Utilisation of a Suite of Screening Tools to Determine Adverse Healthcare Outcomes in an Older Frail Population Admitted to a Community Virtual Ward
Abstract
1. Introduction
2. Methods and Materials
2.1. Study Design
2.2. Sample
2.3. Ethics Approval
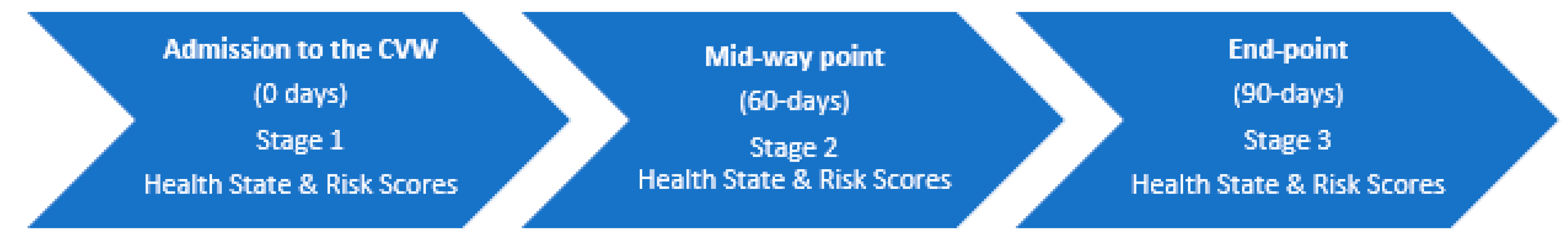
2.4. Data Collection and Outcomes
2.5. Measures
2.6. Data Analysis
3. Results
3.1. Relationship between Instruments and Clinical Stability or Decline
3.1.1. Correlations
3.1.2. Associations
3.2. Relationship between Instrument Scores on Admission and Risk of Institutionalisation, Hospitalisation and Death
3.2.1. Institutionalisation
3.2.2. Hospitalisation
3.2.3. Death
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- WHO. World Report on Ageing and Health; WHO: Geneva, Switzerland, 2015; pp. 1–260. [Google Scholar]
- O’Caoimh, R.; Gao, Y.; Svendrovski, A.; Healy, E.; O’Connell, E.; O’Keeffe, G.; Cronin, U.; O’Herlihy, E.; Cornally, N.; Molloy, W.D. Screening for Markers of Frailty and Perceived Risk of Adverse Outcomes Using the Risk Instrument for Screening in the Community (RISC). BMC Geriatr. 2014, 14, 104. [Google Scholar] [CrossRef] [PubMed]
- Violan, C.; Foguet-Boreu, Q.; Flores-Mateo, G.; Salisbury, C.; Blom, J.; Freitag, M.; Glynn, L.; Muth, C.; Valderas, J.M. Prevalence, determinants and patterns of multimorbidity in primary care: A systematic review of observational studies. PLoS ONE 2014, 9, e102149. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.Y.; Skirbekk, V.F.; Tyrovolas, S.; Kassebaum, N.J.; Dieleman, J.L. Measuring population ageing: An analysis of the global burden of disease study. Lancet Public Health 2017, 4, e159–e167. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Sezgin, D.; O’Donovan, M.R.; Molloy, D.W.; Clegg, A.; Rockwood, K.; Liew, A. Prevalence of frailty in 62 countries across the world: A systematic review and meta-analysis of population-level studies. Age Ageing 2020. [Google Scholar] [CrossRef]
- Lewis, C.; Moore, Z.; Doyle, F.; Martin, A.; Patton, D.; Nugent, L.E. A community virtual ward model to support older persons with complex health care and social care needs. Clin. Interv. Aging 2017, 12, 985–993. [Google Scholar] [CrossRef]
- Lewis, C.; O’Caoimh, R.; Patton, D.; O’Connor, T.; Moore, Z.; Nugent, L.E. Risk Prediction for Adverse Outcomes for Frail Older Persons with Complex Healthcare and Social Care Needs Admitted to a Community Virtual Ward Model. Clin. Interv. Aging 2020, 15, 915–926. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Cornally, N.; Weathers, E.; O’Sullivan, R.; Fitzgerald, C.; Orfila, F.; Clarnette, R.; Paúl, C.; Molloy, D.W. Risk prediction in the community: A systematic review of case-finding instruments that predict adverse healthcare outcomes in community-dwelling older adults. Maturitas 2015, 82, 3–21. [Google Scholar] [CrossRef]
- WHO. Framework on Integrated, People Centred Health; Sixty Ninth World Health Assembly; World Health Organisation: Geneva, Switzerland, 2016; Volume 16, pp. 1–32. [Google Scholar]
- You, E.C.; Dunt, D.; Doyle, C.; Hsueh, A. Effects of case management in community aged care on client and carer outcomes: A systematic review of randomized trials and comparative observational studies. BMC Health Serv. Res. 2012, 12, 395. [Google Scholar] [CrossRef]
- Halm, E.A.; Fine, M.J.; Marrie, T.J.; Coley, C.M.; Kapoor, W.N.; Obrosky, D.S.; Singer, D.E. Time to clinical stability in patients hospitalized with community-acquired pneumonia: Implications for practice guidelines. JAMA 1998, 279, 1452–1457. [Google Scholar] [CrossRef]
- Aliberti, S.; Zanaboni, A.M.; Wiemken, T.; Nahas, A.; Uppatla, S.; Morlacchi, L.C.; Peyrani, P.; Blasi, F.; Ramirez, J. Criteria for clinical stability in hospitalised patients with community-acquired pneumonia. Eur. Respir. J. 2013, 42, 742. [Google Scholar] [CrossRef]
- Poulos, C.J.; Magee, C.; Bashford, G.; Eagar, K. Determining level of care appropriateness in the patient journey from acute care to rehabilitation. BMC Health Serv. Res. 2011, 11, 291. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.H.; Georghiou, T.; Steventon, A. Impact of Virtual Wards on Hospital Use: A Research Study Using Propensity Matched Controls and a Cost Analysis; National Institute for Health Research Service Delivery and Organisation Programme: London, UK, 2013; pp. 1–157. [Google Scholar]
- Lewis, G.; Curry, N.; Bardsley, M. Choosing a predictive riks model: A guide for commissioners in England. Nuffield Trust 2011, 20, 1–18. [Google Scholar]
- Dhalla, I.A.; O’Brien, T.; Moora, D.; Thorpe, K.E.; Wong, B.M.; Mehta, R.M.; Frost, D.W.; Abrams, H.; Ko, F.; Van Rooyen, P.; et al. Effect of Postdischarge Virtual Ward on Readmission or Death for High-Risk Patients: A randomized clinical trail. J. Am. Med. Assoc. 2014, 312, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.P.; Lee, D.T.F.; Lee, I.F.K.; Lam, L.W.; Lee, S.W.Y.; Chan, M.W.M.; Lam, Y.M.; Leung, S.H.; Chiu, P.C.; Ho, N.K.F.; et al. The effect of a virtual ward program on emergency services utilization and quality of life in frail elderly patients after discharge: A pilot study. Clin. Interv. Aging 2015, 10, 413–420. [Google Scholar] [CrossRef] [PubMed]
- HSE. National Consent Policy, Health Service Executive Quality and Patient Safety Directorate; HSE: Dublin, Ireland, 2013. [Google Scholar]
- Kahlon, S.; Pederson, J.; Majumdar, S.R.; Belga, S.; Lau, D.; Fradette, M.; Boyko, D.; Bakal, J.A.; Johnston, C.; Padwal, R.S.; et al. Association between frailty and 30-day outcomes after discharge from hospital. CMAJ 2015, 187, 799–804. [Google Scholar] [CrossRef]
- Mitnitski, A.; Collerton, J.; Martin-Ruiz, C.; Jagger, C.; von Zglinicki, T.; Rockwood, K.; Kirkwood, T.B. Age-related frailty and its association with biological markers of ageing. BMC Med. 2015, 13, 161. [Google Scholar] [CrossRef]
- Rockwood, K.; Howlett, S.E.; Macknight, C.; Beattie, B.L.; Bergman, H.; Hebert, R.; Hogan, D.B.; Wolfson, C.; McDowell, I. Frailty in community-dwelling older adults: Report from the Canadian study of health and aging. J. Gerontol. Biol. Sci. Med. Sci. 2004, 59, 1310–1317. [Google Scholar] [CrossRef]
- Barry, E.; Galvin, R.; Keogh, C.; Horgan, F.; Fahey, T. Is the Timed Up and Go test a useful predictor of risk of falls in community dwelling older adults: A systematic review and meta- analysis. BMC Geriatr. 2014, 14, 14. [Google Scholar] [CrossRef]
- Gabbe, B.J.; Sutherland, A.M.; Wolfe, R.; Williamson, O.D.; Cameron, P.A. Can the modified functional independence measure be reliably obtained from the patient medical record by different raters? J. Trauma 2007, 63, 1374–1379. [Google Scholar] [CrossRef]
- Chumney, D.; Nollinger, K.; Shesko, K.; Skop, K.; Spencer, M.; Newton, R.A. Ability of Functional Independence Measure to accurately predict functional outcome of stroke-specific population: Systematic review. J. Rehabil. Res. Dev. 2010, 47, 17–29. [Google Scholar] [CrossRef]
- Quinn, T.J.; Langhorne, P.; Stott, D.J. Barthel index for stroke trials: Development, properties, and application. Stroke 2011, 42, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Chaloner, D.M.; Franks, P.J. Validity of the Walsall Community Pressure Sore Risk Calculator. Br. J. Community Nurs. 2000, 5, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Cawood, A.; Elia, M.; Sharp, S.; Stratton, R. Malnutrition Self-Screening by Using MUST in Hospital Outpatients: Validity, Reliability, and Ease of Use. Am. J. Clin. Nutr. 2012, 96, 1000–1007. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mountford, C.G.; Okonkwo, A.C.O.; Hart, K.; Thompson, N.P. Managing Malnutrition in Older Persons Residing in Care Homes: Nutritional and Clinical Outcomes Following a Screening and Intervention Program. J. Nutr. Gerontol. Geriatr. 2016, 35, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Jacqmin-Gadda, H.; Fabrigoule, C.; Commenges, D.; Dartigues, J.F. A 5-Year Longitudinal Study of the Mini-Mental State Examination in Normal Aging. Am. J. Epidemiol. 1997, 146, 498–506. [Google Scholar] [CrossRef]
- Korner, A.; Lauritzen, L.; Abelskov, K.; Gulman, N.; Brodersen, A.M.; Wedervang-Jensen, T.; Kjeldgaard, K.M. The Geriatric Depression Scale and the Cornell Scale for Depression in Dementia. A validity study. Nord J. Psychiatry 2006, 60, 360–364. [Google Scholar] [CrossRef]
- McCusker, J.; Bellavance, F.; Cardin, S.; Trépanier, S.; Verdon, J.; Ardman, O.D. Detection of older people at increased risk of adverse health outcomes after an emergency visit: The ISAR screening tool. Am. Geriatr. Soc. 1999, 47, 1229–1237. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using IBM SPSS, 4th ed.; Sage Publications: London, UK, 2013. [Google Scholar]
- Counsell, S.R.; Callahan, C.M.; Buttar, A.B.; Clark, D.O.; Frank, K.I. Geriatric Resources for Assessment and Care of Elders (GRACE): A new model of primary care for low-income seniors. J. Am. Geriatr. Soc. 2006, 54, 1136–1141. [Google Scholar] [CrossRef]
- Hinman, R.S.; Allen, K.D.; Bennell, K.L.; Berenbaum, F.; Betteridge, N.; Briggs, A.M.; Campbell, P.K.; Dahlberg, L.E.; Dziedzic, K.S.; Eyles, J.P.; et al. Development of a core capability framework for qualified health professionals to optimise care for people with osteoarthritis: An OARSI initiative. Osteoarthr. Cartil. 2020, 28, 154–166. [Google Scholar] [CrossRef]
- Donoghue, O.A.; Horgan, N.F.; Savva, G.M.; Cronin, H.; O’Regan, C.; Kenny, R.A. Association between timed up-and-go and memory, executive function, and processing speed. J. Am. Geriatr. Soc. 2012, 60, 1681–1686. [Google Scholar] [CrossRef]
- Boulos, C.; Salameh, P.; Barberger-Gateau, P. Malnutrition and frailty in community dwelling older adults living in a rural setting. Clin. Nutr. (Edinb. Scotl.) 2016, 35, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Edmans, J.; Bradshaw, L.; Gladman, J.R.F.; Franklin, M.; Berdunov, V.; Elliott, R.; Conroy, S.P. The Identification of Seniors at Risk (ISAR) score to predict clinical outcomes and health service costs in older people discharged from UK acute medical units. Age Ageing 2013, 42, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Marengoni, A.; Nobili, A.; Romano, V.; Tettamanti, M.; Pasina, L.; Djade, S.; Corrao, S.; Salerno, F.; Iorio, A.; Marcucci, M.; et al. Adverse Clinical Events and Mortality During Hospitalization and 3 Months After Discharge in Cognitively Impaired Elderly Patients. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Hendry, A.; Vanhecke, E.; Samaniego, L.L.; Espinosa, J.M.; O’Caoimh, R.; Liew, A.; Hannar, T.; Ferry, P.; Vella, A.; Bacairoa, A.O.; et al. Models of care for frailty: A systematic review. Advant. Eur. Union Health Programme 2017, 2014–2020, 1–21. [Google Scholar]
- Baker, M.B.; Oliver, D.; Burns, E.; Paynton, D.; Bullard, E.; Cooke, C. Integrated care for older people with frailty: Innovative approaches in practice. In British Geriatrics Society. Improving Healthcare for Older People, Royal College of General Practitioners, London; Royal College of General Practitioners & British Geriatric Society: London, UK, 2016; pp. 1–37. [Google Scholar]
- Viccaro, L.J.; Perera, S.; Studenski, S.A. Is Timed Up and Go better than gait speed in predicting health, function, and falls in older adults? J. Am. Geriatr. Soc. 2011, 59. [Google Scholar] [CrossRef]
- Elia, M.; Russell, C.A.; Stratton, R.; Todorovic, V.; Evans, L.; Farrer, K. The MUST Explanatory Booklet: A Guide to the Malnutrition Universal Screening Tool (MUST) for Adults; British Dietetic Association: Birmingham, UK, 2011; pp. 1–23. [Google Scholar]
- Frank, M.; Sivagnanaratnam, A.; Bernstein, J. Nutritional assessment in elderly care: A MUST! BMJ Qual. Improv. Rep. 2015, 4, u204810–w2031. [Google Scholar] [CrossRef]
- Martins, A.C.; Moreira, J.; Silva, C.; Silva, J.; Tonelo, C.; Baltazar, D.; Rocha, C.; Pereira, T.; Sousa, I. Multifactorial Screening Tool for Determining Fall Risk in Community-Dwelling Adults Aged 50 Years or Over (FallSensing): Protocol for a Prospective Study. JMIR Res. Protoc. 2018, 7, e10304. [Google Scholar] [CrossRef]
- Kelly, S.; O’Brien, I.; Smuts, K.; O’Sullivan, M.; Warters, A. Prevalence of frailty among community dwelling older adults in receipt of low level home support: A cross-sectional analysis of the North Dublin Cohort. BMC Geriatr. 2017, 17, 121. [Google Scholar] [CrossRef]
- Counsell, S.R.; Callahan, C.M.; Tu, W.; Stump, T.E.; Arling, G.W. Cost analysis of the Geriatric Resources for Assessment and Care of Elders care management intervention. J. Am. Geriatr. Soc. 2009, 57, 1420–1426. [Google Scholar] [CrossRef]
- Clegg, A.; Bates, C.; Young, J.; Ryan, R.; Nichols, L.; Ann Teale, E.; Mohammed, M.A.; Parry, J.; Marshall, T. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing 2016, 45, 353–360. [Google Scholar] [CrossRef]
- HSE. National Service Plan 2021. Health Serv. Exec. Available online: https://www.hse.ie/eng/services/publications/serviceplans/national-service-plan-2021.pdf (accessed on 28 March 2021).
- Liddy, C.; Drosinis, P.; Joschko, J.; Keely, E. Improving Access to Specialist Care for an Aging Population. Gerontol. Geriatr. Med. 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Cooper, R.; Shardell, M.; Simonsick, E.M.; Schrack, J.A.; Kuh, D. Age-Related Change in Mobility: Perspectives From Life Course Epidemiology and Geroscience. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- O’Caoimh, R.; Galluzzo, L.; Rodriguez-Laso, A.; Van der Heyden, J.; Ranhoff, A.H.; Carcaillon-Bentata, L.; Beltzer, N.; Kennelly, S.; Liew, A. Transitions and trajectories in frailty states overtime: A systematic review of the Advantage Joint Action. Advant. Ann. dell’Istituto Super. Sanità 2018, 54, 137–143. [Google Scholar] [CrossRef]
| Stable | Ability to eat and drink returned (if previously diminished) Mental status considered normal or back to previous status if recently changed Functionally returning to their usual activities of daily living either independently or with support Improvements in emotional/psychological state or no evidence of deterioration No subsequent events in the last 30 days. |
| Unstable | Reduced or inadequate oral and/or nutritional intake Gradual cognitive decline or change in mental state Functionally unable to undertake their normal activities of daily living Social care needs exceeding supports within the home Secondary event resulting in above |
| Deteriorating | Increase in events and episodes (set of services provided to treat a clinical condition or procedure) Decrease in function and mobility (activities of daily living) Deterioration in mental status Further weight loss despite interventions |
| Admission to CVW | CVW Selection |
|---|---|
Diagnosis of frailty with evidence of at least one of the following:
| Red CVW Event(s) occurred in the last 30 days or the patient was discharged from hospital in the last 30 days |
| Amber CVW Event(s) occurred >30 days with evidence of more gradual decline in the last 3 months | |
| Green CVW Admission to the green VW can only occur following a period of monitoring either in the Red or Amber CVW. Admission to this ward is part of enhanced discharge planning including members of the primary care team and/or Specialist Geriatric Services. |
| Variable | Number (%)/ Mean and Standard Deviation (SD) |
|---|---|
| Demographics Age (years) | 82.83 (SD 6.406) |
| Sex | |
| Female | 58 (65.9) |
| Male | 30 (34.1) |
| Living Alone | |
| Yes | 33 (37.5) |
| No | 55 (62.5) |
| Co-morbidity Number of co-morbidities | 2.82 (SD 1.034) |
| Number of medications | 8.24 (SD 3.655) |
| Number of falls (last 3 months) | |
| No Falls | 37 (42) |
| 1 Fall | 20 (22.7) |
| 2 or More | 31 (35.2) |
| Incontinence | |
| Yes | 64 (72.7) |
| No | 24 (27.3) |
| Unscheduled Healthcare Utilisation Unplanned admissions (3 months prior to CVW admission) | |
| 1 hospital admission | 36 (40.9) |
| 2 or more hospital admissions | 21 (23.9) |
| Emergency Department Presentations (last 3 months) | |
| 1 ED presentation | 36 (40.9) |
| 2 or more ED presentations | 31 (35.2) |
| Signs of Self-Neglect | |
| Yes | 44 (51.1) |
| No | 42 (48.9) |
| Risk-Screening Tool | Cut-Off Scores |
|---|---|
| Rockwood Clinical Frailty Scale | 3 |
| Timed up and Go Test | >13 s |
| Modified Functional Independence Measure | >1 |
| Modified Barthel Index | 16 |
| Walsall Pressure Ulcer Risk Tool | >3 |
| Malnutritional Universal Screening Tool | 0 |
| Mini Mental State Examination | 25 |
| Geriatric Depression Scale | 4 |
| Identification of Seniors at Risk tool | ≥2 |
| Risk Scores | Correlation (r) | p Value |
|---|---|---|
| 60 days | ||
| Rockwood CFS | 0.57 | <0.001 *** |
| Walsall | 0.59 | <0.001 *** |
| Mobility (FIM) | 0.56 | <0.001 *** |
| MUST | 0.22 | 0.039 * |
| TUG | 0.15 | 0.154 |
| ISAR | 0.44 | <0.001 *** |
| MMSE | 0.26 | 0.015 * |
| Barthel | 0.54 | <0.001 *** |
| GDS | 0.09 | 0.431 |
| 90 days | ||
| Rockwood CFS | 0.44 | <0.001 *** |
| Walsall | 0.68 | <0.001 *** |
| Mobility (FIM) | 0.58 | <0.001 *** |
| MUST | 0.32 | 0.002 ** |
| TUG | 0.09 | 0.393 |
| ISAR | 0.45 | <0.001 *** |
| MMSE | 0.46 | <0.001 *** |
| Barthel | 0.60 | <0.001 *** |
| GDS | 0.01 | 0.947 |
| Risk Scores | Odds Ratio | Lower 95% CI | Upper 95% CI | p Value |
|---|---|---|---|---|
| 60 days | ||||
| Rockwood CFS | 1.77 | 0.79 | 22 | 0.960 |
| Walsall | 4.92 ^ | 2.48 | 9.74 | <0.001 *** |
| Mobility (FIM) | 2.97 ^ | 1.81 | 4.86 | <0.001 *** |
| MUST | 1.73 | 1.01 | 2.98 | 0.049 * |
| TUG | 1.09 | 0.74 | 1.62 | 0.669 |
| ISAR | 3.25 ^ | 1.84 | 5.74 | <0.001 *** |
| MMSE | 2.08 | 1.11 | 3.92 | 0.02 * |
| Barthel | 6.41 ^ | 2.77 | 14.8 | <0.001 *** |
| GDS | 1.40 | 0.83 | 2.38 | 0.213 |
| 90 days | ||||
| Rockwood CFS | 3.29 ^ | 1.55 | 6.99 | 0.002 ** |
| Walsall | 8.86 ^ | 3.48 | 22.5 | <0.001 *** |
| Mobility (FIM) | 3.08 ^ | 1.89 | 5.03 | <0.001 *** |
| MUST | 2.33 ^ | 1.24 | 4.35 | 0.008 ** |
| TUG | 1.03 ^ | 0.78 | 1.35 | 0.849 |
| ISAR | 3.07 ^ | 1.75 | 5.40 | <0.001 *** |
| MMSE | 4.23 ^ | 1.98 | 9.07 | <0.001 *** |
| Barthel | 7.73 ^ | 3.20 | 18.6 | <0.001 *** |
| GDS | 1.08 ^ | 0.62 | 1.91 | 0.778 |
| Baseline Risk Scores | OR | 95 CI Lower | 95 CI Upper | p Value |
|---|---|---|---|---|
| Institutionalisation | ||||
| Rockwood CFS | 2.29 | 1.02 | 5.16 | 0.045 * |
| Walsall | 2.00 | 1.20 | 3.33 | 0.008 ** |
| Mobility (FIM) | 1.19 | 0.98 | 1.43 | 0.080 |
| MUST | 0.84 | 0.48 | 1.46 | 0.530 |
| TUG | 1.27 | 1.03 | 1.57 | 0.023 * |
| ISAR | 1.47 | 0.91 | 2.37 | 0.118 |
| MMSE | 1.12 | 0.59 | 2.14 | 0.722 |
| Barthel | 1.70 | 0.88 | 3.26 | 0.114 |
| GDS | 1.20 | 0.70 | 2.06 | 0.515 |
| Hospitalisation | ||||
| Rockwood CFS | 1.30 ^ | 0.56 | 3.01 | 0.542 |
| Walsall | 0.79 | 0.48 | 1.29 | 0.347 |
| Mobility (FIM) | 0.98 | 0.79 | 1.23 | 0.890 |
| MUST | 0.77 | 0.40 | 1.50 | 0.440 |
| TUG | 1.29 | 1.01 | 1.65 | 0.039 * |
| ISAR | 1.55 | 0.87 | 2.77 | 0.137 |
| MMSE | 1.13 | 0.53 | 2.41 | 0.749 |
| Barthel | 0.73 | 0.35 | 1.54 | 0.407 |
| GDS | 1.42 | 0.75 | 2.68 | 0.283 |
| Death | ||||
| Rockwood CFS | 2.80 | 1.18 | 8.23 | 0.049 * |
| Walsall | 1.69 | 0.83 | 3.46 | 0.150 |
| Mobility (FIM) | 1.12 | 0.87 | 1.43 | 0.390 |
| MUST | 0.83 | 0.37 | 1.86 | 0.651 |
| TUG | 0.92 | 0.69 | 1.23 | 0.561 |
| ISAR | 1.69 | 0.84 | 3.43 | 0.144 |
| MMSE | 3.16 | 1.09 | 9.12 | 0.034 * |
| Barthel | 2.75 | 1.04 | 7.25 | 0.041 * |
| GDS | 1.10 | 0.53 | 2.29 | 0.800 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewis, C.; O’Caoimh, R.; Patton, D.; O’Connor, T.; Moore, Z.; Nugent, L.E. Utilisation of a Suite of Screening Tools to Determine Adverse Healthcare Outcomes in an Older Frail Population Admitted to a Community Virtual Ward. Int. J. Environ. Res. Public Health 2021, 18, 5601. https://doi.org/10.3390/ijerph18115601
Lewis C, O’Caoimh R, Patton D, O’Connor T, Moore Z, Nugent LE. Utilisation of a Suite of Screening Tools to Determine Adverse Healthcare Outcomes in an Older Frail Population Admitted to a Community Virtual Ward. International Journal of Environmental Research and Public Health. 2021; 18(11):5601. https://doi.org/10.3390/ijerph18115601
Chicago/Turabian StyleLewis, Clare, Rónán O’Caoimh, Declan Patton, Tom O’Connor, Zena Moore, and Linda E. Nugent. 2021. "Utilisation of a Suite of Screening Tools to Determine Adverse Healthcare Outcomes in an Older Frail Population Admitted to a Community Virtual Ward" International Journal of Environmental Research and Public Health 18, no. 11: 5601. https://doi.org/10.3390/ijerph18115601
APA StyleLewis, C., O’Caoimh, R., Patton, D., O’Connor, T., Moore, Z., & Nugent, L. E. (2021). Utilisation of a Suite of Screening Tools to Determine Adverse Healthcare Outcomes in an Older Frail Population Admitted to a Community Virtual Ward. International Journal of Environmental Research and Public Health, 18(11), 5601. https://doi.org/10.3390/ijerph18115601







