Comparison of Physical Activity and Sedentary Behaviour between Schoolchildren with Cystic Fibrosis and Healthy Controls: A Gender Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Instruments and Ethical Considerations
2.3. Data Analysis
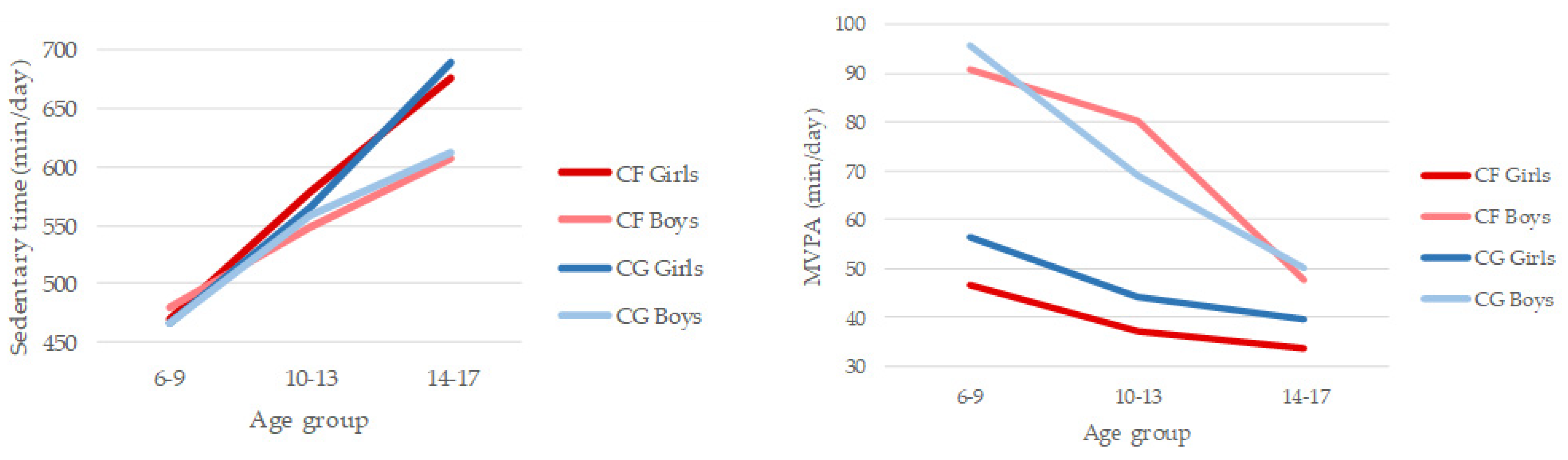
3. Results
3.1. Patterns of Physical Activity and Sedentary Behaviour
3.2. Accomplishment of PA Guidelines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaput, J.P.; Willumsen, J.; Bull, F.; Chou, R.; Ekelund, U.; Firth, J.; Jago, R.; Ortega, F.B.; Katzmarzyk, P.T. 2020 WHO guidelines on physical activity and sedentary behaviour for children and adolescents aged 5–17 years: Summary of the evidence. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 1–9. [Google Scholar] [CrossRef]
- Cystic Fibrosis Foundation. Why Fitness Matters. Available online: http://www.cff.org/Life-With-CF/Daily-Life/Fitness-and-Nutrition/Fitness/Why-Fitness-Matters (accessed on 4 March 2020).
- Wilkes, D.L.; Schneiderman, J.E.; Nguyen, T.; Heale, L.; Moola, F.; Ratjen, F.; Coates, A.L.; Wells, G.D. Exercise and physical activity in children with cystic fibrosis. Paediatr. Respir. Rev. 2009, 10, 105–109. [Google Scholar] [CrossRef]
- Jackson, A.D.; Goss, C.H. Epidemiology of CF: How registries can be used to advance our understanding of the cf population. J. Cyst. Fibros. 2018, 17, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry 2017 Annual Data Report; Bethesda: Rockville, MD, USA, 2018. [Google Scholar]
- Salcedo-Posadas, A.; Girón-Moreno, R.; Beltrán-Bengoechea, B. Complementary therapies in cystic fibrosis: Evidence of therapeutic benefits and treatment recommendations. Pediatr 2003, 58, 39–44. [Google Scholar] [CrossRef]
- Philpott, J.; Houghton, K.; Luke, A. Physical activity recommendations for children with specific chronic health conditions: Juvenile idiopathic arthritis, hemophilia, asthma and cystic fibrosis. Paediatr. Child Health 2010, 15, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Swisher, A.K.; Hebestreit, H.; Mejia-Downs, A.; Lowman, J.D.; Gruber, W.; Nippins, M.; Alison, J.; Schneiderman, J. Exercise and habitual physical activity for people with cystic fibrosis: Expert consensus, evidence-based guide for advising patients. Cardiopulm. Phys. Ther. J. 2015, 26, 85–98. [Google Scholar] [CrossRef]
- Williams, C.A.; Benden, C.; Stevens, D.; Radtke, T. Exercise training in children and adolescents with cystic fibrosis: Theory into practice. Int. J. Pediatr. 2010, 670640. [Google Scholar] [CrossRef] [PubMed]
- McIlwaine, M. Chest physical therapy, breathing techniques and exercise in children with CF. Paediatr. Respir. Rev. 2007, 8, 8–16. [Google Scholar] [CrossRef]
- Hebestreit, A.; Basler, B.; Jeschke, R.; Hebestreit, H. Exercise inhibits epithelial sodium channels in patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2001, 164, 443–446. [Google Scholar] [CrossRef]
- Schneiderman-Walker, J.; Pollock, S.L.; Corey, M.; Wilkes, D.D.; Canny, G.J.; Pedder, L.; Reisman, J.J. A randomized controlled trial of a 3-year home exercise program in cystic fibrosis. J. Pediatr. 2000, 136, 304–310. [Google Scholar] [CrossRef]
- Buntain, H.M.; Greer, R.M.; Schluter, P.J.; Wong, J.C.H.; Batch, J.A.; Potter, J.M.; Lewindon, J.M.; Powell, E.; Wainwright, C.E.; Bell, S.C. Bone mineral density in Australian children, adolescents and adults with cystic fibrosis: A controlled cross sectional study. Thorax 2004, 59, 149–155. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Selvadurai, H.C.; Blimkie, C.J.; Cooper, P.J.; Mellis, C.M.; Van Asperen, P.P. Gender differences in habitual activity in children with cystic fibrosis. Arch. Dis. Child. 2004, 89, 928–933. [Google Scholar] [CrossRef]
- Denford, S.; Cox, N.S.; Mackintosh, K.A.; McNarry, M.A.; O’Halloran, P.; Holland, A.E.; Tomlinson, O.W.; Barker, A.R.; Williams, C.A. Physical activity for cystic fibrosis: Perceptions of people with cystic fibrosis, parents and healthcare professionals. ERJ Open Res. 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Global trends in insufficient physical activity among adolescents: A pooled analysis of 298 population-based surveys with 1.6 million participants. Lancet Child Adolesc. Health 2020, 4, 23–35. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines on Physical Activity and Sedentary Behavior; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Aznar, S.; Gallardo, C.; Fiuza-Luces, C.; Santana-Sosa, E.; López-Mojares, L.M.; Santalla, A.; Rodríguez-Romo, G.; Pérez, M.; Garatachea, N.; Lucia, A. Levels of moderate–vigorous physical activity is low in Spanish children with cystic fibrosis: A comparison with healthy controls. J. Cyst. Fibros. 2014, 13, 335–340. [Google Scholar] [CrossRef]
- Mackintosh, K.A.; Ridgers, N.D.; Evans, R.E.; McNarry, M.A. Physical activity and sedentary time patterns in children and adolescents with cystic fibrosis and age-and sex-matched healthy controls. J. Phys. Act. Health 2018, 15, 82–88. [Google Scholar] [CrossRef]
- Poitras, V.J.; Gray, C.E.; Borghese, M.M.; Carson, V.; Chaput, J.P.; Janssen, I.; Katzmarzyk, P.T.; Pate, R.R.; Connor, S.; Kho, M.E.; et al. Systematic review of the relationships between objectively measured physical activity and health indicators in school-aged children and youth. Appl. Physiol. Nutr. Metab. 2016, 41, S197–S239. [Google Scholar] [CrossRef]
- Tremblay, M.S.; LeBlanc, A.G.; Kho, M.E.; Saunders, T.J.; Larouche, R.; Colley, R.C.; Goldfield, G.; Gorber, S.C. Systematic review of sedentary behaviour and health indicators in school-aged children and youth. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 98. [Google Scholar] [CrossRef]
- Prasad, S.A.; Cerny, F.J. Factors that influence adherence to exercise and their effectiveness: Application to cystic fibrosis. Pediatr. Pulmonol. 2002, 34, 66–72. [Google Scholar] [CrossRef]
- Swisher, A.K.; Erickson, M. Perceptions of physical activity in a group of adolescents with cystic fibrosis. Cardiopulm. Phys. Ther. J. 2008, 19, 107–113. [Google Scholar] [CrossRef]
- Williams, C.A.; Stevens, D. Physical activity and exercise training in young people with cystic fibrosis: Current recommendations and evidence. J. Sport. Health Sci. 2013, 2, 39–46. [Google Scholar] [CrossRef]
- Moola, F.J.; Faulkner, G.E.; Schneiderman, J.E. No time to play: Perceptions toward physical activity in youth with cystic fibrosis. Adapt. Phys. Act. Quart. 2012, 29, 44–62. [Google Scholar] [CrossRef]
- Shields, N.; Synnot, A.J.; Barr, M. Perceived barriers and facilitators to physical activity for children with disability: A systematic review. Br. J. Sports Med. 2012, 46, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Britto, M.T.; Garrett, J.M.; Konrad, T.R.; Majure, J.M.; Leigh, M.W. Comparison of physical activity in adolescents with cystic fibrosis versus age-matched controls. Pediatr. Pulm. 2000, 30, 86–91. [Google Scholar] [CrossRef]
- Nixon, P.A.; Orenstein, D.M.; Kelsey, S.F. Habitual physical activity in children and adolescents with cystic fibrosis. Med. Sci. Sport. Exer. 2001, 33, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Boucher, G.P.; Lands, L.C.; Hay, J.A.; Hornby, L. Activity levels and the relationship to lung function and nutritional status in children with cystic fibrosis. Am. J. Phys. Med. Rehab. 1997, 76, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Jantzen, A.; Opoku-Pare, M.; Bieli, C.; Ruf, K.; Hebestreit, H.; Moeller, A. Perspective on cystic fibrosis and physical activity: Is there a difference compared to healthy individuals? Pediatr. Pulm. 2016, 51, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Mackintosh, K.A.; Ridgers, N.D.; McNarry, M.A. Compensatory changes in physical activity and sedentary time in children and adolescents with cystic fibrosis. J. Sport. Sci. 2019, 37, 1506–1511. [Google Scholar] [CrossRef] [PubMed]
- Schneiderman-Walker, J.; Wilkes, D.L.; Strug, L.; Lands, L.C.; Pollock, S.L.; Selvadurai, H.C.; Hay, J.; Coates, A.L.; Corey, M. Sex differences in habitual physical activity and lung function decline in children with cystic fibrosis. J. Pediatr. 2005, 147, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Currie, C.; Molcho, M.; Boyce, W.; Holstein, B.; Torsheim, T.; Richter, M. Researching health inequalities in adolescents: The development of the Health Behaviour in School-Aged Children (HBSC) Family Affluence Scale. Soc. Sci. Med. 2008, 66, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Freedson, P.; Pober, D.; Janz, K.F. Calibration of Accelerometer Output for Children. Med. Sci. Sport. Exer. 2005, 37, S523–S530. [Google Scholar] [CrossRef]
- Gabel, L.; Ridgers, N.D.; Della Gatta, P.A.; Arundell, L.; Cerin, E.; Robinson, S.; Daly, R.M.; Dunstan, W.; Salmon, J. Associations of sedentary time patterns and TV viewing time with inflammatory and endothelial function biomarkers in children. Pediatr. Obes. 2016, 11, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.M.; Forde, C.; Hussey, J.M.; Gormley, J. Comparison of patterns of physical activity and sedentary behavior between children with cerebral palsy and children with typical development. Phys. Ther. 2015, 95, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Mattocks, C.; Ness, A.; Leary, S.; Tilling, K.; Blair, S.N.; Shield, J.; Deere, K.; Saunders, J.; Kirkby, J.; Davey, G.; et al. Use of accelerometers in a large field-based study of children: Protocols, design issues, and effects on precision. J. Phys. Activ. Health 2008, 5, S98–S111. [Google Scholar] [CrossRef]
- Cardoso, M.; Schmidt, C.J.; Motter, G.; Borba, G.C.; Rech, T.H.; Marostica, P.J.C. The Level of Physical Activity, Lung Function and Exercise Capacity of Children and Adolescents with Cystic Fibrosis Compared to Healthy Controls. Res. Sq. 2020. under review. [Google Scholar] [CrossRef]
- McNarry, M.A.; Stevens, D.; Stone, M.; Roberts, S.; Hall, S.; Mackintosh, K.A. Physical activity, sedentary time and sleep in cystic fibrosis youth: A bidirectional relationship? Pediatr. Pulm. 2021, 56, 450–456. [Google Scholar] [CrossRef]
- Puppo, H.; Torres-Castro, R.; Vasconcello-Castillo, L.; Acosta-Dighero, R.; Sepúlveda-Cáceres, N.; Quiroga-Marabolí, P.; Romero, J.E.; Vilaró, J. Physical activity in children and adolescents with cystic fibrosis: A systematic review and meta-analysis. Pediatr. Pulm. 2020, 55, 2863–2876. [Google Scholar] [CrossRef] [PubMed]
- Mackintosh, K.A.; Evans, R.E.; Barry, M.; Clarke, J.; McNarry, M.A. Physical activity levels of children and adolescents with cystic fibrosis in Wales. J. Cyst. Fibros. 2015, 14, S98. [Google Scholar] [CrossRef]
- Valencia-Peris, A.; Lizandra, J.; Ubeda-Colomer, J.; Pans, M. Physical activity in youth with cystic fibrosis: A brief review. J. Phys. Act. Health 2018, 15, S141. [Google Scholar] [CrossRef]
- Cooper, A.R.; Goodman, A.; Page, A.S.; Sherar, L.B.; Esliger, D.W.; van Sluijs, E.M.F.; Andersen, L.B.; Anderssen, S.; Cardon, G.; Davey, R.; et al. Objectively measured physical activity and sedentary time in youth: The International children’s accelerometry database (ICAD). Int. J. Behav. Nutr. Phys. Act. 2015, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.; Timperio, A.; Salmon, J.; Villanueva, K.; Brown, H.; Esteban-Cornejo, I.; Cabanas-Sánchez, V.; Castro-Piñero, J.; Sánchez-Oliva, D.; Veiga, O.L. Correlates of dual trajectories of physical activity and sedentary time in youth: The UP & DOWN longitudinal study. Scand. J. Med. Sci. Sport 2021. [Google Scholar] [CrossRef]
- Schneiderman, J.E.; Wilkes, D.L.; Atenafu, E.G.; Nguyen, T.; Wells, G.D.; Alarie, N.; Ratjen, F. Longitudinal relationship between physical activity and lung health in patients with cystic fibrosis. Eur. Respir. J. 2014, 43, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Scott, J.L.; Caldwell, L.L. Urban adolescents’ physical activity experience, physical activity levels, and use of screen-based media during leisure time: A structural model. Front. Psychol. 2018, 8, 2317. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Peris, A.; Devís-Devís, J.; García-Massó, X.; Lizandra, J.; Pérez-Gimeno, E.; Peiró-Velert, C. Competing effects between screen media time and physical activity in adolescent girls: Clustering a self-organizing maps analysis. J. Phys. Act. Health 2016, 13, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Lizandra, J.; Devís-Devís, J.; Valencia-Peris, A.; Tomás, J.M.; Peiró-Velert, C. Screen time and moderate-to-vigorous physical activity changes and displacement in adolescence: A prospective cohort study. Eur. J. Sport. Sci. 2019, 19, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Gorely, T.; Marshall, S.J.; Biddle, S.J.; Cameron, N. The prevalence of leisure time sedentary behaviour and physical activity in adolescent girls: An ecological momentary assessment approach. Int. J. Pediatr. Obes. 2007, 2, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Willis, E.; Miller, R.; Wyn, J. Gendered embodiment and survival for young people with cystic-fibrosis. Soc. Sci. Med. 2001, 53, 1163–1174. [Google Scholar] [CrossRef]
- Berge, J.M.; Patterson, J.M.; Goetz, D.; Milla, C. Gender differences in young adults’ perceptions of living with cystic fibrosis during the transition to adulthood: A qualitative investigation. Fam. Syst. Health 2007, 25, 190–203. [Google Scholar] [CrossRef]
- Slater, A.; Tiggemann, M. Gender differences in adolescent sport participation, teasing, self-objectification and body image concerns. J. Adolesc. 2011, 34, 455–463. [Google Scholar] [CrossRef]

| All (N = 89) | CF Group (N = 44) | Control Group (N = 45) | p | |
|---|---|---|---|---|
| Sociodemographic variables | ||||
| Gender (girls), n (%) | 48 (53.9) | 24 (54.5) | 24 (53.3) | 0.909 |
| Age (years), M(SD) | 11.0 (3.1) | 11.0 (3.2) | 11.1 (3.0) | 0.816 |
| Socioeconomic status, M(SD) | 5.4 (1.8) | 5.3 (2.01) | 5.5 (1.7) | 0.503 |
| Anthropometric variables, M(SD) | ||||
| Height, cm | 146.1 (16.8) | 142.4 (16.3) | 149.7 (16.7) | 0.041 |
| Body mass, kg | 40.1 (13.6) | 37.5 (13.6) | 42.6 (13.2) | 0.063 |
| BMI, kg.m−2 | 18.2 (2.9) | 17.9 (3.1) | 18.5 (2.8) | 0.390 |
| BMI, z-score | 0.13 (1.1) | −0.03 (1.2) | 0.28 (1.1) | 0.211 |
| Fat mass, % | 22.0 (7.8) | 22.9 (9.3) | 21.3 (6.3) | 0.433 |
| PA variables, n (%) | ||||
| Organized PA | 68 (76.4) | 32 (72.7) | 36 (80.0) | 0.506 |
| Number of activities/sports | ||||
| None | 18 (20.2) | 10 (22.7) | 8 (17.8) | 0.018 |
| 1 | 39 (43.8) | 13 (29.5) | 26 (57.8) | |
| 2 | 20 (22.5) | 11 (25.0) | 9 (20.0) | |
| ≥3 | 12 (13.5) | 10 (22.7) | 2 (4.4) | |
| Type of activity/sport | ||||
| Swimming | 22 (24.7) | 14 (31.8) | 8 (17.8) | 0.125 |
| Individual sports | 55 (61.8) | 29 (65.9) | 26 (57.8) | 0.430 |
| Team sports | 29 (32.6) | 15 (34.1) | 14 (31.1) | 0.764 |
| Individual and team sports | 15 (16.9) | 12 (27.3) | 3 (6.7) | 0.009 |
| CF Group | Control Group | F | p | η2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| All | Girls | Boys | All | Girls | Boys | ||||
| Sedentary time (min·day−1) | 558 (106) | 574 (96) | 538 (117) | 553 (92) | 566 (99) | 539 (83) | 0.794 | 0.501 | 0.027 |
| Light PA (min·day−1) | 240 (64) | 224 (61) | 260 (64) | 230 (63) | 220 (61) | 241 (64) | 1.710 | 0.171 | 0.057 |
| Moderate PA (min·day−1) | 39 (20) | 30 (13) a,c | 49 (22) a,b | 41 (15) | 35 (10) a,b | 49 (16) a,c | 8.968 | <0.001 | 0.240 |
| Vigorous PA (min·day−1) | 15 (13) | 10 (7) a,c | 20 (17) a,b | 17 (15) | 10 (5) a,b | 26 (17) a,c | 7.855 | <0.001 | 0.217 |
| MVPA (min·day−1) | 54 (31) | 40 (19) a,c | 70 (36) a,b | 59 (27) | 45 (14) a,b | 76 (29) a,c | 10.566 | <0.001 | 0.272 |
| CF Group | Control Group | |||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Gender (boys) | 8.02 | (1.63–39.42) | 9.59 | (1.99–46.28) |
| Age | 0.94 | (0.70–1.24) | 0.88 | (0.61–1.26) |
| Sedentary time (min/day) | 0.66 | (0.44–0.99) | 0.65 | (0.34–1.22) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valencia-Peris, A.; Lizandra, J.; Moya-Mata, I.; Gómez-Gonzalvo, F.; Castillo-Corullón, S.; Escribano, A. Comparison of Physical Activity and Sedentary Behaviour between Schoolchildren with Cystic Fibrosis and Healthy Controls: A Gender Analysis. Int. J. Environ. Res. Public Health 2021, 18, 5375. https://doi.org/10.3390/ijerph18105375
Valencia-Peris A, Lizandra J, Moya-Mata I, Gómez-Gonzalvo F, Castillo-Corullón S, Escribano A. Comparison of Physical Activity and Sedentary Behaviour between Schoolchildren with Cystic Fibrosis and Healthy Controls: A Gender Analysis. International Journal of Environmental Research and Public Health. 2021; 18(10):5375. https://doi.org/10.3390/ijerph18105375
Chicago/Turabian StyleValencia-Peris, Alexandra, Jorge Lizandra, Irene Moya-Mata, Fernando Gómez-Gonzalvo, Silvia Castillo-Corullón, and Amparo Escribano. 2021. "Comparison of Physical Activity and Sedentary Behaviour between Schoolchildren with Cystic Fibrosis and Healthy Controls: A Gender Analysis" International Journal of Environmental Research and Public Health 18, no. 10: 5375. https://doi.org/10.3390/ijerph18105375
APA StyleValencia-Peris, A., Lizandra, J., Moya-Mata, I., Gómez-Gonzalvo, F., Castillo-Corullón, S., & Escribano, A. (2021). Comparison of Physical Activity and Sedentary Behaviour between Schoolchildren with Cystic Fibrosis and Healthy Controls: A Gender Analysis. International Journal of Environmental Research and Public Health, 18(10), 5375. https://doi.org/10.3390/ijerph18105375








