Spatial Uncertainty in Modeling Inhalation Exposure to Volatile Organic Compounds in Response to the Application of Consumer Spray Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Test Chambers
2.3. Exposure Scenarios
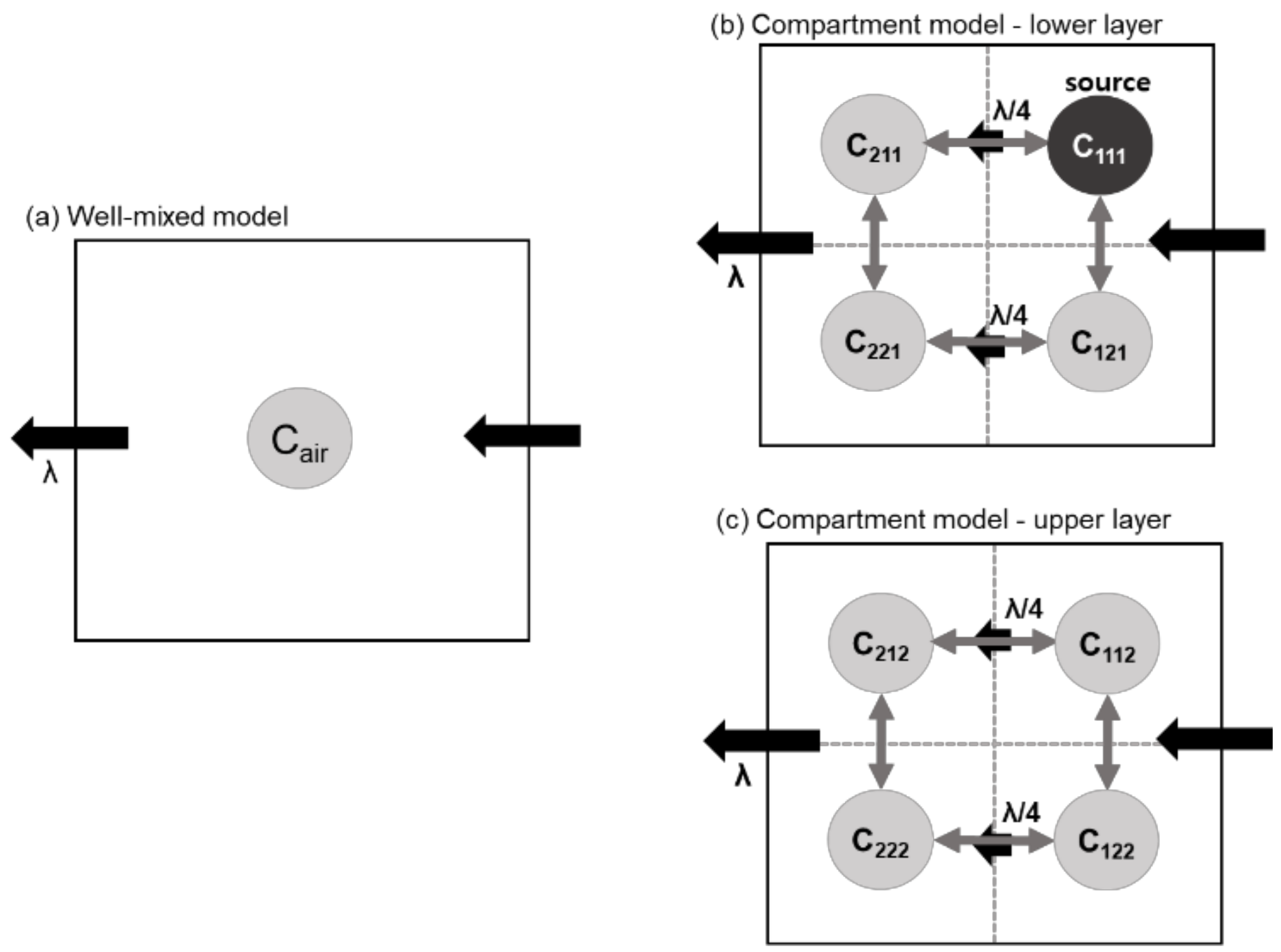
2.4. Modeling
2.5. Real-Time Monitoring of VOCs in the Test Chamber
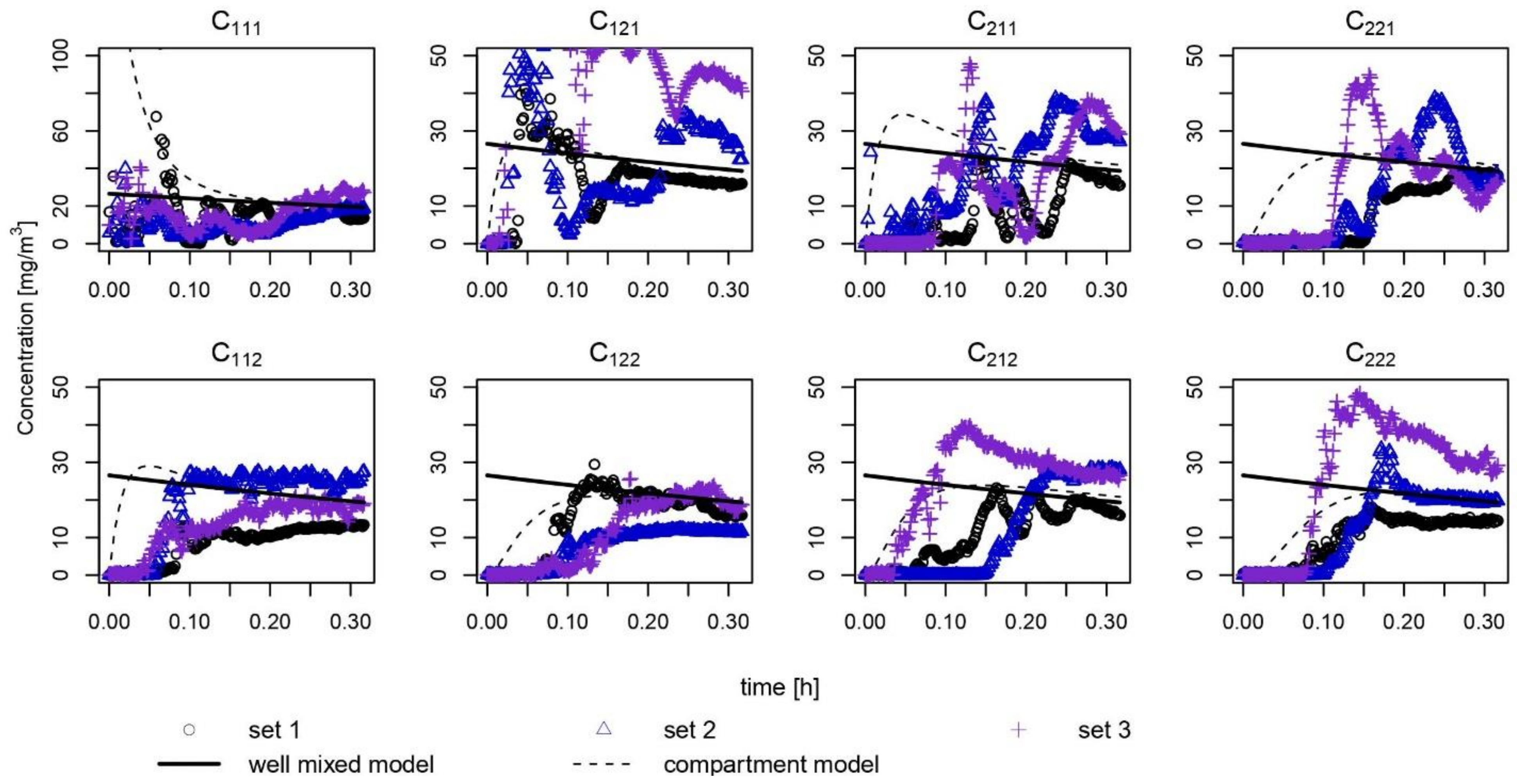
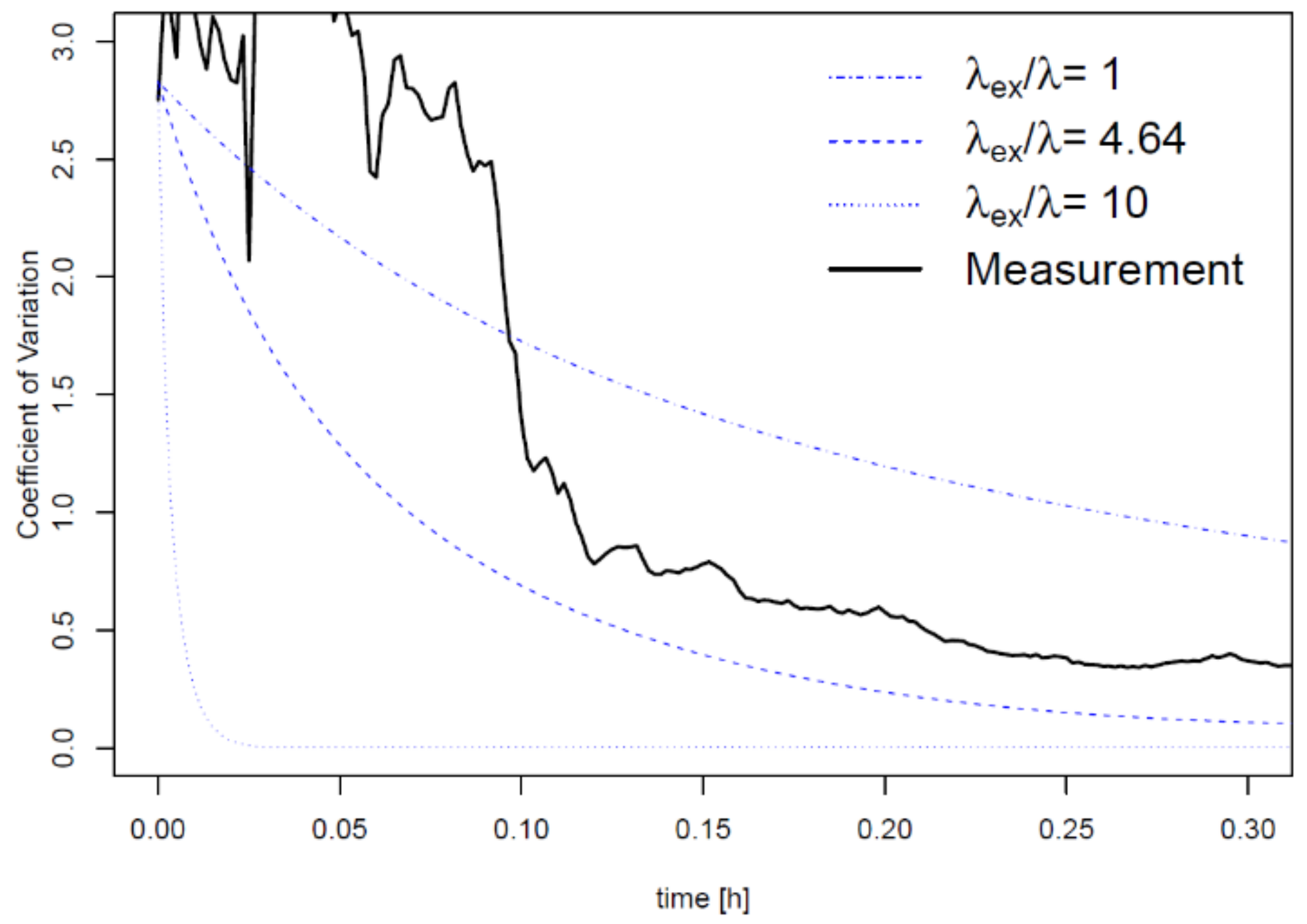
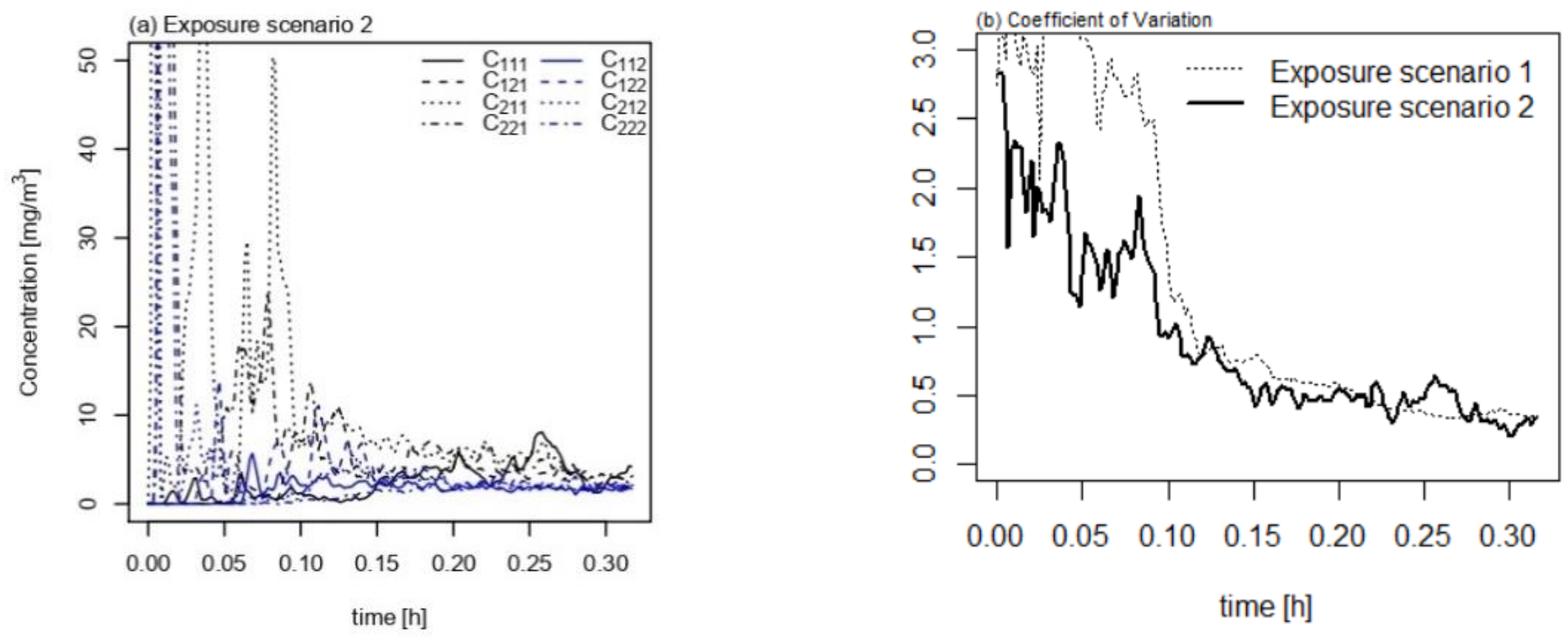
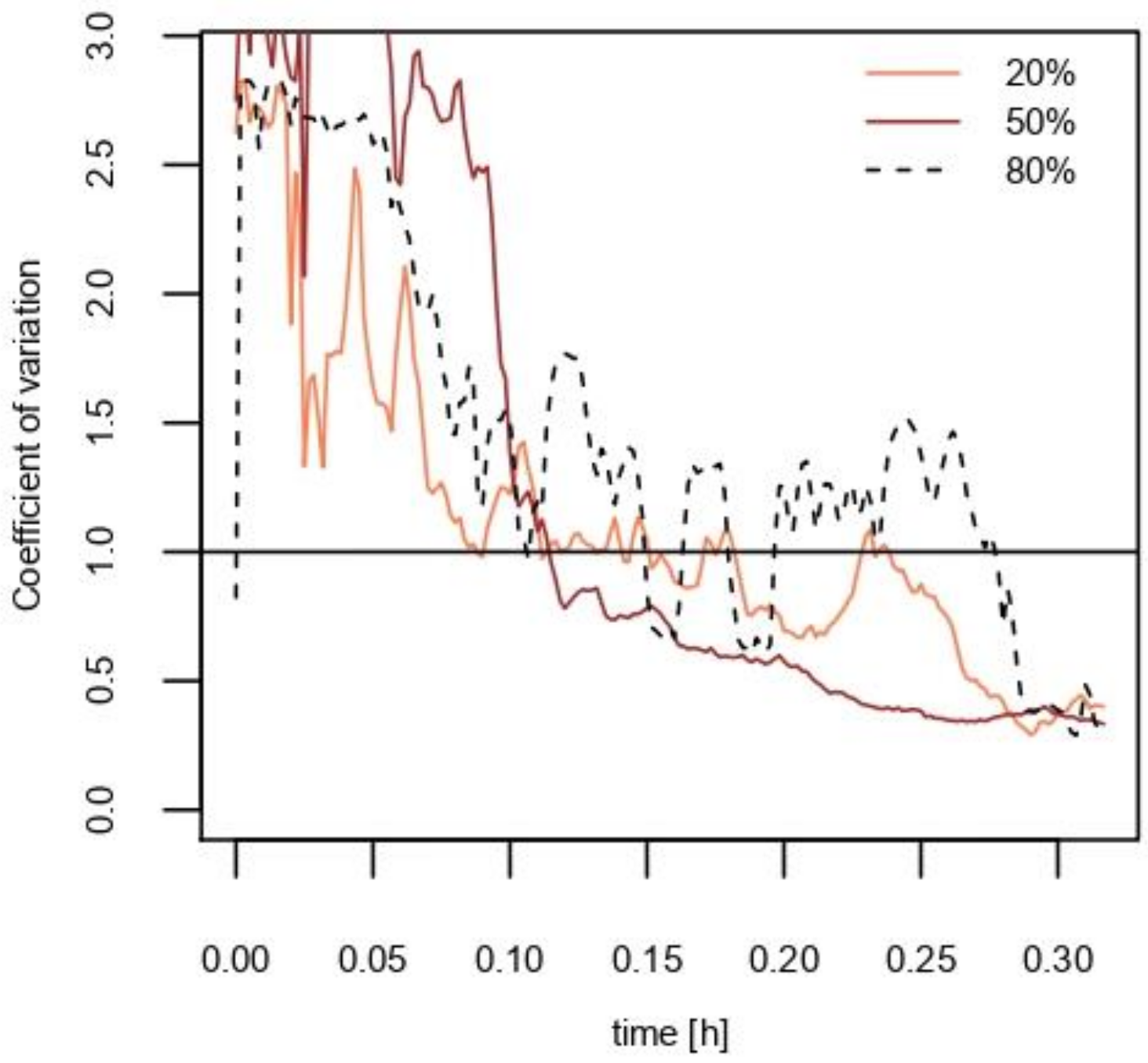
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, B.C.; Coleman, B.K.; Destaillats, H.; Hodgson, A.T.; Lunden, M.M.; Weschler, C.J.; Nazaroff, W.W. Indoor secondary pollutants from cleaning product and air freshener use in the presence of ozone. Atmos. Environ. 2006, 40, 6696–6710. [Google Scholar] [CrossRef]
- Rahman, M.M.; Kim, K.H. Potential hazard of volatile organic compounds contained in household spray products. Atmos. Environ. 2014, 85, 266–274. [Google Scholar] [CrossRef]
- Clausen, P.A.; Mørck, T.A.; Jensen, A.C.Ø.; Schou, T.W.; Kofoed-Sørensen, V.; Koponen, I.K.; Frederiksen, M.; Detmer, A.; Fink, M.; Nørgaard, A.W.; et al. Biocidal spray product exposure: Measured gas, particle, and surface concentrations compared with spray model simulations. J. Occup. Environ. Hyg. 2020, 17, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Mendell, M.J. Indoor residential chemical emissions as risk factors for respiratory and allergic effects in children: A review. Indoor Air 2007, 17, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, C.; Liu, W.; Jin, Y. Potential mechanisms of neurobehavioral disturbances in mice caused by sub-chronic exposure to low-dose VOCs. Inhal. Toxicol. 2014, 26, 250–258. [Google Scholar] [CrossRef]
- Zock, J.P.; Plana, E.; Jarvis, D.; Antó, J.M.; Kromhout, H.; Kennedy, S.M.; Künzli, N.; Villani, S.; Olivieri, M.; Torén, K.; et al. The use of household cleaning sprays and adult asthma: An international longitudinal study. Am. J. Respir. Crit. Care Med. 2007, 176, 735–741. [Google Scholar] [CrossRef]
- Wichmann, F.A.; Müller, A.; Busi, L.E.; Cianni, N.; Massolo, L.; Schlink, U.; Porta, A.; Sly, P.D. Increased asthma and respiratory symptoms in children exposed to petrochemical pollution. J. Allergy Clin. Immun. 2009, 123, 632–638. [Google Scholar] [CrossRef]
- OECD (Organisation for Economic Co-operation and Development). Report on International Consumer Product Safety Risk Assessment Practices. Working Party on Consumer Product Safety, Directorate for Science, Technology, and Innovation Committee on Consumer Policy. 2016. Available online: https://www.oecd.org/sti/consumer/Report%20on%20International%20Consumer%20Product%20Safety%20Risk%20Assessment%20Practices.pdf (accessed on 8 May 2021).
- Delmaar, J.E.; Schuur, A.G. ConsExpo Web, Consumer Exposure Models. Model Documentation, Update for ConsExpo Web 1.0.2. RIVM Report 2017-0197; National Institute for Public Health and the Environment (RIVM): Bilthoven, The Netherlands, 2017.
- Jayjock, M.A.; Chaisson, C.F.; Arnold, S.; Dederick, E.J. Modeling framework for human exposure assessment. J. Expo. Sci. Environ. Epidemiol. 2007, 17, S81–S89. [Google Scholar] [CrossRef] [PubMed]
- Dimitroulopoulou, C.; Ashmore, M.R.; Byrne, M.A.; Kinnersley, R.P. Modelling of indoor exposure to nitrogen dioxide in the UK. Atmos. Environ. 2001, 35, 269–279. [Google Scholar] [CrossRef]
- Milner, J.; Vardoulakis, S.; Chalabi, Z.; Wilkinson, P. Modelling inhalation exposure to combustion-related air pollutants in residential buildings: Application to health impact assessment. Environ. Int. 2011, 37, 268–279. [Google Scholar] [CrossRef]
- Delmaar, C.; Meesters, J. Modeling consumer exposure to spray products: An evaluation of the ConsExpo Web and ConsExpo nano models with experimental data. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Rosemberg, M.-A.; Li, W.; Meza-Wilson, E.; Godwin, C.; Batterman, S. Occupational exposure and health risks of volatile organic compounds (vocs) of hotel housekeepers field measurements of exposure and health risks. Indoor Air 2020, 31, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ye, M.; He, B.J. CFD simulation research on residential indoor air quality. Sci. Total Environ. 2014, 472, 1137–1144. [Google Scholar] [CrossRef]
- US EPA (United States Environmental Protection Agency) Consumer Exposure Model (CEM) Version 2.0 User’s Guide. 2017. Available online: https://www.epa.gov/tsca-screening-tools/consumer-exposure-model-cem-version-20-users-guide (accessed on 12 April 2021).
- Hofstetter, E.; Spencer, J.W.; Hiteshew, K.; Coutu, M.; Nealley, M. Evaluation of recommended reach exposure modeling tools and near-field, far-field model in assessing occupational exposure to toluene from spray paint. Ann. Occup. Hyg. 2013, 57, 210–220. [Google Scholar] [PubMed]
- Park, S.-K.; Seol, H.-S.; Park, H.-J.; Kim, Y.-S.; Ryu, S.-H.; Kim, J.; Kim, S.; Lee, J.-H.; Kwon, J.-H. Experimental determination of indoor air concentration of 5-chloro-2-methylisothiazol-3(2H)-one/2-methylisothiazol-3(2H)-one (CMIT/MIT) emitted by the use of humidifier disinfectant. Environ. Anal. Health Toxicol. 2020, 35, e2020008. [Google Scholar] [CrossRef]
- American Society for Testing and Materials (ASTM). Standard Practice for Full-Scale Chamber Determination of Volatile Organic Emissions from Indoor Material/Products. 2007. Available online: https://www.astm.org/Standards/D6670.htm (accessed on 6 June 2019).
- US EPA (United States Environmental Protection Agency). Large Chamber Test Protocol for Measuring Emissions of VOCs and Aldehydes; United States Environmental Protection Agency: Research Triangle Park, NC, USA, 1999.
- Soetaert, K.; Petzoldt, T.; Setzer, R. Solving Differential Equations in R: Package deSolve. J. Stat. Softw. 2010, 33, 1–25. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R. 2015. Available online: https://www.rstudio.com/products/rstudio/download/ (accessed on 18 January 2019).
- Amador-Muñoz, O.; Misztal, P.K.; Weber, R.; Worton, D.R.; Zhang, H.; Drozd, G.; Goldstein, A.H. Sensitive detection of n-alkanes using a mixed ionization mode proton-transfer-reaction mass spectrometer. Atmos. Meas. Tech. 2016, 9, 5315–5329. [Google Scholar] [CrossRef]
- Warneke, C.; Veres, P.; Murphy, S.M.; Soltis, J.; Field, R.A.; Graus, M.G.; Koss, A.; Li, S.-M.; Li, R.; Yuan, B.; et al. PTR-QMS versus PTR-TOF comparison in a region with oil and natural gas extraction industry in the Uintah Basin in 2013. Atmos. Meas. Tech. 2015, 8, 411–420. [Google Scholar] [CrossRef]
- Hewitt, C.N.; Hayward, S.; Tani, A. The application of proton transfer reaction- mass spectrometry (PTR-MS) to the monitoring and analysis of volatile organic compounds in the atmosphere. J. Environ. Monit. 2002, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Taipale, R.; Ruuskanen, T.M.; Rinne, J.; Kajos, M.K.; Hakola, H.; Pohja, T.; Kulmala, M. Technical note: Quantitative long-term measurements of VOC concentrations by PTR-MS—Measurement, calibration, and volume mixing ratio calculation methods. Atmos. Chem. Phys. 2008, 8, 6681–6698. [Google Scholar] [CrossRef]
- Nielson, P.V. Numerical Predictions of Air Distribution in Rooms—Status and Potentials: Status and Potentials; Indoor Environmental Technology; Department of Building Technology and Structural Engineering, Aalborg University: Aalborg, Denmark, 1988; Volume R8823. [Google Scholar]
- Srebric, J.; Vukovic, V.; He, G.; Yang, X. CFD boundary conditions for contaminant dispersion, heat transfer and airflow simulations around human occupants in indoor environments. Build. Environ. 2008, 43, 294–303. [Google Scholar] [CrossRef]
- Hagendorfer, H.; Lorenz, C.; Kaegi, R.; Sinnet, B.; Gehrig, R.; Goetz, N.V.; Scheringer, M.; Ludwig, C.; Ulrich, A. Size-fractionated characterization and quantification of nanoparticle release rates from a consumer spray product containing engineered nanoparticles. J. Nanopart. Res. 2010, 12, 2481–2494. [Google Scholar] [CrossRef]
- Nazarenko, Y.; Han, T.W.; Lioy, P.J.; Mainelis, G. Potential for exposure to engineered nanoparticles from nanotechnology-based consumer spray products. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Quadros, M.E.; Marr, L.C. Silver nanoparticles and total aerosols emitted by nanotechnology-related consumer spray products. Environ. Sci. Technol. 2011, 45, 10713–10719. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Ham, S.; Jang, M.; Lee, J.; Kim, S.; Kim, S.; Lee, K.; Park, D.; Kwon, J.; Kim, H.; et al. Spatial-temporal dispersion of aerosolized nanoparticles during the use of consumer spray products and estimates of inhalation exposure. Environ. Sci. Technol. 2017, 51, 7624–7638. [Google Scholar] [CrossRef]
- Park, J.; Ham, S.; Kim, S.; Jang, M.; Lee, J.; Kim, S.; Park, D.; Lee, K.; Kim, H.; Kim, P.; et al. Physicochemical characteristics of colloidal nanomaterial suspensions and aerosolized particulates from nano-enabled consumer spray products. Indoor Air 2020, 30, 925–941. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.A. Activities of Aliphatic Acids and Alcohols in Binary Aqueous Solutions. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 1953. [Google Scholar]
- Posner, J.D.; Buchanan, C.R.; Dunn-Rankin, D. Measurement and prediction of indoor air flow in a model room. Energy Build. 2003, 35, 515–526. [Google Scholar] [CrossRef]
- Lim, E.; Sagara, K.; Yamanaka, T.; Kotani, H. CFD analysis of airflow characteristics inside office room with hybrid air-conditioning system. In Proceedings of the 29th AIVC Conference, Advanced Building Ventilation and Environmental Technology for Addressing Climate Change Issues, Kyoto, Japan, 14–16 October 2008; pp. 89–94. [Google Scholar]
- Hagyard, J.; Brimmell, J.; Edwards, E.J.; Vaughan, R.S. Inhibitory control across athletic expertise and its relationship with sport performance. J. Sport Exerc. Psychol. 2021, 43, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Salthammer, T.; Schripp, T. Application of the Junge- and Pankow-equation for estimating indoor gas/particle distribution and exposure to SVOCs. Atmos. Environ. 2015, 106, 467–476. [Google Scholar] [CrossRef]

| Spray Type | Chemical | Exposure Duration (h) | TWA Exposure (mg m−3) | |||
|---|---|---|---|---|---|---|
| Real-Time Measurement in Compartments | Well-Mixed Modeling | |||||
| C111 | C112 | C222 | ||||
| Trigger | Ethanol | 0.1 | 40.1 | 4.9 | 1.7 | 25.2 |
| 0.3 | 22.3 | 13.4 | 16.1 | 22.9 | ||
| Propellant | n-butane | 0.1 | 0.91 | 1.0 | 10.3 | - |
| 0.3 | 2.3 | 1.7 | 4.5 | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, Y.; Kim, Y.; Seol, H.-S.; Lee, J.-H.; Kwon, J.-H. Spatial Uncertainty in Modeling Inhalation Exposure to Volatile Organic Compounds in Response to the Application of Consumer Spray Products. Int. J. Environ. Res. Public Health 2021, 18, 5334. https://doi.org/10.3390/ijerph18105334
Jung Y, Kim Y, Seol H-S, Lee J-H, Kwon J-H. Spatial Uncertainty in Modeling Inhalation Exposure to Volatile Organic Compounds in Response to the Application of Consumer Spray Products. International Journal of Environmental Research and Public Health. 2021; 18(10):5334. https://doi.org/10.3390/ijerph18105334
Chicago/Turabian StyleJung, Yerin, Yoonsub Kim, Hwi-Soo Seol, Jong-Hyeon Lee, and Jung-Hwan Kwon. 2021. "Spatial Uncertainty in Modeling Inhalation Exposure to Volatile Organic Compounds in Response to the Application of Consumer Spray Products" International Journal of Environmental Research and Public Health 18, no. 10: 5334. https://doi.org/10.3390/ijerph18105334
APA StyleJung, Y., Kim, Y., Seol, H.-S., Lee, J.-H., & Kwon, J.-H. (2021). Spatial Uncertainty in Modeling Inhalation Exposure to Volatile Organic Compounds in Response to the Application of Consumer Spray Products. International Journal of Environmental Research and Public Health, 18(10), 5334. https://doi.org/10.3390/ijerph18105334






