Cognitive Neural Mechanism of Social Anxiety Disorder: A Meta-Analysis Based on fMRI Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Selection
2.2. Data Extraction
2.3. Study Quality
2.4. ALE Meta-Analysis
3. Results
3.1. Study Selection
3.2. Characteristics of the Included Studies
3.3. Study Quality
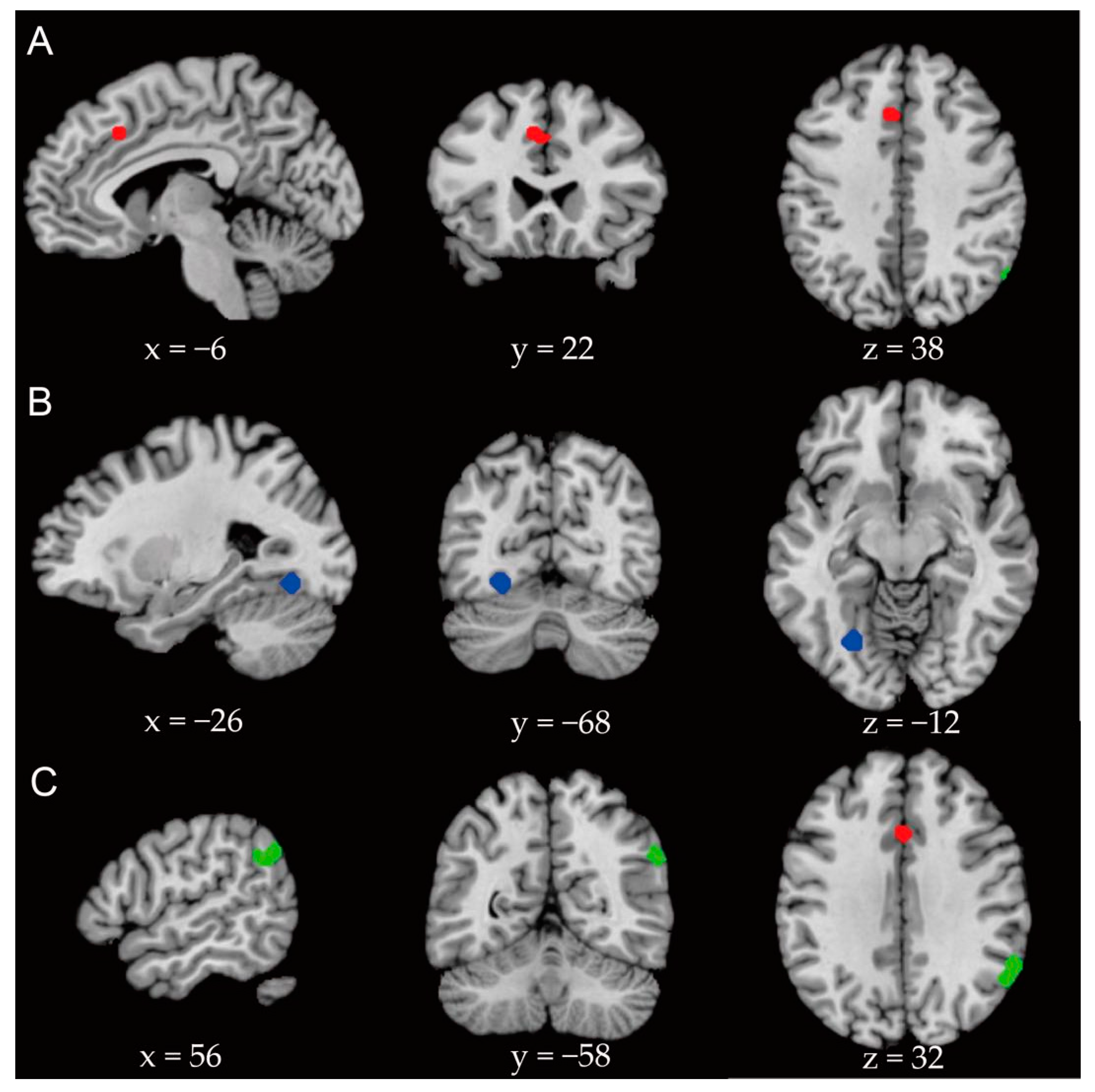
3.4. Activation Likelihood Estimation
4. Discussion
4.1. Anterior Cingulate Cortex
4.2. Angular Gyrus/Supramarginal Gyrus
4.3. Cerebellar Slope/Fusiform Gyrus
4.4. Different Task Types Affect Activation Patterns in Brain Regions
4.5. Advantages and Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5™, 5th ed.; American Psychiatric Publishing: Arlington VA, USA, 2013. [Google Scholar]
- Watson, H.J.; Swan, A.; Nathan, P.R. Psychiatric diagnosis and quality of life: The additional burden of psychiatric comorbidity. Compr. Psychiatry 2011, 52, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.C.; Bar-Haim, Y.; Fox, N.A.; Pine, D.S.; Britton, J.C. Neural mechanisms underlying heterogeneous expression of threat-related attention in social anxiety. Behav. Res. Ther. 2020, 132. [Google Scholar] [CrossRef]
- Xing, M.; Fitzgerald, J.M.; Klumpp, H. Classification of Social Anxiety Disorder with Support Vector Machine Analysis using neural correlates of social signals of threat. Front. Psychiatry 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Gentili, C.; Gobbini, M.I.; Ricciardi, E.; Vanello, N.; Pietrini, P.; Haxby, J.V.; Guazzelli, M. Differential modulation of neural activity throughout the distributed neural system for face perception in patients with Social Phobia and healthy subjects. Brain Res. Bull. 2008, 77, 286–292. [Google Scholar] [CrossRef]
- Frick, A.; Howner, K.; Fischer, H.; Kristiansson, M.; Furmark, T. Altered fusiform connectivity during processing of fearful faces in social anxiety disorder. Transl. Psychiatry 2013, 3, e312. [Google Scholar] [CrossRef]
- Lorberbaum, J.P.; Kose, S.; Johnson, M.R.; Arana, G.W.; Sullivan, L.K.; Hamner, M.B.; George, M.S. Neural correlates of speech anticipatory anxiety in generalized social phobia. Neuroreport 2004, 15, 2701–2705. [Google Scholar]
- Etkin, A.; Wager, T.D. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry 2007, 164, 1476–1488. [Google Scholar] [CrossRef]
- Hattingh, C.J.; Ipser, J.; Tromp, S.A.; Syal, S.; Lochner, C.; Brooks, S.J.; Stein, D.J. Functional magnetic resonance imaging during emotion recognition in social anxiety disorder: An activation likelihood meta-analysis. Front. Hum. Neurosci. 2013, 6, 1656–1661. [Google Scholar] [CrossRef]
- Binelli, C.; Muñiz, A.; Subira, S.; Navines, R.; Blanco-Hinojo, L.; Perez-Garcia, D.; Crippa, J.; Farré, M.; Pérez-Jurado, L.; Pujol, J.; et al. Facial emotion processing in patients with social anxiety disorder and Williams-Beuren syndrome: An fMRI study. J. Psychiatry Neurosci. 2016, 41, 182–191. [Google Scholar] [CrossRef]
- Gentili, C.; Messerotti Benvenuti, S.; Lettieri, G.; Costa, C.; Cecchetti, L. ROI and phobias: The effect of ROI approach on an ALE meta-analysis of specific phobias. Hum. Brain Mapp. 2018, 40, 1814–1828. [Google Scholar] [CrossRef]
- Costa, C.; Cristea, I.A.; Dal Bò, E.; Melloni, C.; Gentili, C. Brain activity during facial processing in autism spectrum disorder: An activation likelihood estimation (ALE) meta-analysis of neuroimaging studies. J. Child Psychol. Psychiatry 2021, 3. [Google Scholar] [CrossRef]
- Stein, M.B.; Goldin, P.R.; Sareen, J.; Zorrilla, L.T.E.; Brown, G.G. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch. Gen. Psychiatry 2002, 59, 1027–1034. [Google Scholar] [CrossRef]
- Amir, N.; Klumpp, H.; Elias, J.; Bedwell, J.S.; Yanasak, N.; Miller, L.S. Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biol. Psychiatry 2005, 57, 975–981. [Google Scholar] [CrossRef]
- Phan, K.L.; Fitzgerald, D.A.; Nathan, P.J.; Tancer, M.E. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol. Psychiatry 2006, 59, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.C.; Wright, C.I.; Wedig, M.M.; Gold, A.L.; Pollack, M.H.; Rauch, S.L. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depress. Anxiety 2008, 25, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Gentili, C.; Ricciardi, E.; Gobbini, M.I.; Santarelli, M.F.; Haxby, J.V.; Pietrini, P.; Guazzelli, M. Beyond amygdala: Default Mode Network activity differs between patients with social phobia and healthy controls. Brain Res. Bull. 2009, 79, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Klumpp, H.; Angstadt, M.; Nathan, P.J.; Phan, K.L. Amygdala reactivity to faces at varying intensities of threat in generalized social phobia: An event-related functional MRI study. Psychiatry Res. 2010, 183, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Danti, S.; Ricciardi, E.; Gentili, C.; Gobbini, M.I.; Pietrini, P.; Guazzelli, M. Is Social Phobia a “Mis-Communication” Disorder? Brain Functional Connectivity during Face Perception Differs between Patients with Social Phobia and Healthy Control Subjects. Front. Syst. Neurosci. 2010, 4, 152. [Google Scholar] [CrossRef]
- Klumpp, H.; Angstadt, M.; Phan, K.L. Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biol. Psychol. 2012, 89, 273–276. [Google Scholar] [CrossRef]
- Labuschagne, I.; Phan, K.L.; Wood, A.; Angstadt, M.; Chua, P.; Heinrichs, M.; Stout, J.C.; Nathan, P.J. Medial frontal hyperactivity to sad faces in generalized social anxiety disorder and modulation by oxytocin. Int. J. Neuropsychopharmacol. 2012, 15, 883–896. [Google Scholar] [CrossRef]
- Frick, A.; Howner, K.; Fischer, H.; Kristiansson, M.; Furmark, T. Altered fusiform connectivity during processing of fearful faces in social anxiety disorder. Transl. Psychiatry 2013, 3, e312. [Google Scholar] [CrossRef]
- Wheaton, M.G.; Fitzgerald, D.A.; Phan, K.L.; Klumpp, H. Perceptual load modulates anterior cingulate cortex response to threat distractors in generalized social anxiety disorder. Biol. Psychol. 2014, 101, 13–17. [Google Scholar] [CrossRef]
- Michalowski, J.M.; Matuszewski, J.; Drozdziel, D.; Koziejowski, W.; Rynkiewicz, A.; Jednorog, K.; Marchewka, A. Neural response patterns in spider, blood-injection-injury and social fearful individuals: New insights from a simultaneous EEG/ECG-fMRI study. Brain Imaging Behav. 2017, 11, 829–845. [Google Scholar] [CrossRef] [PubMed]
- Tadayonnejad, R.; Klumpp, H.; Ajilore, O.; Leow, A.; Phan, K.L. Aberrant pulvinar effective connectivity in generalized social anxiety disorder. Medicine 2016, 95, e5358. [Google Scholar] [CrossRef] [PubMed]
- Goldin, P.R.; Manber-Ball, T.; Werner, K.; Heimberg, R.; Gross, J.J. Neural mechanisms of cognitive reappraisal of negative self-beliefs in social anxiety disorder. Biol. Psychiatry 2009, 66, 1091–1099. [Google Scholar] [CrossRef]
- Goldin, P.R.; Manber, T.; Hakimi, S.; Canli, T.; Gross, J.J. Neural bases of social anxiety disorder: Emotional reactivity and cognitive regulation during social and physical threat. Arch. Gen. Psychiatry 2009, 66, 170–180. [Google Scholar] [CrossRef]
- Shah, S.G.; Klumpp, H.; Angstadt, M.; Nathan, P.J.; Phan, K.L. Amygdala and insula response to emotional images in patients with generalized social anxiety disorder. J. Psychiatry Neurosci. 2009, 34, 296–302. [Google Scholar] [PubMed]
- Nakao, T.; Sanematsu, H.; Yoshiura, T.; Togao, O.; Murayama, K.; Tomita, M.; Masuda, Y.; Kanba, S. fMRI of patients with social anxiety disorder during a social situation task. Neurosci. Res. 2011, 69, 67–72. [Google Scholar] [CrossRef]
- Pujol, J.; Gimenez, M.; Ortiz, H.; Soriano-Mas, C.; Lopez-Sola, M.; Farre, M.; Deus, J.; Merlo-Pich, E.; Harrison, B.J.; Cardoner, N.; et al. Neural response to the observable self in social anxiety disorder. Psychol. Med. 2013, 43, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Boehme, S.; Mohr, A.; Becker, M.P.; Miltner, W.H.; Straube, T. Area-dependent time courses of brain activation during video-induced symptom provocation in social anxiety disorder. Biol. Mood Anxiety Disord. 2014, 4, 6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heitmann, C.Y.; Feldker, K.; Neumeister, P.; Zepp, B.M.; Peterburs, J.; Zwitserlood, P.; Straube, T. Abnormal brain activation and connectivity to standardized disorder-related visual scenes in social anxiety disorder. Hum. Brain Mapp. 2016, 37, 1559–1572. [Google Scholar] [CrossRef] [PubMed]
- Heitmann, C.Y.; Feldker, K.; Neumeister, P.; Brinkmann, L.; Schrammen, E.; Zwitserlood, P.; Straube, T. Brain activation to task-irrelevant disorder-related threat in social anxiety disorder: The impact of symptom severity. Neuroimage Clin. 2017, 14, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Boehme, S.; Ritter, V.; Tefikow, S.; Stangier, U.; Strauss, B.; Miltner, W.H.; Straube, T. Brain activation during anticipatory anxiety in social anxiety disorder. Soc. Cogn. Affect Neurosci. 2014, 9, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Bunford, N.; Kujawa, A.; Fitzgerald, K.D.; Monk, C.S.; Phan, K.L. Convergence of BOLD and ERP measures of neural reactivity to emotional faces in children and adolescents with and without anxiety disorders. Biol. Psychol. 2018, 134, 9–19. [Google Scholar] [CrossRef]
- Quadflieg, S.; Mohr, A.; Mentzel, H.J.; Miltner, W.H.; Straube, T. Modulation of the neural network involved in the processing of anger prosody: The role of task-relevance and social phobia. Biol. Psychol. 2008, 78, 129–137. [Google Scholar] [CrossRef]
- Blair, K.S.; Geraci, M.; Hollon, N.; Otero, M.; DeVido, J.; Majestic, C.; Jacobs, M.; Blair, R.J.R.; Pine, D.S. Social norm processing in adult social phobia: Atypically increased ventromedial frontal cortex responsiveness to unintentional (Embarrassing) transgressions. Am. J. Psychiatry 2010, 167, 1526–1532. [Google Scholar] [CrossRef]
- Bruhl, A.B.; Rufer, M.; Delsignore, A.; Kaffenberger, T.; Jancke, L.; Herwig, U. Neural correlates of altered general emotion processing in social anxiety disorder. Brain Res. 2011, 1378, 72–83. [Google Scholar] [CrossRef]
- Blair, K.S.; Geraci, M.; Otero, M.; Majestic, C.; Odenheimer, S.; Jacobs, M.; Blair, R.J.; Pine, D.S. Atypical modulation of medial prefrontal cortex to self-referential comments in generalized social phobia. Psychiatry Res. 2011, 193, 38–45. [Google Scholar] [CrossRef]
- Koric, L.; Volle, E.; Seassau, M.; Bernard, F.A.; Mancini, J.; Dubois, B.; Pelissolo, A.; Levy, R. How cognitive performance-induced stress can influence right VLPFC activation: An fMRI study in healthy subjects and in patients with social phobia. Hum. Brain Mapp. 2012, 33, 1973–1986. [Google Scholar] [CrossRef]
- Giménez, M.; Pujol, J.; Ortiz, H.; Soriano-Mas, C.; López-Solà, M.; Farré, M.; Deus, J.; Merlo-Pich, E.; Martin-Santos, R. Altered brain functional connectivity in relation to perception of scrutiny in social anxiety disorder. Psychiatry Res. 2012, 202, 214–223. [Google Scholar] [CrossRef]
- Gaebler, M.; Daniels, J.K.; Lamke, J.P.; Fydrich, T.; Walter, H. Behavioural and neural correlates of self-focused emotion regulation in social anxiety disorder. J. Psychiatry Neurosci. 2014, 39, 249–258. [Google Scholar] [CrossRef]
- Boehme, S.; Ritter, V.; Tefikow, S.; Stangier, U.; Strauss, B.; Miltner, W.H.; Straube, T. Neural correlates of emotional interference in social anxiety disorder. PLoS ONE 2015, 10, e0128608. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Kim, J.S.; Shin, Y.B.; Choi, S.H.; Lee, S.K.; Kim, J.J. Neural activity during self-referential working memory and the underlying role of the amygdala in social anxiety disorder. Neurosci. Lett. 2016, 627, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Richey, J.A.; Ghane, M.; Valdespino, A.; Coffman, M.C.; Strege, M.V.; White, S.W.; Ollendick, T.H. Spatiotemporal dissociation of brain activity underlying threat and reward in social anxiety disorder. Soc. Cogn. Affect. Neurosci. 2017, 12, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.P.I.; Simon, D.; Miltner, W.H.R.; Straube, T. Altered activation of the ventral striatum under performance-related observation in social anxiety disorder. Psychol. Med. 2017, 47, 2502–2512. [Google Scholar] [CrossRef]
- Petersen, S.E.; Posner, M.I. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 2012, 35, 73–89. [Google Scholar] [CrossRef]
- Eisenberger, N.I.; Lieberman, M.D.; Williams, K.D. Does rejection hurt? An fMRI study of social exclusion. Science 2003, 302, 290–292. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, H.; Liu, T. Neural mechanisms of social exclusion: Evidences from a meta-analysis on fMRI studies. Chin. J. Clin. Psychol. 2019, 27, 436–442. [Google Scholar]
- Wang, W.; Qian, S.; Liu, K.; Li, B.; Xin, K.; Sun, G. Resting-state functional magnetic resonance imaging in neural mechanism of generalized anxiety disorder. Chin. J. Med. Imaging Technol. 2016, 32, 358–362. [Google Scholar]
- Frith, U.; Frith, C.D. Development and neurophysiology of mentalizing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003, 358, 459–473. [Google Scholar] [CrossRef]
- Qiu, C.; Liao, W.; Ding, J.; Feng, Y.; Zhu, C.; Nie, X.; Zhang, W.; Chen, H.; Gong, Q. Regional homogeneity changes in social anxiety disorder: A resting-state fMIRI study. Psychiatry Res. 2011, 194, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Goldin, P.R.; Gross, J.J. Effects of Mindfulness-Based Stress Reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion 2010, 10, 83–91. [Google Scholar] [CrossRef]
- Kim, M.-K.; Yoon, H.-J.; Shin, Y.-B.; Lee, S.-K.; Kim, J.-J. Neural basis of distorted self-face recognition in social anxiety disorder. Neuroimage Clin. 2016, 12, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Baillieux, H.; De Smet, H.J.; Paquier, P.F.; De Deyn, P.P.; Marien, P. Cerebellar neurocognition: Insights into the bottom of the brain. Clin. Neurol. Neurosurg. 2008, 110, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Rius, J. The cerebellum in fear and anxiety-related disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 85, 23–32. [Google Scholar] [CrossRef]
- Silani, G.; Lamm, C.; Ruff, C.C.; Singer, T. Right supramarginal gyrus is crucial to overcome emotional egocentricity bias in social judgments. J. Neurosci. 2013, 33, 15466–15476. [Google Scholar] [CrossRef]
- Alvi, T.; Kouros, C.D.; Lee, J.; Fulford, D.; Tabak, B.A. Social anxiety is negatively associated with theory of mind and Empathic Accuracy. J. Abnorm. Psychol. 2020, 129, 108–113. [Google Scholar] [CrossRef]
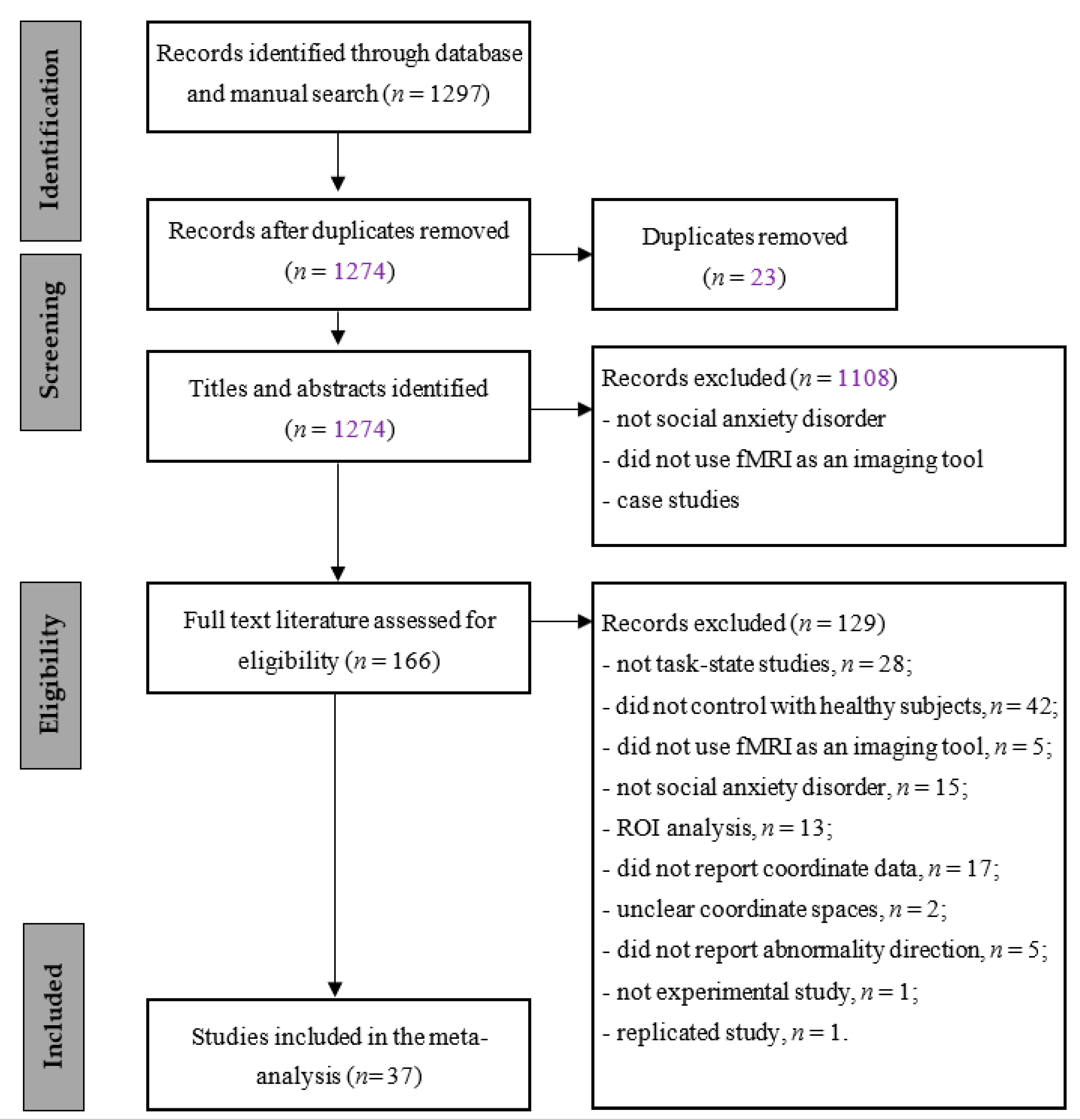
| ID | Ref. ID | Study Year | Number of Participants SAD/Healthy Controls | Number of Extracted Coordinates (SAD Groups) Healthy Controls) | Extract Number of Coordinates (SAD Groups Healthy Controls) | Coordinate System | Task Type |
|---|---|---|---|---|---|---|---|
| 1 | [13] | 2002 | 15/15 | 8 | 0 | Talairach | Emotional face |
| 2 | [14] | 2005 | 11/11 | 40 | 0 | Talairach | Emotional face |
| 3 | [15] | 2006 | 10/10 | 8 | 0 | Talairach | Emotional face |
| 4 | [16] | 2008 | 11/11 | 10 | 2 | MNI | Emotional face |
| 5 | [17] | 2009 | 8/7 | 2 | 0 | Talairach | Emotional face |
| 6 | [18] | 2010 | 12/12 | 12 | 1 | MNI | Emotional face |
| 7 | [19] | 2010 | 8/7 | 11 | 4 | Talairach | Emotional face |
| 8 | [20] | 2012 | 29/26 | 14 | 0 | MNI | Emotional face |
| 9 | [21] | 2012 | 18/18 | 19 | 6 | MNI | Emotional face |
| 10 | [6] | 2013 | 14/12 | 9 | 1 | MNI | Emotional face |
| 11 | [22] | 2013 | 27/27 | 0 | 11 | Talairach | Emotional face |
| 12 | [23] | 2014 | 23/24 | 0 | 1 | MNI | Emotional face |
| 13 | [10] | 2016 | 20/20 | 0 | 8 | MNI | Emotional face |
| 14 | [24] | 2017 | 12/13 | 9 | 0 | MNI | Emotional face |
| 15 | [25] | 2016 | 19/21 | 4 | 1 | MNI | Emotional face |
| 16 | [26] | 2009 | 27/27 | 20 | 27 | Talairach | Specific situations |
| 17 | [27] | 2009 | 15/17 | 14 | 26 | Talairach | Specific situations |
| 18 | [28] | 2009 | 11/11 | 5 | 0 | MNI | Specific situations |
| 19 | [29] | 2011 | 6/9 | 0 | 5 | MNI | Specific situations |
| 20 | [30] | 2013 | 20/20 | 2 | 6 | MNI | Specific situations |
| 21 | [31] | 2014 | 20/20 | 15 | 0 | Talairach | Specific situations |
| 22 | [32] | 2016 | 30/30 | 12 | 0 | Talairach | Specific situations |
| 23 | [33] | 2017 | 24/24 | 11 | 0 | Talairach | Specific situations |
| 24 | [7] | 2004 | 8/6 | 3 | 6 | Talairach | Speech task |
| 25 | [34] | 2014 | 17/17 | 2 | 1 | Talairach | Speech task |
| 26 | [35] | 2018 | 51/13 | 2 | 1 | MNI | Speech task |
| 27 | [36] | 2008 | 12/12 | 1 | 0 | Talairach | Listen to the words and recognize the emotional colors |
| 28 | [37] | 2010 | 16/16 | 5 | 0 | Talairach | Story reading |
| 29 | [38] | 2011 | 16/18 | 21 | 2 | Talairach | Emotional pictures |
| 30 | [39] | 2011 | 15/15 | 10 | 0 | Talairach | Discourse presentation |
| 31 | [40] | 2012 | 15/15 | 2 | 0 | MNI | Cognitive task |
| 32 | [41] | 2011 | 20/20 | 2 | 0 | Talairach | Security review awareness task |
| 33 | [42] | 2014 | 21/23 | 8 | 0 | MNI | Emotion regulation task |
| 34 | [43] | 2015 | 16/16 | 6 | 0 | Talairach | Emotion Stroop task |
| 35 | [44] | 2016 | 20/20 | 15 | 0 | MNI | Memory task |
| 36 | [45] | 2017 | 21/22 | 33 | 3 | MNI | Currency delayed |
| 37 | [46] | 2017 | 16/16 | 0 | 3 | Talairach | Time estimation task |
| Total | 654/594 | 335 | 115 |
| Central Coordinates | Volume mm3 | p–Value | Maximum ALE Value | Cerebral Area | |||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Global analysis | −6 | 22 | 38 | 584 | 0.000124 | 0.015 | Anterior cingulate gyrus |
| Emotional face task | −26 | −68 | −12 | 496 | 0.00000034 | 0.015 | Cerebellar slope, fusiform gyrus |
| Situational task | 58 | −52 | 30 | 784 | 0.0000046 | 0.013 | Supramarginal gyrus and angular gyrus |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Ruan, Y.; Zhang, Y.; Wang, J.; Liu, Y.; Zhang, J.; Zhang, L. Cognitive Neural Mechanism of Social Anxiety Disorder: A Meta-Analysis Based on fMRI Studies. Int. J. Environ. Res. Public Health 2021, 18, 5556. https://doi.org/10.3390/ijerph18115556
Yu X, Ruan Y, Zhang Y, Wang J, Liu Y, Zhang J, Zhang L. Cognitive Neural Mechanism of Social Anxiety Disorder: A Meta-Analysis Based on fMRI Studies. International Journal of Environmental Research and Public Health. 2021; 18(11):5556. https://doi.org/10.3390/ijerph18115556
Chicago/Turabian StyleYu, Xianglian, Yijun Ruan, Yawen Zhang, Jiayi Wang, Yuting Liu, Jibiao Zhang, and Lin Zhang. 2021. "Cognitive Neural Mechanism of Social Anxiety Disorder: A Meta-Analysis Based on fMRI Studies" International Journal of Environmental Research and Public Health 18, no. 11: 5556. https://doi.org/10.3390/ijerph18115556
APA StyleYu, X., Ruan, Y., Zhang, Y., Wang, J., Liu, Y., Zhang, J., & Zhang, L. (2021). Cognitive Neural Mechanism of Social Anxiety Disorder: A Meta-Analysis Based on fMRI Studies. International Journal of Environmental Research and Public Health, 18(11), 5556. https://doi.org/10.3390/ijerph18115556







