Prevalence of High HDL Cholesterol and Its Associated Factors Among Tunisian Women of Childbearing Age: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Study Population
2.2. Socioeconomic and Demographic Variables
2.3. Anthropometric Variables
2.4. Biological Variables
2.4.1. Analysis
2.4.2. Threshold Values
2.5. Data Management and Statistical Analysis
3. Results
3.1. General Characteristics of the Subjects
3.2. Characteristics of Women According to HDL-C Levels
3.3. Individual Association between Socio-Demographic, Lifestyle, and Biological Characteristics with a High HDL-C Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- NIS. National Statistics on the Causes of Death in Tunisia 2015 and 2017; National Institute of Statistics: Tunis, Tunisia, 2020. (In French) [Google Scholar]
- Gordon, D.J.; Probstfield, J.L.; Garrison, R.J.; Neaton, J.D.; Castelli, W.P.; Knoke, J.D.; Jacobs, D.R., Jr.; Bangdiwala, S.; Tyroler, H.A. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989, 79, 8–15. [Google Scholar] [CrossRef]
- The Emerging Risk Factors Collaboration. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009, 302, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Assmann, G.; Gotto, A.M., Jr. HDL cholesterol and protective factors in atherosclerosis. Circulation 2004, 109, III8–III14. [Google Scholar] [CrossRef]
- Marz, W.; Kleber, M.E.; Scharnagl, H.; Speer, T.; Zewinger, S.; Ritsch, A.; Parhofer, K.G.; von Eckardstein, A.; Landmesser, U.; Laufs, U. HDL cholesterol: Reappraisal of its clinical relevance. Clin. Res. Cardiol. 2017, 106, 663–675. [Google Scholar] [CrossRef]
- Marz, W.; Kleber, M.E.; Scharnagl, H.; Speer, T.; Zewinger, S.; Ritsch, A.; Parhofer, K.G.; von Eckardstein, A.; Landmesser, U.; Laufs, U. Clinical importance of HDL cholesterol. Herz 2017, 42, 58–66. [Google Scholar] [CrossRef]
- Brites, F.; Martin, M.; Guillas, I.; Kontush, A. Antioxidative activity of high-density lipoprotein (HDL): Mechanistic insights into potential clinical benefit. BBA Clin. 2017, 8, 66–77. [Google Scholar] [CrossRef]
- Tosheska Trajkovska, K.; Topuzovska, S. High-density lipoprotein metabolism and reverse cholesterol transport: Strategies for raising HDL cholesterol. Anatol. J. Cardiol. 2017, 18, 149–154. [Google Scholar] [CrossRef]
- Estrada-Luna, D.; Ortiz-Rodriguez, M.A.; Medina-Briseno, L.; Carreon-Torres, E.; Izquierdo-Vega, J.A.; Sharma, A.; Cancino-Diaz, J.C.; Perez-Mendez, O.; Belefant-Miller, H.; Betanzos-Cabrera, G. Current Therapies Focused on High-Density Lipoproteins Associated with Cardiovascular Disease. Molecules 2018, 23, 2730. [Google Scholar] [CrossRef]
- Talbot, D.; Delaney, J.A.C.; Sandfort, V.; Herrington, D.M.; McClelland, R.L. Importance of the lipid-related pathways in the association between statins, mortality, and cardiovascular disease risk: The Multi-Ethnic Study of Atherosclerosis. Pharmacoepidemiol. Drug Saf. 2018, 27, 365–372. [Google Scholar] [CrossRef]
- Singh, K.; Rohatgi, A. Examining the paradox of high high-density lipoprotein and elevated cardiovascular risk. J. Thorac. Dis. 2018, 10, 109–112. [Google Scholar] [CrossRef]
- Oh, I.H.; Hur, J.K.; Ryoo, J.H.; Jung, J.Y.; Park, S.K.; Yang, H.J.; Choi, J.M.; Jung, K.W.; Won, Y.J.; Oh, C.M. Very high high-density lipoprotein cholesterol is associated with increased all-cause mortality in South Koreans. Atherosclerosis 2019, 283, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.M.; Varbo, A.; Nordestgaard, B.G. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: Two prospective cohort studies. Eur. Heart J. 2017, 38, 2478–2486. [Google Scholar] [CrossRef]
- El Ati, J.; Traissac, P.; Delpeuch, F.; Aounallah-Skhiri, H.; Beji, C.; Eymard-Duvernay, S.; Bougatef, S.; Kolsteren, P.; Maire, B.; Ben Romdhane, H. Gender obesity inequities are huge but differ greatly according to environment and socio-economics in a North African setting: A national cross-sectional study in Tunisia. PLoS ONE 2012, 7, e48153. [Google Scholar] [CrossRef] [PubMed]
- WHO. Physical Status: The Use and Interpretation of Anthropometry; World Health Organization: Geneva, Switzerland, 1995. [Google Scholar]
- International Society of Hypertension Writing Group. International Society of Hypertension (ISH) statement on management of hypertension. J. Hypertens. 2003, 21, 1983–1992. [Google Scholar] [CrossRef]
- Gavin, J.R., III; Albert, K.G.M.M.; Davidson, M.B.; DeFronzo, R.A. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997, 20, 1183–1197. [Google Scholar] [CrossRef]
- National Cholesterol Education Programs (US). Treatment of High Blood Cholesterol in, A. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Walldius, G.; Jungner, I.; Holme, I.; Aastveit, A.H.; Kolar, W.; Steiner, E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): A prospective study. Lancet 2001, 358, 2026–2033. [Google Scholar] [CrossRef]
- Lavie, C.J.; Milani, R.V.; Ventura, H.O. Obesity and cardiovascular disease: Risk factor, paradox, and impact of weight loss. J Am. Coll. Cardiol. 2009, 53, 1925–1932. [Google Scholar] [CrossRef]
- Epidata. EpiData Data Entry, Data Management and Basic Statistical Analysis System; Epidata Association: Odense, Denmark, 2008. [Google Scholar]
- StataCorp. Stata Statistical Software: Release 14.0; StataCorp LP: College Station, TX, USA, 2015. [Google Scholar]
- Hadj-Taieb, S.; Elasmi, M.; Hammami, M.B.; Marrakchi, R.; Amani, K.; Omar, S.; Sanhaji, H.; Jemaa, R.; Feki, M.; Kaabachi, N. Dyslipidemia in the Greater Tunis population: Prevalence and determinants. Clin. Lab. 2012, 58, 763–770. [Google Scholar] [CrossRef]
- Soyama, Y.; Miura, K.; Morikawa, Y.; Nishijo, M.; Nakanishi, Y.; Naruse, Y.; Kagamimori, S.; Nakagawa, H.; Oyabe, S. High-density lipoprotein cholesterol and risk of stroke in Japanese men and women: The Oyabe Study. Stroke 2003, 34, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Han, J.H.; Park, H.S. Prevalence of low HDL-cholesterol levels and associated factors among Koreans. Circ. J. 2006, 70, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Young, K.A.; Maturu, A.; Lorenzo, C.; Langefeld, C.D.; Wagenknecht, L.E.; Chen, Y.I.; Taylor, K.D.; Rotter, J.I.; Norris, J.M.; Rasouli, N. The triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio as a predictor of insulin resistance, beta-cell function, and diabetes in Hispanics and African Americans. J. Diabetes Complicat. 2019, 33, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.T.; Alter, D.A.; Guo, H.; Koh, M.; Lau, G.; Austin, P.C.; Booth, G.L.; Hogg, W.; Jackevicius, C.A.; Lee, D.S.; et al. High-Density Lipoprotein Cholesterol and Cause-Specific Mortality in Individuals Without Previous Cardiovascular Conditions: The CANHEART Study. J. Am. Coll. Cardiol. 2016, 68, 2073–2083. [Google Scholar] [CrossRef]
- Ford, E.S.; Liu, S. Glycemic index and serum high-density lipoprotein cholesterol concentration among us adults. Arch. Intern. Med. 2001, 161, 572–576. [Google Scholar] [CrossRef]
- Bentley, A.R.; Chen, G.; Shriner, D.; Doumatey, A.P.; Zhou, J.; Huang, H.; Mullikin, J.C.; Blakesley, R.W.; Hansen, N.F.; Bouffard, G.G.; et al. Gene-based sequencing identifies lipid-influencing variants with ethnicity-specific effects in African Americans. PLoS Genet. 2014, 10, e1004190. [Google Scholar] [CrossRef]
- Karthikeyan, G.; Teo, K.K.; Islam, S.; McQueen, M.J.; Pais, P.; Wang, X.; Sato, H.; Lang, C.C.; Sitthi-Amorn, C.; Pandey, M.R.; et al. Lipid profile, plasma apolipoproteins, and risk of a first myocardial infarction among Asians: An analysis from the INTERHEART Study. J. Am. Coll. Cardiol. 2009, 53, 244–253. [Google Scholar] [CrossRef]
- Rysz, J.; Gluba-Brzozka, A.; Rysz-Gorzynska, M.; Franczyk, B. The Role and Function of HDL in Patients with Chronic Kidney Disease and the Risk of Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 601. [Google Scholar] [CrossRef]
- Walter, M. Interrelationships among HDL metabolism, aging, and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1244–1250. [Google Scholar] [CrossRef]
- Droomers, M.; Schrijvers, C.T.; Mackenbach, J.P. Educational level and decreases in leisure time physical activity: Predictors from the longitudinal GLOBE study. J. Epidemiol. Community Health 2001, 55, 562–568. [Google Scholar] [CrossRef]
- Robinson, S.M.; Crozier, S.R.; Borland, S.E.; Hammond, J.; Barker, D.J.; Inskip, H.M. Impact of educational attainment on the quality of young women’s diets. Eur. J. Clin. Nutr. 2004, 58, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Schultz, N.S.; Chui, K.K.H.; Economos, C.D.; Lichtenstein, A.H.; Volpe, S.L.; Sacheck, J.M. Impact of physical activity, diet quality and stress on cardiometabolic health in school employees. Prev. Med. Rep. 2020, 20, 101243. [Google Scholar] [CrossRef]
- Kritz-Silverstein, D.; Barrett-Connor, E.; Wingard, D.L. The relationship between multiparity and lipoprotein levels in older women. J. Clin. Epidemiol. 1992, 45, 761–767. [Google Scholar] [CrossRef]
- Lv, H.; Yang, X.; Zhou, Y.; Wu, J.; Liu, H.; Wang, Y.; Pan, Y.; Xia, Y. Parity and serum lipid levels: A cross-sectional study in chinese female adults. Sci. Rep. 2016, 6, 33831. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ferreira, D.L.S.; Nelson, S.M.; Sattar, N.; Ala-Korpela, M.; Lawlor, D.A. Metabolic characterization of menopause: Cross-sectional and longitudinal evidence. BMC Med. 2018, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Agongo, G.; Nonterah, E.A.; Debpuur, C.; Amenga-Etego, L.; Ali, S.; Oduro, A.; Crowther, N.J.; Ramsay, M.; as members of AWI-Gen and the H3Africa Consortium. The burden of dyslipidaemia and factors associated with lipid levels among adults in rural northern Ghana: An AWI-Gen sub-study. PLoS ONE 2018, 13, e0206326. [Google Scholar] [CrossRef] [PubMed]
- Muennig, P.; Sohler, N.; Mahato, B. Socioeconomic status as an independent predictor of physiological biomarkers of cardiovascular disease: Evidence from NHANES. Prev. Med. 2007, 45, 35–40. [Google Scholar] [CrossRef] [PubMed]
- King, A.C.; Haskell, W.L.; Young, D.R.; Oka, R.K.; Stefanick, M.L. Long-term effects of varying intensities and formats of physical activity on participation rates, fitness, and lipoproteins in men and women aged 50 to 65 years. Circulation 1995, 91, 2596–2604. [Google Scholar] [CrossRef]
- Gupta, A.K.; Ross, E.A.; Myers, J.N.; Kashyap, M.L. Increased reverse cholesterol transport in athletes. Metabolism 1993, 42, 684–690. [Google Scholar] [CrossRef]
- Nakamura, M.; Yamamoto, Y.; Imaoka, W.; Kuroshima, T.; Toragai, R.; Ito, Y.; Kanda, E.; Schaefer, E.J.; Ai, M. Relationships between Smoking Status, Cardiovascular Risk Factors, and Lipoproteins in a Large Japanese Population. J. Atheroscler. Thromb. 2020. [Google Scholar] [CrossRef]
- Jain, R.B.; Ducatman, A. Associations between smoking and lipid/lipoprotein concentrations among US adults aged ≥ 20 years. J. Circ. Biomark. 2018, 7, 1849454418779310. [Google Scholar] [CrossRef]
- van der Gaag, M.S.; van Tol, A.; Vermunt, S.H.; Scheek, L.M.; Schaafsma, G.; Hendriks, H.F. Alcohol consumption stimulates early steps in reverse cholesterol transport. J. Lipid. Res. 2001, 42, 2077–2083. [Google Scholar] [CrossRef]
- Brien, S.E.; Ronksley, P.E.; Turner, B.J.; Mukamal, K.J.; Ghali, W.A. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: Systematic review and meta-analysis of interventional studies. BMJ 2011, 342, d636. [Google Scholar] [CrossRef]
- Traissac, P.; Pradeilles, R.; El Ati, J.; Aounallah-Skhiri, H.; Eymard-Duvernay, S.; Gartner, A.; Beji, C.; Bougatef, S.; Martin-Prevel, Y.; Kolsteren, P.; et al. Abdominal vs. overall obesity among women in a nutrition transition context: Geographic and socio-economic patterns of abdominal-only obesity in Tunisia. Popul. Health Metr. 2015, 13, 1. [Google Scholar] [CrossRef]
- Woudberg, N.J.; Goedecke, J.H.; Blackhurst, D.; Frias, M.; James, R.; Opie, L.H.; Lecour, S. Association between ethnicity and obesity with high-density lipoprotein (HDL) function and subclass distribution. Lipids Health Dis. 2016, 15, 92. [Google Scholar] [CrossRef]
- Yu, S.; Guo, X.; Li, G.X.; Yang, H.; Zheng, L.; Sun, Y. Lower or higher HDL-C levels are associated with cardiovascular events in the general population in rural China. Lipids Health Dis. 2020, 19, 152. [Google Scholar] [CrossRef] [PubMed]
- Dalal, J.J.; Padmanabhan, T.N.; Jain, P.; Patil, S.; Vasnawala, H.; Gulati, A. LIPITENSION: Interplay between dyslipidemia and hypertension. Indian J. Endocrinol. Metab. 2012, 16, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Opoku, S.; Gan, Y.; Fu, W.; Chen, D.; Addo-Yobo, E.; Trofimovitch, D.; Yue, W.; Yan, F.; Wang, Z.; Lu, Z. Prevalence and risk factors for dyslipidemia among adults in rural and urban China: Findings from the China National Stroke Screening and prevention project (CNSSPP). BMC Public Health 2019, 19, 1500. [Google Scholar] [CrossRef] [PubMed]
- Halperin, R.O.; Sesso, H.D.; Ma, J.; Buring, J.E.; Stampfer, M.J.; Gaziano, J.M. Dyslipidemia and the risk of incident hypertension in men. Hypertension 2006, 47, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Tohidi, M.; Hatami, M.; Hadaegh, F.; Azizi, F. Triglycerides and triglycerides to high-density lipoprotein cholesterol ratio are strong predictors of incident hypertension in Middle Eastern women. J. Hum. Hypertens. 2012, 26, 525–532. [Google Scholar] [CrossRef]
- Steyn, K.; Fourie, J.; Benade, A.J.; Rossouw, J.E.; Langenhoven, M.L.; Joubert, G.; Chalton, D.O. Factors associated with high density lipoprotein cholesterol in a population with high high density lipoprotein cholesterol levels. Arteriosclerosis 1989, 9, 390–397. [Google Scholar] [CrossRef]
- Freedman, D.S.; Srinivasan, S.R.; Shear, C.L.; Franklin, F.A.; Webber, L.S.; Berenson, G.S. The relation of apolipoproteins A-I and B in children to parental myocardial infarction. N. Engl. J. Med. 1986, 315, 721–726. [Google Scholar] [CrossRef]
- Pirro, M.; Ricciuti, B.; Rader, D.J.; Catapano, A.L.; Sahebkar, A.; Banach, M. High density lipoprotein cholesterol and cancer: Marker or causative? Prog. Lipid. Res. 2018, 71, 54–69. [Google Scholar] [CrossRef]
- Hokanson, J.E.; Austin, M.A. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: A meta-analysis of population-based prospective studies. J. Cardiovasc. Risk 1996, 3, 213–219. [Google Scholar] [CrossRef]
- Bolanos-Garcia, V.M.; Miguel, R.N. On the structure and function of apolipoproteins: More than a family of lipid-binding proteins. Prog. Biophys. Mol. Biol. 2003, 83, 47–68. [Google Scholar] [CrossRef]
- Walldius, G.; Jungner, I. The apoB/apoA-I ratio: A strong, new risk factor for cardiovascular disease and a target for lipid-lowering therapy—A review of the evidence. J. Intern. Med. 2006, 259, 493–519. [Google Scholar] [CrossRef]
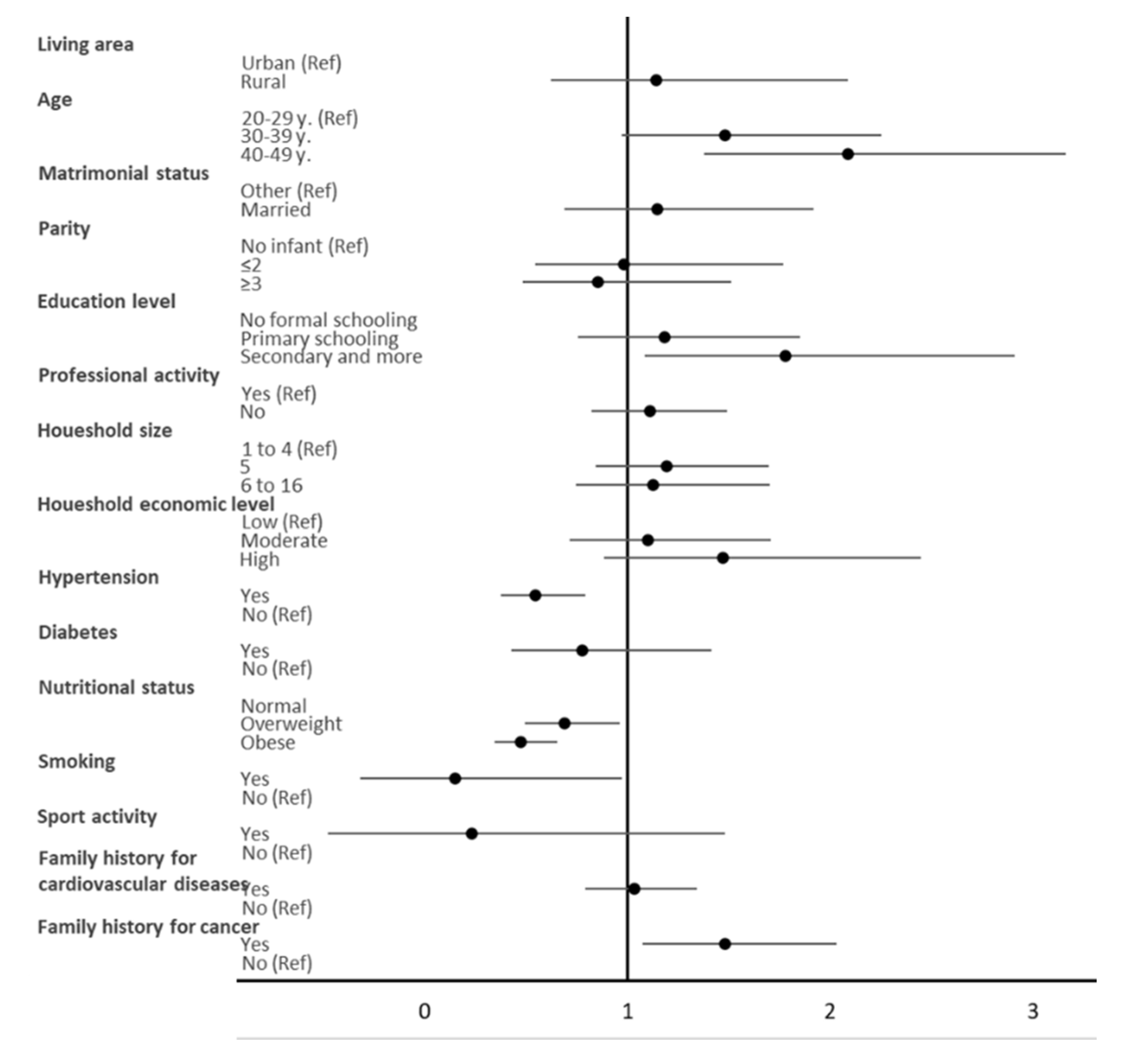
| Variable | High HDL-C ≥60 mg/dL (n = 446) | Normal and Low HDL-C <60 mg/dL (n = 1243) | p-Value 1 |
|---|---|---|---|
| Age (%) | |||
| 20–29 years | 32.0 | 28.1 | 0.35 |
| 30–39 years | 28.2 | 31.3 | |
| 40–49 years | 39.8 | 40.6 | |
| Area of living (%) | |||
| Rural | 6.8 | 7.5 | 0.68 |
| Urbain | 93.2 | 92.5 | |
| Marital status (%) | |||
| Other 2 | 61.4 | 69.2 | 0.005 |
| Married | 38.6 | 30.8 | |
| Parity (%) | |||
| Three and more children | 34.8 | 42.2 | 0.029 |
| One or two children | 26.6 | 26.9 | |
| 0 children | 38.5 | 30.9 | |
| Menopause (%) | |||
| No | 90.5 | 92.9 | 0.081 |
| Yes | 9.5 | 7.1 | |
| Level of education (%) | |||
| No schooling | 7.7 | 12.0 | <10−4 |
| Primary and secondary school | 28.3 | 38.8 | |
| Secondary complete and graduate | 64.0 | 49.2 | |
| Professional activity (%) | |||
| No | 33.9 | 32.4 | 0.67 |
| Yes | 66.1 | 67.6 | |
| Economic level (%) | |||
| Low | 42.5 | 35.1 | 0.007 |
| Medium | 31.3 | 34.9 | |
| High | 26.2 | 30.0 | |
| Smoking (%) | |||
| No | 94.8 | 93.6 | 0.21 |
| Yes | 5.2 | 6.4 | |
| Drinking alcohol (%) | |||
| No | 100 | 99.4 | 0.24 |
| Yes | 0 | 0.6 | |
| Sport activity (%) | |||
| No | 93.2 | 93.8 | 0.70 |
| Yes | 6.8 | 6.2 | |
| Hypertension (%) | 4.0 | 6.4 | 0.074 |
| Diabetes Mellitus (%) | 13.1 | 24.4 | <10−4 |
| Metabolic syndrome (%) | 28.3 | 33.2 | 0.059 |
| Family history of cancer (%) | 61.2 | 71.8 | 0.002 |
| Family history of CVD 3 (%) | 61.9 | 66.1 | 0.17 |
| Family history of hypertension (%) | 62.1 | 64.4 | 0.59 |
| Family history of diabetes (%) | 60.0 | 57.7 | 0.48 |
| Family history of obesity (%) | 55.2 | 54.6 | 0.86 |
| Lipid lowering treatment (%) | 1.3 | 1.3 | 0.97 |
| Fasting blood glucose (mmol/L) | 4.93 ± 0.08 | 5.07 ± 0.06 | 0.102 |
| TC 4 (mmol/L) | 5.17 ± 0.06 | 4.62 ± 0.05 | <10−4 |
| Triglyceridemia (mmol/L) | 0.89 ± 0.03 | 1.12 ± 0.03 | <10−4 |
| LDL-C 5 (mmol/L) | 2.98 ± 0.06 | 2.91 ± 0.04 | 0.27 |
| TC/HDL-C 6 | 2.92 ± 0.04 | 3.91 ± 0.04 | <10−4 |
| ApoA-I 7 (mmol/L) | 1.58 ± 0.03 | 1.31 ± 0.02 | <10−4 |
| ApoB 8 (mmol/L) | 0.79 ± 0.02 | 0.86 ± 0.02 | 0.003 |
| ApoB/Apo AI | 0.50 ± 0.03 | 0.66 ± 0.02 | <10−4 |
| SBP 9 | 119.5 ± 0.9 | 122.8 ± 0.8 | 0.001 |
| DBP 10 | 74.5 ± 0.5 | 76.85 ± 0.51 | 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Cherifa, F.; El Ati, J.; Doggui, R.; El Ati-Hellal, M.; Traissac, P. Prevalence of High HDL Cholesterol and Its Associated Factors Among Tunisian Women of Childbearing Age: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 5461. https://doi.org/10.3390/ijerph18105461
Ben Cherifa F, El Ati J, Doggui R, El Ati-Hellal M, Traissac P. Prevalence of High HDL Cholesterol and Its Associated Factors Among Tunisian Women of Childbearing Age: A Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2021; 18(10):5461. https://doi.org/10.3390/ijerph18105461
Chicago/Turabian StyleBen Cherifa, Fatma, Jalila El Ati, Radhouene Doggui, Myriam El Ati-Hellal, and Pierre Traissac. 2021. "Prevalence of High HDL Cholesterol and Its Associated Factors Among Tunisian Women of Childbearing Age: A Cross-Sectional Study" International Journal of Environmental Research and Public Health 18, no. 10: 5461. https://doi.org/10.3390/ijerph18105461
APA StyleBen Cherifa, F., El Ati, J., Doggui, R., El Ati-Hellal, M., & Traissac, P. (2021). Prevalence of High HDL Cholesterol and Its Associated Factors Among Tunisian Women of Childbearing Age: A Cross-Sectional Study. International Journal of Environmental Research and Public Health, 18(10), 5461. https://doi.org/10.3390/ijerph18105461







