Temporal Trends in Notification and Mortality of Tuberculosis in China, 2004–2019: A Joinpoint and Age–Period–Cohort Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Statistical Analysis
2.2.1. Joinpoint Regression Analysis
2.2.2. Age–Period–Cohort Model Analysis
3. Results
3.1. Joinpoint Regression Model Analysis
3.2. Description Analysis and Age–Period–Cohort Model Analysis
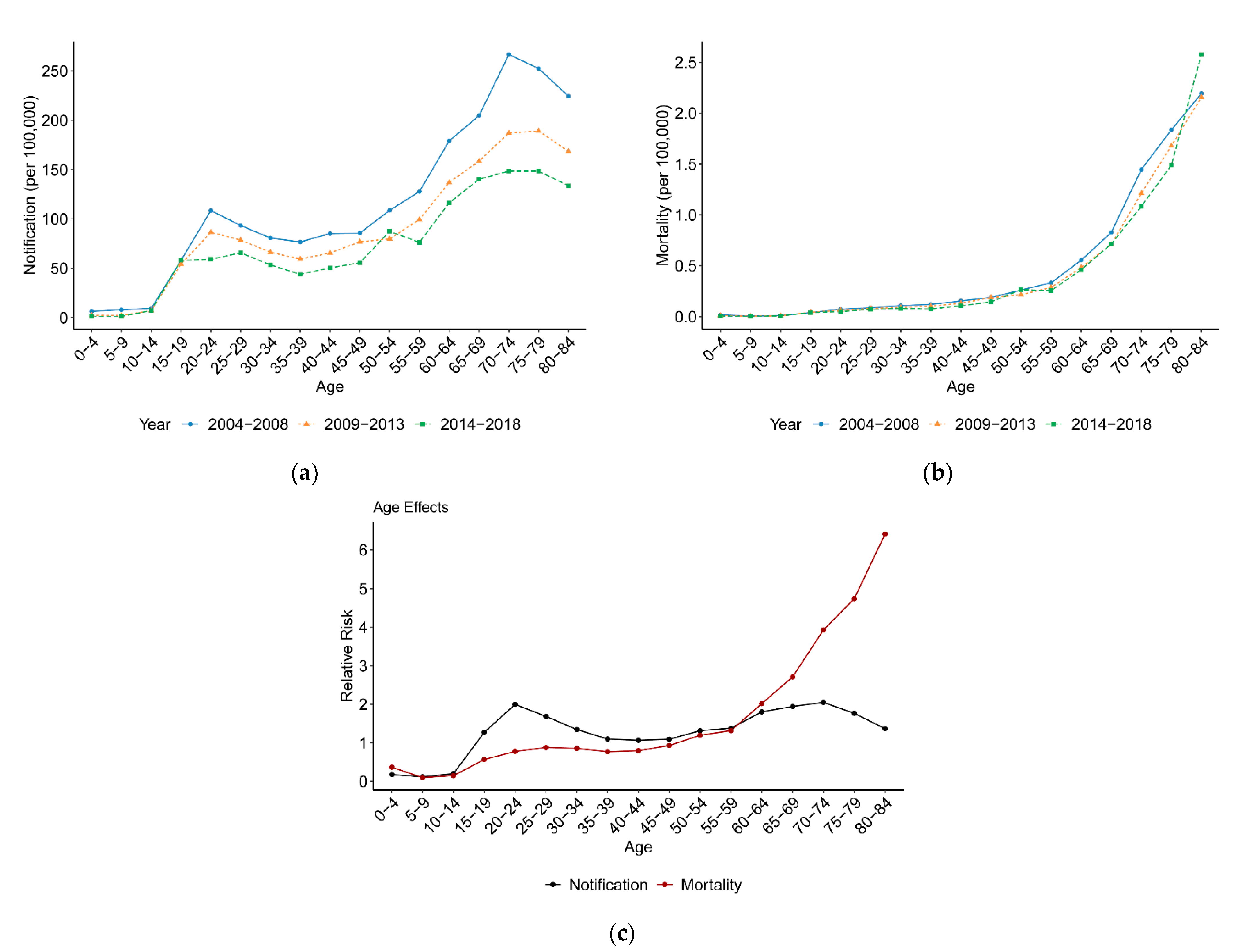
3.2.1. Variation with Age
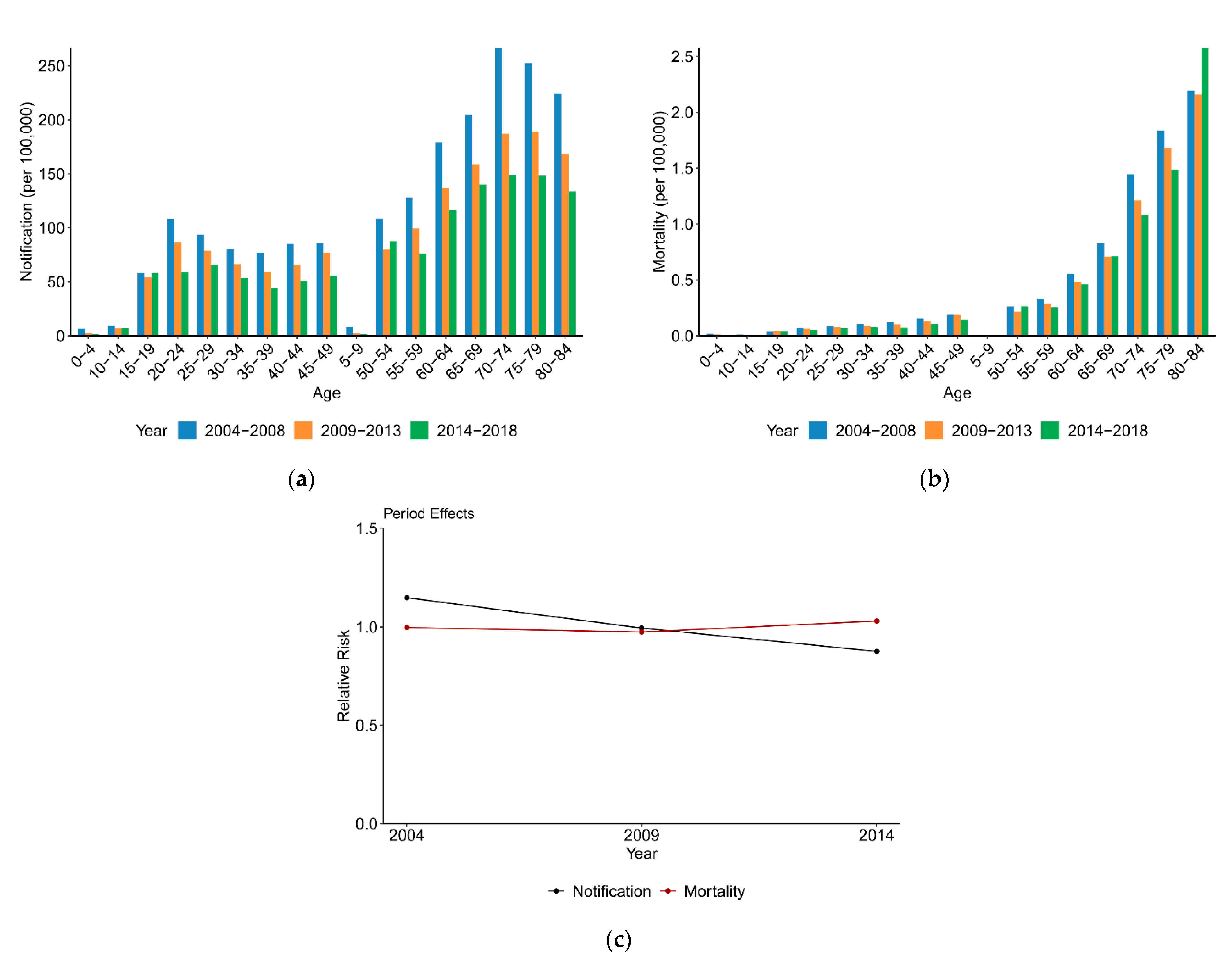
3.2.2. Variation with Period
3.2.3. Variation with Cohort
4. Discussion
4.1. Temporal Variation in Tuberculosis Incidence and Mortality
4.2. Age, Period and Cohort Effects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kolia-Diafouka, P.; Carrère-Kremer, S.; Lounnas, M.; Bourdin, A.; Kremer, L.; Van de Perre, P.; Godreuil, S.; Tuaillon, E. Detection of Mycobacterium tuberculosis in paucibacillary sputum: Performances of the Xpert MTB/RIF ultra compared to the Xpert MTB/RIF, and IS6110 PCR. Diagn. Microbiol. Infect. Dis. 2019, 94, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Pasa, F.; Golkov, V.; Pfeiffer, F.; Cremers, D.; Pfeiffer, D. Efficient Deep Network Architectures for Fast Chest X-Ray Tuberculosis Screening and Visualization. Sci. Rep. 2019, 9, 6268. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wu, J.; Ding, C.; Cui, Y.; Zhou, Y.; Li, Y.; Deng, M.; Wang, C.; Xu, K.; Ren, J.; et al. Epidemiological features of and changes in incidence of infectious diseases in China in the first decade after the SARS outbreak: An observational trend study. Lancet Infect. Dis. 2017, 17, 716–725. [Google Scholar] [CrossRef]
- Harding, E. WHO global progress report on tuberculosis elimination. Lancet Respir. Med. 2020, 8, 19. [Google Scholar] [CrossRef]
- Zuo, Z.; Wang, M.; Cui, H.; Wang, Y.; Wu, J.; Qi, J.; Pan, K.; Sui, D.; Liu, P.; Xu, A. Spatiotemporal characteristics and the epidemiology of tuberculosis in China from 2004 to 2017 by the nationwide surveillance system. BMC Public Health 2020, 20, 1284. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Dou, X.; Yan, C.; Wan, L.; Liu, H.; Li, M.; Wang, R.; Li, G.; Zhao, L.; Liu, Z.; et al. Epidemiological characteristics and trends of notifiable infectious diseases in China from 1986 to 2016. J. Glob. Health 2020, 10, 020803. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wang, Y.; Li, T.; Chen, W.; Wang, W. Gap to End-TB targets in eastern China: A joinpoint analysis from population-based notification data in Zhejiang Province, China, 2005-2018. Int. J. Infect. Dis. 2021, 104, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Meira, K.C.; Silva, G.; Dos Santos, J.; Guimarães, R.M.; de Souza, D.L.B.; Ribeiro, G.P.C.; Dantas, E.S.O.; Carvalho, J.B.L.; Jomar, R.T.; Simões, T.C. Analysis of the effects of the age-period-birth cohort on cervical cancer mortality in the Brazilian Northeast. PLoS ONE 2020, 15, e0226258. [Google Scholar] [CrossRef]
- Li, Z.; Wang, P.; Gao, G.; Xu, C.; Chen, X. Age-period-cohort analysis of infectious disease mortality in urban-rural China, 1990–2010. Int. J. Equity Health 2016, 15, 55. [Google Scholar] [CrossRef]
- Ding, C.; Wang, S.; Shangguan, Y.; Feng, X.; Guo, W.; Shi, P.; Ji, Z.; Xu, K. Epidemic Trends of Tuberculosis in China from 1990 to 2017: Evidence from the Global Burden of Disease Study. Infect. Drug Resist. 2020, 13, 1663–1672. [Google Scholar] [CrossRef]
- Wen, H.; Xie, C.; Wang, L.; Wang, F.; Wang, Y.; Liu, X.; Yu, C. Difference in Long-Term Trends in COPD Mortality between China and the U.S., 1992–2017: An Age⁻Period⁻Cohort Analysis. Int. J. Environ. Res. Public Health 2019, 16, 1529. [Google Scholar] [CrossRef]
- Iqbal, S.A.; Winston, C.A.; Bardenheier, B.H.; Armstrong, L.R.; Navin, T.R. Age-Period-Cohort Analyses of Tuberculosis Incidence Rates by Nativity, United States, 1996–2016. Am. J. Public Health 2018, 108, S315–S320. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Cowling, B.J.; Schooling, C.M.; Wong, I.O.; Johnston, J.M.; Leung, C.C.; Tam, C.M.; Leung, G.M. Age-period-cohort analysis of tuberculosis notifications in Hong Kong from 1961 to 2005. Thorax 2008, 63, 312–316. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, L.T.; Chen, C.J.; Lee, W.C.; Luh, K.T.; Hsieh, W.C.; Lin, R.S. Age-period-cohort analysis of pulmonary tuberculosis mortality in Taiwan: 1961 to 1990. J. Formos. Med. Assoc. 1994, 93, 657–662. [Google Scholar] [PubMed]
- Yun, J.W.; Son, M. Forecasting Cause-Specific Mortality in Korea up to Year 2032. J. Korean Med. Sci. 2016, 31, 1181–1189. [Google Scholar] [CrossRef]
- Cui, Y.; Shen, H.; Wang, F.; Wen, H.; Zeng, Z.; Wang, Y.; Yu, C. A Long-Term Trend Study of Tuberculosis Incidence in China, India and United States 1992–2017: A Joinpoint and Age-Period-Cohort Analysis. Int. J. Environ. Res. Public Health 2020, 17, 3334. [Google Scholar] [CrossRef]
- Llanes-Álvarez, C.; Llano, J.M.A.; Álvarez-Navares, A.I.; Roncero, C.; Pastor-Hidalgo, M.T.; Garmendia-Leiza, J.R.; Andrés-Alberola, I.; Franco-Martín, M.A. Hospitalization and Socio-Health Care for Dementia in Spain. J. Clin. Med. 2020, 9, 3875. [Google Scholar] [CrossRef]
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef]
- Goovaerts, P.; Xiao, H. Geographical, temporal and racial disparities in late-stage prostate cancer incidence across Florida: A multiscale joinpoint regression analysis. Int. J. Health Geogr. 2011, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Hagiya, H.; Koyama, T.; Zamami, Y.; Minato, Y.; Tatebe, Y.; Mikami, N.; Teratani, Y.; Ohshima, A.; Shinomiya, K.; Kitamura, Y.; et al. Trends in incidence and mortality of tuberculosis in Japan: A population-based study, 1997–2016. Epidemiol. Infect. 2018, 147. [Google Scholar] [CrossRef]
- Mason, K.O.; Mason, W.M.; Winsborough, H.H.; Poole, W.K. Some methodological issues in cohort analysis of archival data. Am. Sociol. Rev. 1973, 38, 242–258. [Google Scholar] [CrossRef]
- Yang, Y.; Schulhofer-Wohl, S.; Fu, W.J.; Land, K.C. The intrinsic estimator for age-period-cohort analysis: What it is and how to use it. Am. J. Sociol. 2008, 113, 1697–1736. [Google Scholar] [CrossRef]
- Luo, L. Assessing validity and application scope of the intrinsic estimator approach to the age-period-cohort problem. Demography 2013, 50, 1945–1967. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Zhang, F.; Sun, Y. Urban and rural stroke mortality rates in China between 1988 and 2013: An age-period-cohort analysis. J. Int. Med. Res. 2017, 45, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, C.; Bi, Y.; Zhang, Z.J. Trends and age-period-cohort effect on incidence and mortality of prostate cancer from 1990 to 2017 in China. Public Health 2019, 172, 70–80. [Google Scholar] [CrossRef]
- Wang, L.; Yu, C.; Liu, Y.; Wang, J.; Li, C.; Wang, Q.; Wang, P.; Wu, S.; Zhang, Z.J. Lung Cancer Mortality Trends in China from 1988 to 2013: New Challenges and Opportunities for the Government. Int. J. Environ. Res. Public Health 2016, 13, 1052. [Google Scholar] [CrossRef]
- Tzeng, I.S.; Chen, K.H.; Lee, Y.L.; Yang, W.S. Trends and Age-Period-Cohort Effects of Fertility Rate: Analysis of 26,224 Married Women in Taiwan. Int. J. Environ. Res. Public Health 2019, 16, 4952. [Google Scholar] [CrossRef]
- Li, T.; Du, X.; Chen, W.; Huang, F.; Zhao, Y.L.; Zhang, H. Review and prospect of information monitoring and control of tuberculosis management in China. Chin. J. Antituberc. 2020, 42, 657–661. (In Chinese) [Google Scholar]
- Schwartz, N.G.; Price, S.F.; Pratt, R.H.; Langer, A.J. Tuberculosis-United States, 2019. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 286–289. [Google Scholar] [CrossRef]
- Kirenga, B.J.; Ssengooba, W.; Muwonge, C.; Nakiyingi, L.; Kyaligonza, S.; Kasozi, S.; Mugabe, F.; Boeree, M.; Joloba, M.; Okwera, A. Tuberculosis risk factors among tuberculosis patients in Kampala, Uganda: Implications for tuberculosis control. BMC Public Health 2015, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Narita, M.; Sullivan Meissner, J.; Burzynski, J. Use of Modeling to Inform Tuberculosis Elimination Strategies. Am. J. Respir. Crit. Care Med. 2020, 201, 272–274. [Google Scholar] [CrossRef]
- Tuberculosis Guidelines (ICN). Available online: https://www.icn.ch/sites/default/files/inline-files/tb_mdrtb_guideline.pdf (accessed on 21 May 2021).
- Wang, F.; Mubarik, S.; Zhang, Y.; Wang, L.; Wang, Y.; Yu, C.; Li, H. Long-Term Trends of Liver Cancer Incidence and Mortality in China 1990-2017: A Joinpoint and Age-Period-Cohort Analysis. Int. J. Environ. Res. Public Health 2019, 16, 2878. [Google Scholar] [CrossRef] [PubMed]
- Negin, J.; Abimbola, S.; Marais, B.J. Tuberculosis among older adults--time to take notice. Int. J. Infect. Dis. 2015, 32, 135–137. [Google Scholar] [CrossRef]
- Fletcher, J.M. The effects of in utero exposure to the 1918 influenza pandemic on family formation. Econ. Hum. Biol. 2018, 30, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.R.; Utz, R.L.; Keyes, K.M.; Martin, C.L.; Yang, Y. Birth cohort effects on abdominal obesity in the United States: The Silent Generation, Baby Boomers and Generation X. Int. J. Obes. 2013, 37, 1129–1134. [Google Scholar] [CrossRef] [PubMed]


| Age Group (Year) | Notification | Mortality | ||
|---|---|---|---|---|
| AAPC (%) | 95%CI | AAPC (%) | 95%CI | |
| 0–4 | −14.7 * | (−16.5, −12.8) | −13.6 * | (−18.6, −8.4) |
| 5–9 | −12.3 * | (−16.6, −7.7) | −11.5 * | (−16.2, −6.6) |
| 10–14 | −4.5 * | (−6.1, −2.9) | −5.0 * | (−9.2, −0.6) |
| 15–19 | −2.2 * | (−3.6, 0.8) | −0.8 | (−3.6, 2.0) |
| 20–24 | −4.8 * | (−6.6, 3.0) | −7.2 * | (−9.1, −5.2) |
| 25–29 | −1.9 * | (−3.4, −0.3) | −4.1 * | (−6.4, −1.8) |
| 30–34 | −4.9 * | (−7.0, −2.7) | −6.0 * | (−8.7, −3.2) |
| 35–39 | −5.2 * | (−6.1, −4.3) | −8.1 * | (−10.4, −5.9) |
| 40–44 | −3.1 * | (−4.3, −1.9) | −6.7 * | (−8.6, −4.7) |
| 45–49 | −0.3 | (−1.9, −1.5) | −6.8 * | (−9.2, −4.3) |
| 50–54 | −2.0 * | (−3.4, −0.6) | −2.3 | (−4.9, 0.3) |
| 55–59 | −1.3 | (−3.4, 0.8) | −5.8 * | (−7.6, −4.0) |
| 60–64 | −4.3 * | (−5.2, −3.4) | −5.5 * | (−8.3, −2.5) |
| 65–69 | −1.2 | (−2.4, 0.1) | −5.4 * | (−7.7, −3.0) |
| 70–74 | −3.4 * | (−4.9, −2.0) | −6.7 * | (−8.6, −4.7) |
| 75–79 | −4.9 * | (−6.0, −3.8) | −6.2 * | (−8.3, −4.1) |
| 80–84 | 2.1 | (−1.3, 5.6) | −5.5 * | (−8.2, −2.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Wang, W. Temporal Trends in Notification and Mortality of Tuberculosis in China, 2004–2019: A Joinpoint and Age–Period–Cohort Analysis. Int. J. Environ. Res. Public Health 2021, 18, 5607. https://doi.org/10.3390/ijerph18115607
Wang L, Wang W. Temporal Trends in Notification and Mortality of Tuberculosis in China, 2004–2019: A Joinpoint and Age–Period–Cohort Analysis. International Journal of Environmental Research and Public Health. 2021; 18(11):5607. https://doi.org/10.3390/ijerph18115607
Chicago/Turabian StyleWang, Luqi, and Weibing Wang. 2021. "Temporal Trends in Notification and Mortality of Tuberculosis in China, 2004–2019: A Joinpoint and Age–Period–Cohort Analysis" International Journal of Environmental Research and Public Health 18, no. 11: 5607. https://doi.org/10.3390/ijerph18115607
APA StyleWang, L., & Wang, W. (2021). Temporal Trends in Notification and Mortality of Tuberculosis in China, 2004–2019: A Joinpoint and Age–Period–Cohort Analysis. International Journal of Environmental Research and Public Health, 18(11), 5607. https://doi.org/10.3390/ijerph18115607







