged-Race Resident Population Estimates online database, downloaded from the Centers for Disease Control and Prevention WONDER (Wide-ranging Online Data for Epidemiologic Research) [41].
Stratified analyses were conducted to explore potential effect modification by factors that may be associated with the food environment or with disparities in HCC incidence. We examined potential interactions with age at diagnosis, sex, race/ethnicity, year of diagnosis, region, state, median household income, poverty, unemployment, educational attainment, urbanicity, alcohol consumption, smoking, diabetes, obesity, and foreign-born. Tests for interaction were conducted by adding an interaction term to the model and using likelihood ratio tests to determine statistical significance. We performed sensitivity analyses using mRFEI scores calculated using the number of food retailers in 2014, and using log-transformed mRFEI scores to account for the relatively diminishing beneficial effects of higher mRFEI values compared to lower values [42]. A p-value < 0.05 was considered statistically significant. All statistical analyses were performed using SAS version 9.4 (Cary, NC, USA).
3. Results
A total of 90,578 HCC cases, diagnosed between 2000 and 2016, were included in the analysis. HCC cases were on average 62.7 years of age, mostly male (77.5%), non-Hispanic white (50.0%), and/or resided in the Western region of the U.S. (46.1%) (Table 1). Using county-level data from the underlying population from which HCC cases were sampled, HCC cases at the time of diagnosis resided in counties where the average mRFEI scores were 16.9 ± 8.3. HCC cases lived in counties where an average of 8.4% of the population consumed alcohol, 29.0% smoked cigarettes, 28.4% were obese, and 12.2% had diabetes. HCC cases resided in counties with a median household income of $36,300, where an average of 16.9% had a bachelor’s degree or higher, 6.3% were unemployed, 15.1% were living below the poverty level, and 4.6% were foreign-born.

Table 1.
Population characteristics of hepatocellular carcinoma cases in the U.S. (SEER 2000–2016).
Figure 1 shows mRFEI scores in 2009, categorized by quintiles, calculated using the 727 counties included in the study. The mRFEI values ranged between 4.55 and 50. In general, higher mRFEI scores were observed in the Midwest (SEER registries in Detroit and Iowa) and lower mRFEI scores were observed in the Northeast (SEER registries in Connecticut, Massachusetts, New Jersey, and New York) regions of the U.S.
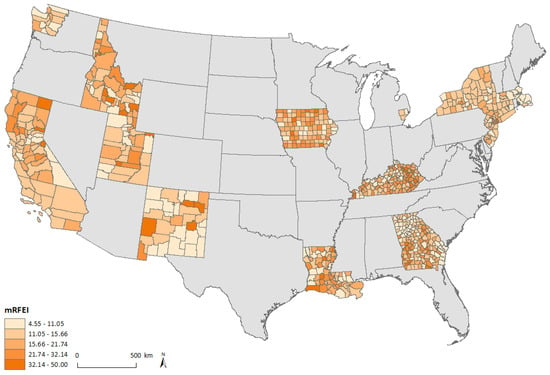
Figure 1.
Map of mRFEI in SEER catchment areas of the contiguous United States.
mRFEI scores were not associated with HCC risk in basic models, adjusted for age at diagnosis, sex, race/ethnicity, year of diagnosis, and SEER registry (adjusted IRR per IQR increase: 1.00, 95% CI: 0.97, 1.03, p = 0.97) (Table 2). After additional adjustment by county-level urbanicity, median household income, percentage with a bachelor’s degree or higher, percentage unemployed, percentage of individuals below the poverty level, percentage of foreign-born individuals, and prevalence of alcohol consumption, smoking, obesity, and diabetes, we observed no association between mRFEI scores and HCC risk (adjusted IRR per IQR increase: 0.99, 95% CI: 0.96, 1.01, p = 0.36). Similar null results were observed when examining quintiles of mRFEI scores.

Table 2.
Associations between mRFEI exposure and HCC incidence (SEER 2000–2016).
We observed statistically significant interactions (p < 0.05) between mRFEI scores and age at diagnosis, race/ethnicity, year of diagnosis, and region (Supplemental Table S1). However, the effect estimates for mRFEI scores across strata defined by these variables were similar. The association between mRFEI scores and HCC risk did not differ by sex, state, median household income, poverty, unemployment, educational attainment, urbanicity, alcohol consumption, smoking, diabetes, obesity, or foreign-born (p > 0.05). In sensitivity analyses, we observed similar results when examining mRFEI estimated in 2014 and using log-transformed mRFEI values (results not shown).
4. Discussion
This study showed no association between the county-level food environment (estimated using the mRFEI) and HCC incidence, after adjusting for individual- and county-level information on demographics, health conditions, lifestyle factors, and SES. To our knowledge, this is the first study examining the association between the food environment and HCC risk in the U.S.
Food environments may represent an important context in which cancer risk is shaped, informing disease processes and enabling the identification of vulnerable populations [43]. The food environment can include both health-promoting resources (e.g., healthy food retailers) and less healthy amenities (e.g., fast food restaurants), and has been shown to influence healthier dietary attitudes and behaviors [19,44,45,46,47]. For example, one study found that higher mRFEI scores (indicating a healthier food environment) were associated with increased levels of objectively assessed fruit and vegetable consumption [48]. Another study showed that perceptions about the food environment are important in promoting self-efficacy for consuming healthier foods, such as fruits and vegetables, among U.S. adults [49]. Access to healthier foods and healthier dietary patterns may affect cancer risk through mechanisms related to reducing chronic inflammation [50]. Population-based research on the impact of the food environment on cancer risk has not been well established, although two studies found some evidence of a positive association between an unhealthy food environment and colorectal cancer [20,21]. For HCC in particular, several studies have suggested that diet alteration can affect HCC risk. For example, the consumption of nutrients derived from plants [9,10,11,12] and dairy and proteins, such as white meat and eggs [13,14,15], may play a role in reducing HCC risk. When examining dietary patterns, adherence to the AHEI has been inversely associated with HCC risk [16].
In this study using SEER data, we observed no association between the county-level food environment and HCC risk after adjustment. Established risk factors for HCC have shown geographic variation across the U.S., including age [51,52], sex [52], race/ethnicity [53], obesity [54], diabetes [55], alcohol consumption [56], HBV, and HCV [57]. The median age of the population varies by state, with higher median ages (40 years or older) observed in parts of the Northeast and South [52]. Sex ratios, or the number of males per 100 females, were higher in the West and lower in the Northeast [52]. Race/ethnicity also differed geographically, with California, Texas, Washington, D.C., Hawaii, and New Mexico being characterized by a relatively higher population of race/ethnicities other than non-Hispanic white [53,58]. Obesity and diabetes also varied by state, with a higher diabetes prevalence and average BMI in parts of the South such as Mississippi (mean 30.5 kg/m2) [54,55]. Alcohol consumption was highest in areas including New England, and lower in parts of the South [56]. HCV also exhibits geographic variation, as the following nine states included 51.9% of all individuals living with HCV in the U.S.: California, Texas, Florida, New York, Pennsylvania, Ohio, Michigan, Tennessee, and North Carolina [57]. As geographic variability in HCC incidence across the U.S. may be explained by geographic differences in the underlying prevalence of risk factors [59], we adjusted for many of these variables at the individual or county level in our analyses. However, future research including information on individual-level HCC risk factors would be valuable.
Although there were statistically significant interactions with age at diagnosis, race/ethnicity, year at diagnosis, and region, stratified effect estimates were similar and not meaningfully different. Several factors may have contributed to our observing overall null results. The usage of administrative boundaries, such as counties, may be limited when representing the food environment. When asked to define neighborhood boundaries, residents created maps that resulted in differing spaces (30% smaller in size) than those determined by census-defined boundaries [60,61]. Thus, county-level data may not accurately represent the food environment to which individuals are exposed. Furthermore, food availability may be modified by factors such as urbanicity and transportation. For example, car ownership may expand the boundaries of the local food environment. One study found that among non-car owners, those who resided in a food environment with a higher concentration of fast-food outlets had higher BMIs compared to those who resided in an environment with relatively fewer fast-food outlets [62]. Future research could consider examining more granular locational and food environment data and other relevant covariate factors such as transportation, time-use, and household composition. In addition, our study did not account for pesticide use, a risk factor for HCC, which may be related to the food environment such as through the amount of pesticides used in fresh produce at the food retailers [63,64].
The limitations of our study include its ecological design, preventing inference to the individual-level risk of developing HCC. County administrative boundaries may not accurately represent the individual-level residential environment [65,66]. The usage of more granular data, such as census tracts, would increase exposure variability compared to county-level analyses of mRFEI scores [67]. Although the mRFEI score is an established measure for the food environment [23,48,68], there may be potential exposure misclassification, as there was no information on the specific food options provided at each food retailer. Furthermore, the binary grouping of establishments into healthy vs. unhealthy may not accurately represent the food environment. Alternative measures of the food environment, such as quick-service food retail availability, may capture other aspects of the food environment not considered in the mRFEI. There may also be residual confounding due to unmeasured factors. Although the prevalence of HCV in the U.S. is low (1.0–1.9%), and HCV infection may be more common in urban areas, urbanicity as a proxy for HCV infection may not adequately capture geographic variation in HCV [34,69]. Future research should include information on individual-level HCV status. There are also regional differences in the quality of food environments across the U.S., such as a lack of access to supermarkets in areas including the Great Plains, Deep South, and Appalachian region [70]. Although we addressed these regional differences through stratified analyses by region and state, additional research using locational data that are more granular than the county level is warranted. The strengths of our study include a large sample size of confirmed HCC cases from population-based cancer registries across the U.S. The study area included counties across the contiguous U.S., characterized by a wide range of mRFEI scores. Data on many potential confounders and effect modifiers at the individual and county levels were available.
5. Conclusions
In conclusion, our results showed no association between the food environment and HCC risk. Future research examining the association of the food environment and HCC risk could examine higher resolution locational and food environment data, and account for transportation and dietary preferences.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijerph18115740/s1, Table S1: Associations between mRFEI exposure and HCC incidence stratified by age at diagnosis, year at diagnosis, race/ethnicity, and region (SEER 2000–2016).
Author Contributions
Conceptualization, T.V.; methodology, M.T., M.J.W., P.J. and T.V.; formal analysis, M.T.; data curation, T.V., writing—original draft, M.T. and T.V.; writing—review and editing, M.T., M.J.W., P.J. and T.V.; supervision, T.V.; project administration, T.V.; funding acquisition, T.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institute of Health/National Institute of Diabetes and Digestive and Kidney Diseases, grant number K01 DK125612 to T.V.; and the National Institute of Health/National Cancer Institute, grant number T32 CA094880 to M.T.
Institutional Review Board Statement
SEER data do not include personal identifiers. This study was exempt from an Institutional Review Board review.
Informed Consent Statement
Not applicable.
Data Availability Statement
Restrictions apply to the availability of this data. Data were obtained from SEER registries and are available with the permission of SEER.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- El–Serag, H.B.; Rudolph, K.L. Hepatocellular Carcinoma: Epidemiology and Molecular Carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef]
- VoPham, T. Environmental Risk Factors for Liver Cancer and Nonalcoholic Fatty Liver Disease. Curr. Epidemiol. Rep. 2019, 6, 50–66. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Petrick, J.L.; London, W.T. Global Epidemiology of Hepatocellular Carcinoma: An Emphasis on Demographic and Regional Variability. Clin. Liver Dis. 2015, 19, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.W.; Hsieh, H.H.; Pan, W.H.; Yang, C.S.; Chen, C.J. Vegetable Consumption, Serum Retinol Level, and Risk of Hepatocellular Carcinoma. Cancer Res. 1995, 55, 1301. [Google Scholar] [PubMed]
- Yu, M.-W.; Horng, I.-S.; Hsu, K.-H.; Chiang, Y.-C.; Liaw, Y.F.; Chen, C.-J. Plasma Selenium Levels and Risk of Hepatocellular Carcinoma among Men with Chronic Hepatitis Virus Infection. Am. J. Epidemiol. 1999, 150, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.D.; Cross, A.J.; McGlynn, K.A.; Abnet, C.C.; Park, Y.; Hollenbeck, A.R.; Schatzkin, A.; Everhart, J.E.; Sinha, R. Association of Meat and Fat Intake with Liver Disease and Hepatocellular Carcinoma in the NIH-AARP Cohort. J. Natl. Cancer Inst. 2010, 102, 1354–1365. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, W.; Li, T.; Liu, Y.; Simon, T.G.; Sui, J.; Wu, K.; Giovannucci, E.L.; Chan, A.T.; Zhang, X. Meat Intake and Risk of Hepatocellular Carcinoma in Two Large US Prospective Cohorts of Women and Men. Int. J. Epidemiol. 2019, 48, 1863–1871. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Sui, J.; Ma, Y.; Simon, T.G.; Petrick, J.L.; Lai, M.; McGlynn, K.A.; Campbell, P.T.; Giovannucci, E.L.; Chan, A.T.; et al. High Dietary Intake of Vegetable or Polyunsaturated Fats Is Associated with Reduced Risk of Hepatocellular Carcinoma. Clin. Gastroenterol. Hepatol. 2020, 18, 2775–2783. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, W.; VoPham, T.; Ma, Y.; Simon, T.G.; Gao, X.; Chan, A.T.; Meyerhardt, J.A.; Giovannucci, E.L.; Zhang, X. Plant-Based and Animal-Based Low-Carbohydrate Diets and Risk of Hepatocellular Carcinoma Among US Men and Women. Hepatology 2021, 73, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ma, Y.; Liu, Y.; Smith-Warner, S.A.; Simon, T.G.; Chong, D.Q.; Qi, Q.; Meyerhardt, J.A.; Giovannucci, E.L.; Chan, A.T.; et al. Association of Intake of Whole Grains and Dietary Fiber with Risk of Hepatocellular Carcinoma in US Adults. JAMA Oncol. 2019, 5, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Yang, W.; Ma, Y.; Li, T.Y.; Simon, T.G.; Meyerhardt, J.A.; Liang, G.; Giovannucci, E.L.; Chan, A.T.; Zhang, X. A Prospective Study of Nut Consumption and Risk of Primary Hepatocellular Carcinoma in the U.S. Women and Men. Cancer Prev. Res. 2019, 12, 367–374. [Google Scholar] [CrossRef]
- Talamini, R.; Polesel, J.; Montella, M.; Maso, L.D.; Crispo, A.; Tommasi, L.G.; Izzo, F.; Crovatto, M.; La Vecchia, C.; Franceschi, S. Food Groups and Risk of Hepatocellular Carcinoma: A Multicenter Case-control Study in Italy. Int. J. Cancer 2006, 119, 2916–2921. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Sui, J.; Ma, Y.; Simon, T.G.; Chong, D.; Meyerhardt, J.A.; Willett, W.C.; Giovannucci, E.L.; Chan, A.T.; Zhang, X. A Prospective Study of Dairy Product Intake and the Risk of Hepatocellular Carcinoma in U.S. Men and Women. Int. J. Cancer 2020, 146, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Sauvaget, C.; Nagano, J.; Hayashi, M.; Spencer, E.; Shimizu, Y.; Allen, N. Vegetables and Fruit Intake and Cancer Mortality in the Hiroshima/Nagasaki Life Span Study. Br. J. Cancer 2003, 88, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, W.; Simon, T.G.; Smith-Warner, S.A.; Fung, T.T.; Sui, J.; Chong, D.; Vo Pham, T.; Meyerhardt, J.A.; Wen, D.; et al. Dietary Patterns and Risk of Hepatocellular Carcinoma among U.S. Men and Women. Hepatology 2019, 70, 577–586. [Google Scholar] [CrossRef]
- French, A.S.; Story, M.; Jeffery, R.W. Environmental Influences on Eating and Physical Activity. Annu. Rev. Public Health 2001, 22, 309–335. [Google Scholar] [CrossRef]
- Inagami, S.; Cohen, D.A.; Finch, B.K.; Asch, S.M. You Are Where You Shop: Grocery Store Locations, Weight, and Neighborhoods. Am. J. Prev. Med. 2006, 31, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Morland, K.; Wing, S.; Roux, A.D. The Contextual Effect of the Local Food Environment on Residents’ Diets: The Ather-osclerosis Risk in Communities Study. Am. J. Public Health 2002, 92, 1761–1768. [Google Scholar] [CrossRef]
- Gibson, D.C.; Prochaska, J.D.; Yu, X.; Kaul, S. An Examination between Census Tract Unhealthy Food Availability and Colorectal Cancer Incidence. Cancer Epidemiol. 2020, 67, 101761. [Google Scholar] [CrossRef]
- Canchola, A.J.; Shariff-Marco, S.; Yang, J.; Albright, C.; Hertz, A.; Park, S.Y.; Gomez, L.S.; Monroe, K.R.; Wilkens, L.R.; Cheng, I.; et al. Association between the Neighborhood Obesogenic Environment and Colorectal Cancer Risk in the Multi-ethnic Cohort. Cancer Epidemiol. 2017, 50, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Mitacek, E.J.; Brunnemann, K.D.; Suttajit, M.; Caplan, L.S.; Gagna, C.E.; Bhothisuwan, K.; Siriamornpun, S.; Hummel, C.F.; Ohshima, H.; Roy, R.; et al. Geographic Distribution of Liver and Stomach Cancers in Thailand in Relation to Estimated Dietary Intake of Nitrate, Nitrite, and Nitrosodimethylamine. Nutr. Cancer 2008, 60, 196–203. [Google Scholar] [CrossRef]
- Mahendra, A.; Polsky, J.Y.; Robitaille, E.; Lefebvre, M.; McBrien, T.; Minaker, M.L. Status Report-Geographic Retail Food Environment Measures for Use in Public Health. Health Promot. Chronic Dis. Prev. Can. 2017, 37, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Duggan, M.A.; Anderson, W.F.; Altekruse, S.; Penberthy, L.; Sherman, M.E. The Surveillance, Epidemiology, and End Results (SEER) Program and Pathology: Toward Strengthening the Critical Relationship. Am. J. Surg. Pathol. 2016, 40, e94–e102. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Lloyd, S.; Decker, R.H.; Wilson, L.D.; Yu, J.B. Overview of the Surveillance, Epidemiology, and End Results Database: Evolution, Data Variables, and Quality Assurance. Curr. Probl. Cancer 2012, 36, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Enewold, L.; Parsons, H.; Zhao, L.; Bott, D.; Rivera, D.R.; Barrett, M.J.; Virnig, B.A.; Warren, J.L. Updated Overview of the SEER-Medicare Data: Enhanced Content and Applications. JNCI Monogr. 2020, 2020, 3–13. [Google Scholar]
- VoPham, T.; Bertrand, K.A.; Tamimi, R.M.; Laden, F.; Hart, J.E. Ambient PM2.5 Air Pollution Exposure and Hepatocellular Carcinoma Incidence in the United States. Cancer Causes Control 2018, 29, 563–572. [Google Scholar] [CrossRef] [PubMed]
- VoPham, T.; Hart, J.E.; Bertrand, K.A.; Sun, Z.; Tamimi, R.M.; Laden, F. Spatiotemporal Exposure Modeling of Ambient Erythemal Ultraviolet Radiation. Environ. Health 2016, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fritz, A.; Percy, C.; Shanmugaratnam, K.; Sobin, L.; Parkin, D.M.; Whelan, S. International Classification of Diseases for Oncology (ICD-O-3); World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- VoPham, T.; Weaver, M.D.; Vetter, C.; Tamimi, R.M.; Laden, F.; Bertrand, K.A.; Hart, E.J. Circadian Misalignment and Hepatocellular Carcinoma Incidence in the United States. Cancer Epidemiol. Biomark. Prev. 2018, 27, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Census Tract Level State Maps of the Modified Retail Food Environment Index (mRFEI); Centers for Disease Control and Prevention: Atlanta, GA, USA, 2013.
- Food Environment Atlas; U.S. Department of Agriculture Economic Research Service: Washington, DC, USA, 2020.
- Carr, B. Heptaocellular Carcinoma: Diagnosis and Treatment; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Kauhl, B.; Heil, J.; Hoebe, C.J.P.A.; Schweikart, J.; Krafft, T.; Dukers-Muijrers, N.H.T.M. The Spatial Distribution of Hepatitis C Virus Infections and Associated Determinants—An Application of a Geographically Weighted Poisson Regression for Evidence-Based Screening Interventions in Hotspots. PLoS ONE 2015, 10, e0135656. [Google Scholar] [CrossRef]
- Surveillance, Epidemiology, and End Results: County Attributes; National Cancer Institute: Bethesda, MD, USA, 2019.
- Dwyer-Lindgren, L.; Flaxman, A.D.; Ng, M.; Hansen, G.M.; Murray, C.J.L.; Mokdad, A.H. Drinking Patterns in US Counties From 2002 to 2012. Am. J. Public Health 2015, 105, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Dwyer-Lindgren, L.; Mokdad, A.H.; Srebotnjak, T.; Flaxman, A.D.; Hansen, G.M.; Murray, C.J. Cigarette Smoking Prevalence in US Counties: 1996–2012. Popul. Health Metr. 2014, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Diagnosed and Undiagnosed Diabetes Prevalence by County in the U.S., 1999–2012; Institute for Health Metrics and Evaluation: Seattle, WA, USA, 2016.
- Dwyer-Lindgren, L.; MacKenbach, J.P.; Van Lenthe, F.J.; Flaxman, A.D.; Mokdad, A.H. Diagnosed and Undiagnosed Diabetes Prevalence by County in the U.S., 1999–2012. Diabetes Care 2016, 39, 1556–1562. [Google Scholar] [CrossRef] [PubMed]
- Dwyer-Lindgren, L.; Freedman, G.; Engell, E.R.; Fleming, T.D.; Lim, S.S.; Murray, C.J.; Mokdad, A.H. Prevalence of Physical Activity and Obesity in US Counties, 2001–2011: A Road Map for Action. Popul. Health Metr. 2013, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Bridged-Race Population Estimates 1990–2014; National Center for Health Statistics: Hyattsville, MD, USA, 2018.
- Liu, B.; Widener, M.; Burgoine, T.; Hammond, D. Association between Time-weighted Activity Space-based Exposures to Fast Food Outlets and Fast Food Consumption among Young Adults in Urban Canada. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 1–13. [Google Scholar] [CrossRef]
- Gomez, S.L.; Shariff-Marco, S.; De Rouen, M.; Keegan, T.H.M.; Yen, I.H.; Mujahid, M.; Satariano, W.A.; Glaser, S.L. The Impact of Neighborhood Social and Built Environment Factors Across the Cancer Continuum: Current Research, Methodological Considerations, and Future Directions. Cancer 2015, 121, 2314–2330. [Google Scholar] [CrossRef] [PubMed]
- Bodor, J.N.; Rose, D.; Farley, A.T.; Swalm, C.; Scott, S.K. Neighbourhood Fruit and Vegetable Availability and Consumption: The Role of Small Food Stores in an Urban Environment. Public Health Nutr. 2008, 11, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Cheadle, A.; Psaty, B.M.; Curry, S.; Wagner, E.; Diehr, P.; Koepsell, T.; Kristal, A. Community-level Comparisons between the Grocery Store Environment and Individual Dietary Practices. Prev. Med. 1991, 20, 250–261. [Google Scholar] [CrossRef]
- Moore, L.V.; Roux, A.V.D.; Brines, S. Comparing Perception-Based and Geographic Information System (GIS)-Based Characterizations of the Local Food Environment. J. Hered. 2008, 85, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.; Richards, R. Food Store Access and Household Fruit and Vegetable Use among Participants in the US Food Stamp Program. Public Health Nutr. 2004, 7, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- McGuirt, J.T.; Pitts, S.B.J.; Gustafson, A. Association between Spatial Access to Food Outlets, Frequency of Grocery Shopping, and Objectively-Assessed and Self-Reported Fruit and Vegetable Consumption. Nutrients 2018, 10, 1974. [Google Scholar] [CrossRef]
- Erinosho, T.O.; Oh, A.; Moser, R.; Davis, K.; Nebeling, L.; Yaroch, A. Association Between Perceived Food Environment and Self-Efficacy for Fruit and Vegetable Consumption Among US Adults, 2007. Prev. Chronic Dis. 2011, 9, E10. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Lee, J.H.; Yu, G.-Y.; He, G.; Ali, S.R.; Holzer, R.G.; Österreicher, C.H.; Takahashi, H.; Karin, M. Dietary and Genetic Obesity Promote Liver Inflammation and Tumorigenesis by Enhancing IL-6 and TNF Expression. Cell 2010, 140, 197–208. [Google Scholar] [CrossRef]
- Bean, F.D.; Myers, G.C.; Angel, J.L.; Galle, O.R. Geographic Concentration, Migration, and Population Redistribution Among the Elderly. In Demography of Aging; Martin, L.G., Preston, S.H., Eds.; National Academies Press (US): Washington, DC, USA, 1994. [Google Scholar]
- Howden, L.M.; Meyer, J.A. Age and Sex Composition: 2010. In 2010 Census Briefs. 2011; United States Census Bureau: Suitland, MD, USA, 2015. [Google Scholar]
- Humes, K.R.; Jones, N.A.; Ramirez, R.R. Overview of Race and Hispanic Origin: 2010; US Department of Commerce, Economics and Statistics Administration: Washington, DC, USA, 2011. [Google Scholar]
- Le, A.; Judd, S.E.; Allison, D.B.; Oza-Frank, R.; Affuso, O.; Safford, M.M.; Howard, V.J.; Howard, G. The Geographic Distribution of Obesity in the US and the Potential Regional Differences in Misreporting of Obesity. Obesity 2013, 22, 300–306. [Google Scholar] [CrossRef]
- Barker, L.E.; Kirtland, K.A.; Gregg, E.W.; Geiss, L.S.; Thompson, T.J. Geographic Distribution of Diagnosed Diabetes in the U.S.: A Diabetes Belt. Am. J. Prev. Med. 2011, 40, 434–439. [Google Scholar] [CrossRef]
- Kerr, W.C. Categorizing US State Drinking Practices and Consumption Trends. Int. J. Environ. Res. Public Health 2010, 7, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.S.; Rosenthal, E.; Hall, E.W.; Barker, L.; Hofmeister, M.G.; Sullivan, P.S.; Dietz, P.; Mermin, J.; Ryerson, A.B. Prevalence of Hepatitis C Virus Infection in US States and the District of Columbia, 2013 to 2016. JAMA Netw. Open 2018, 1, e186371. [Google Scholar] [CrossRef] [PubMed]
- White, D.L.; Thrift, A.P.; Kanwal, F.; Davila, J.; El-Serag, H.B. Incidence of Hepatocellular Carcinoma in All 50 United States, From 2000 Through 2012. Gastroenterology 2017, 152, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Davila, A.J.; Petersena, N.J.; Nelson, A.H.; El-Serag, H.B. Geographic Variation within the United States in the Incidence of Hepatocellular Carcinoma. J. Clin. Epidemiol. 2003, 56, 487–493. [Google Scholar] [CrossRef]
- Coulton, C.J.; Korbin, J.; Chan, T.; Su, M. Mapping Residents’ Perceptions of Neighborhood Boundaries: A Methodological Note. Am. J. Community Psychol. 2001, 29, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Coulton, C.J.; Jennings, M.Z.; Chan, T. How Big is My Neighborhood? Individual and Contextual Effects on Perceptions of Neighborhood Scale. Am. J. Community Psychol. 2012, 51, 140–150. [Google Scholar] [CrossRef]
- Inagami, S.; Cohen, D.A.; Brown, A.F.; Asch, S.M. Body Mass Index, Neighborhood Fast Food and Restaurant Concentration, and Car Ownership. J. Hered. 2009, 86, 683–695. [Google Scholar] [CrossRef] [PubMed]
- VoPham, T.; Brooks, M.M.; Yuan, J.-M.; Talbott, E.O.; Ruddell, D.; Hart, J.E.; Chang, C.-C.H.; Weissfeld, J.L. Pesticide Exposure and Hepatocellular Carcinoma Risk: A Case-control Study Using a Geographic Information System (GIS) to Link SEER-Medicare and California Pesticide Data. Environ. Res. 2015, 143, 68–82. [Google Scholar] [CrossRef] [PubMed]
- VoPham, T.; Bertrand, K.A.; Hart, J.E.; Laden, F.; Brooks, M.M.; Yuan, J.-M.; Talbott, E.O.; Ruddell, D.; Chang, C.-C.H.; Weissfeld, J.L. Pesticide Exposure and Liver Cancer: A Review. Cancer Causes Control. 2017, 28, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Nicotera, N. Measuring Neighborhood: A Conundrum for Human Services Researchers and Practitioners. Am. J. Community Psychol. 2007, 40, 26–51. [Google Scholar] [CrossRef] [PubMed]
- Rainham, D.; McDowell, I.; Krewski, D.; Sawada, M. Conceptualizing the Healthscape: Contributions of Time Geography, Location Technologies and Spatial Ecology to Place and Health Research. Soc. Sci. Med. 2010, 70, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Salinas, J.J.; Abdelbary, B.; Klaas, K.; Tapia, B.; Sexton, K. Socioeconomic Context and the Food Landscape in Texas: Results from Hotspot Analysis and Border/Non-Border Comparison of Unhealthy Food Environments. Int. J. Environ. Res. Public Health 2014, 11, 5640–5650. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Howard, V.; Harrington, K.F.; Creger, T.; Judd, S.E.; Fontaine, K.R. Does Adherence to Mediterranean Diet Mediate the Association Between Food Environment and Obesity Among Non-Hispanic Black and White Older US Adults? A Path Analysis. Am. J. Health Promot. 2020, 34, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Alter, M.J. Epidemiology of Hepatitis C Virus Infection. World J. Gastroenterol. 2007, 13, 2436–2441. [Google Scholar] [CrossRef] [PubMed]
- Story, M.; Kaphingst, K.M.; Robinson-O’Brien, R.; Glanz, K. Creating Healthy Food and Eating Environments: Policy and Environmental Approaches. Annu. Rev. Public Health 2008, 29, 253–272. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).