Scutellaria baicalensis and Their Natural Flavone Compounds as Potential Medicinal Drugs for the Treatment of Nicotine-Induced Non-Small-Cell Lung Cancer and Asthma
Abstract
1. Introduction
2. Methods
3. In Vitro Effects of Nicotine on NSCLC Development
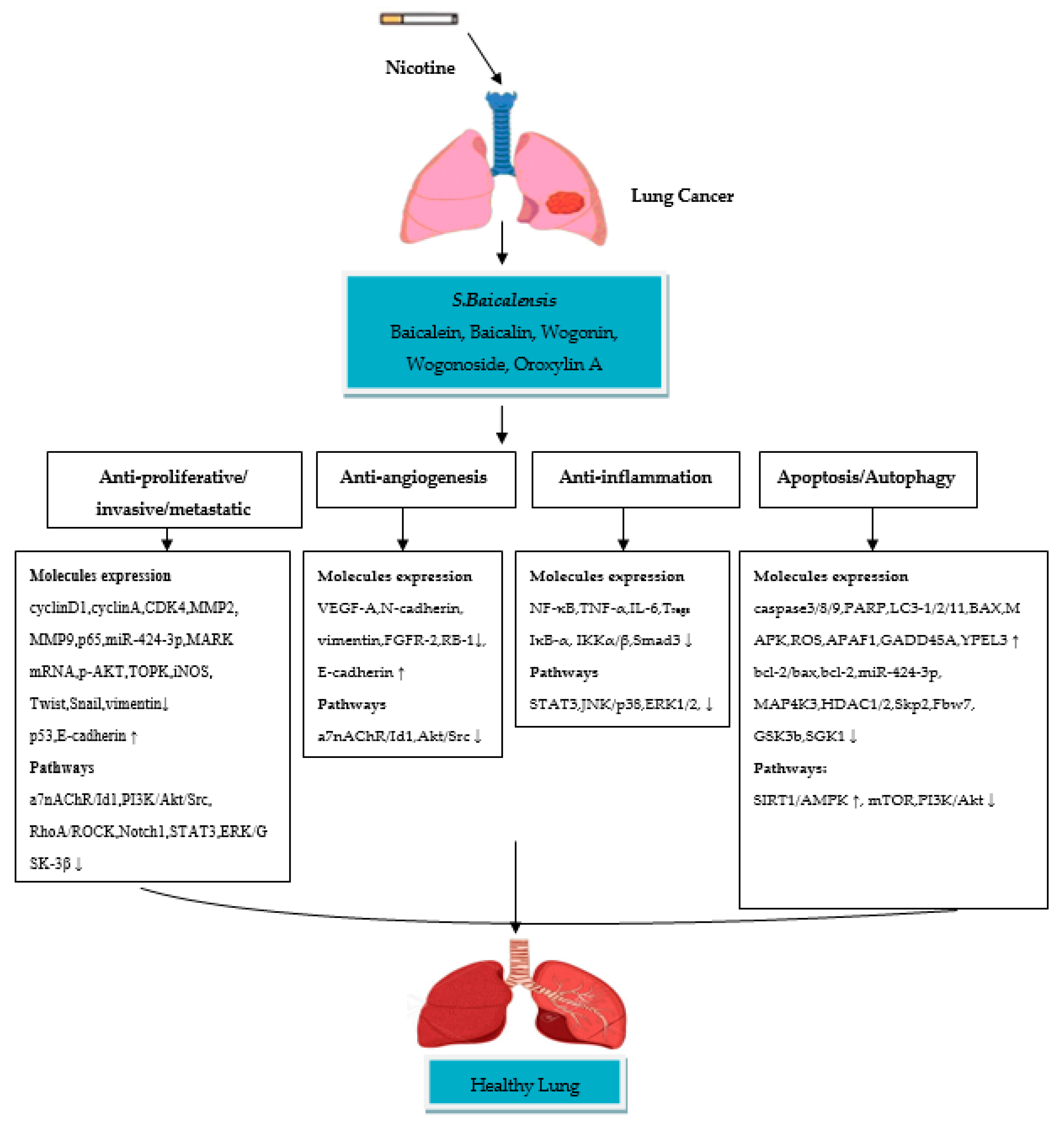
4. Therapeutic Role of Scutellaria baicalensis and Their Flavone Compounds in Nicotine-Induced NSCLC
4.1. Baicalein
4.2. Baicalin
4.3. Wogonin
4.4. Wogonoside
4.5. Oroxylin A
5. In Vitro Effects of Nicotine on Asthma Development
6. Therapeutic Role of Scutellaria baicalensis and Their Flavone Compounds in Nicotine-Induced Asthma
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Jacques, F.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Dillekås, H.; Rogers, M.S.; Straume, O. Are 90% of deaths from cancer caused by metastases? Cancer Med. 2019, 8, 5574–5576. [Google Scholar] [CrossRef]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Institute for Health Metrics and Evaluation. Global Burden of Disease. 2017. Available online: http://vizhub.healthdata.org/gbd-compare/# (accessed on 2 February 2020).
- Sanner, T.; Grimsrud, T.K. Nicotine: Carcinogenicity and effects on response to cancer treatment—A review. Front. Oncol. 2015, 5, 196. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S. Lung carcinogenesis by tobacco smoke. Int. J. Cancer 2012, 131, 2724–2732. [Google Scholar] [CrossRef] [PubMed]
- Warren, G.W.; Singh, A.K. Nicotine and lung cancer. J. Carcinog. 2013, 12, 1. [Google Scholar] [CrossRef]
- Nagathihalli, N.S.; Massion, P.P.; Gonzalez, A.L.; Lu, P.; Datta, P.K. Smoking induces epithelial-to-mesenchymal transition in non-small cell lung cancer through HDAC-mediated downregulation of E-cadherin. Mol. Cancer Ther. 2012, 11, 2362–2372. [Google Scholar] [CrossRef]
- Vu, T.; Jin, L.; Datta, P.K. Effect of cigarette smoking on epithelial to mesenchymal transition (EMT) in lung cancer. J. Clin. Med. 2016, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, A.; Phandthong, R.; Chaili, A.; Lemark, G.; Talbot, P. Epithelial-to-mesenchymal transition of A549 lung cancer cells exposed to electronic cigarettes. Lung Cancer 2018, 122, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.; Ma, N.; Wang, Y.; Li, H.; Liu, X.; Su, Y.; Yang, J. Anti-PD-1/PD-L1 therapy for non-small-cell lung cancer: Toward personalized medicine and combination strategies. J. Immunol. Res. 2018, 2018, 6984948. [Google Scholar] [CrossRef] [PubMed]
- Neeve, S.C.; Robinson, B.W.; Fear, V.S. The role and therapeutic implications of T cells in cancer of the lung. Clin. Transl. Immunol. 2019, 8, e1076. [Google Scholar] [CrossRef]
- Palata, O.; Hradilova, N.P.; Mysiková, D.; Kutna, B.; Mrazkova, H.; Lischke, R.; Spisek, R.; Adkins, I. Detection of tumor antigens and tumor-antigen specific T cells in NSCLC patients: Correlation of the quality of T cell responses with NSCLC subtype. Immunol. Lett. 2020, 219, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Doeing, D.C.; Solway, J. Airway smooth muscle in the pathophysiology and treatment of asthma. J. Appl. Physiol. 2013, 114, 834–843. [Google Scholar] [CrossRef]
- Skaaby, T.; Taylor, A.E.; Jacobsen, R.K.; Paternoster, L.; Thuesen, B.H.; Ahluwalia, T.S.; Larsen, S.C.; Zhou, A.; Wong, A.; Gabrielsen, M.E.; et al. Investigating the causal effect of smoking on hay fever and asthma: A Mendelian randomization meta-analysis in the CARTA consortium. Sci. Rep. 2017, 7, 2224. [Google Scholar] [CrossRef] [PubMed]
- McLeish, A.C.; Cougle, J.R.; Zvolensky, M.J. Asthma and cigarette smoking in a representative sample of adults. J. Health Psychol. 2011, 16, 643–652. [Google Scholar] [CrossRef]
- Qu, Y.L.; Liu, J.; Zhang, L.X.; Wu, C.M.; Chu, A.J.; Wen, B.L.; Ma, C.; Yan, X.Y.; Zhang, X.; Wang, D.M.; et al. Asthma and the risk of lung cancer: A meta-analysis. Oncotarget 2017, 8, 48525. [Google Scholar] [CrossRef]
- Cheng, B.; Xiong, S.; Li, C.; Liang, H.; Zhao, Y.; Li, J.; Shi, J.; Ou, L.; Chen, Z.; Liang, P.; et al. An annual review of the remarkable advances in lung cancer clinical research in 2019. J. Thorac. Dis. 2020, 12, 1056–1069. [Google Scholar] [CrossRef] [PubMed]
- Nasser, N.J.; Gorenberg, M.; Agbarya, A. First line immunotherapy for non-small cell lung cancer. Pharmaceuticals 2020, 13, 373. [Google Scholar] [CrossRef]
- Dafni, U.; Tsourti, Z.; Vervita, K.; Peters, S. Immune checkpoint inhibitors, alone or in combination with chemotherapy, as first-line treatment for advanced non-small cell lung cancer. A systematic review and network meta-analysis. Lung Cancer 2019, 134, 127–140. [Google Scholar] [CrossRef]
- Alsharairi, N.A. Supplements for smoking-related lung diseases. Encyclopedia 2021, 1, 76–86. [Google Scholar] [CrossRef]
- Alsharairi, N. The effects of dietary supplements on asthma and lung cancer risk in smokers and non-smokers: A review of the literature. Nutrients 2019, 11, 725. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Chahar, M.K.; Sharma, N.; Dobhal, M.P.; Joshi, Y.C. Flavonoids: A versatile source of anticancer drugs. Pharm. Rev. 2011, 5, 1–12. [Google Scholar]
- Zanoaga, O.; Braicu, C.; Jurj, A.; Rusu, A.; Buiga, R.; Berindan-Neagoe, I. Progress in research on the role of flavonoids in lung cancer. Int. J. Mol. Sci. 2019, 20, 4291. [Google Scholar] [CrossRef] [PubMed]
- Masraksa, W.; Tanasawet, S.; Hutamekalin, P.; Wongtawatchai, T.; Sukketsiri, W. Luteolin attenuates migration and invasion of lung cancer cells via suppressing focal adhesion kinase and non-receptor tyrosine kinase signaling pathway. Nutr. Res. Pract. 2020, 14, 127–133. [Google Scholar] [CrossRef]
- Weng, Z.; Patel, A.B.; Panagiotidou, S.; Theoharides, T.C. The novel flavone tetramethoxyluteolin is a potent inhibitor of human mast cells. J. Allergy Clin. Immunol. 2015, 135, 1044–1052.e5. [Google Scholar] [CrossRef]
- Rouhi-Boroujeni, H.; Heidarian, E.; Rouhi-Boroujeni, H.; Deris, F.; Rafieian-Kopaei, M. Medicinal plants with multiple effects on cardiovascular diseases: A systematic review. Curr. Pharm. Des. 2017, 23, 999–1015. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Lankatillake, C.; Dias, D.A.; Docea, A.O.; Mahomoodally, M.F.; Lobine, D.; Chazot, P.L.; Kurt, B.; Tumer, T.B.; Moreira, A.C.; et al. Impact of natural compounds on neurodegenerative disorders: From preclinical to pharmacotherapeutics. J. Clin. Med. 2020, 9, 1061. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, A.; Miao, J.; Sun, H.; Yan, G.; Wu, F.; Wang, X. Applications and potential mechanisms of herbal medicines for rheumatoid arthritis treatment: A systematic review. RSC Adv. 2019, 9, 26381. [Google Scholar] [CrossRef]
- Amaral-Machado, L.; Oliveira, W.N.; Moreira-Oliveira, S.S.; Pereira, D.T.; Alencar, E.N.; Tsapis, N.; Egito, E.S.T. Use of natural products in asthma treatment. Evid. Based Complement. Altern. Med. 2020, 2020, 1021258. [Google Scholar] [CrossRef] [PubMed]
- De Sousa Monteiro, L.; Bastos, K.X.; Barbosa-Filho, J.M.; de Athayde-Filho, P.F.; Diniz, M.D.; Sobral, M.V. Medicinal plants and other living organisms with antitumor potential against lung cancer. Evid. Based Complement. Altern. Med. 2014, 2014, 604152. [Google Scholar]
- Farzaei, M.H.; Bayrami, Z.; Farzaei, F.; Aneva, I.; Das, S.K.; Patra, J.K.; Das, G.; Abdollahi, M. Poisoning by medical plants. Arch. Iran. Med. 2020, 23, 117–127. [Google Scholar] [PubMed]
- Mali, R.G.; Dhake, A.S. A review on herbal antiasthmatics. Orient. Pharm. Exp. Med. 2011, 11, 77–90. [Google Scholar] [CrossRef]
- Taur, D.J.; Patil, R.Y. Some medicinal plants with antiasthmatic potential: A current status. Asian Pac. J. Trop. Biomed. 2011, 1, 413–418. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, X.; Martin, C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci. Bull. 2016, 61, 1391–1398. [Google Scholar] [CrossRef]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Kuang, Y.; Hu, Z.; Qiao, X.; Ye, M. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm. Biol. 2018, 56, 465–484. [Google Scholar] [CrossRef]
- Khan, T.; Ali, M.; Khan, A.; Nisar, P.; Jan, S.A.; Afridi, S.; Shinwari, Z.H. Anticancer plants: A review of the active phytochemicals, applications in animal models, and regulatory aspects. Biomolecules 2019, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, B.; Sun, J.; Lu, L.; Liu, L.; Qiu, J.; Li, Q.; Yan, C.; Jiang, S.; Mohammadtursun, N.; et al. Scutellaria flavonoids effectively inhibit the malignant phenotypes of non-small cell lung cancer in an Id1-dependent manner. Int. J. Biol. Sci. 2019, 15, 1500–1513. [Google Scholar] [CrossRef] [PubMed]
- Cathcart, M.; Useckaite, Z.; Drakeford, C.; Semik, V.; Lysaght, J.; Gately, K.; O’Byrne, K.J.; Pidgeon, G.P. Anti-cancer effects of baicalein in non-small cell lung cancer in-vitro and in-vivo. BMC Cancer 2016, 16, 707. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, B.; Sun, J.; Lu, L.; Liu, L.; Qiu, J.; Li, Q.; Yan, C.; Jiang, S.; Mohammadtursun, N.; et al. Baicalein inhibits orthotopic human non-small cell lung cancer xenografts via Src/Id1 pathway. Evid. Based Complement. Altern. Med. 2019, 2019, 9806062. [Google Scholar] [CrossRef] [PubMed]
- Schaal, C.; Chellappan, S.P. Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol. Cancer Res. 2014, 12, 14–23. [Google Scholar] [CrossRef]
- Chernyavsky, A.I.; Shchepotin, I.B.; Galitovkiy, V.; Grando, S.A. Mechanisms of tumor-promoting activities of nicotine in lung cancer: Synergistic effects of cell membrane and mitochondrial nicotinic acetylcholine receptors. BMC Cancer 2015, 15, 152. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hu, Y. α7 nicotinic acetylcholine receptors in lung cancer (Review). Oncol. Lett. 2018, 16, 1375–1382. [Google Scholar] [CrossRef]
- Yuan, X.; Wu, H.; Han, N.; Xu, H.; Chu, Q.; Yu, S.; Chen, Y.; Wu, K. Notch signaling and EMT in non-small cell lung cancer: Biological significance and therapeutic application. J. Hematol. Oncol. 2014, 7, 87. [Google Scholar] [CrossRef]
- Xiao, D.; He, J. Epithelial mesenchymal transition and lung cancer. J. Thorac. Dis. 2010, 2, 154–159. [Google Scholar]
- Cheng, W.; Chen, K.; Lee, K.; Feng, P.; Wu, S. Nicotinic-nAChR signaling mediates drug resistance in lung cancer. J. Cancer 2020, 11, 1125–1140. [Google Scholar] [CrossRef] [PubMed]
- Hajiasgharzadeh, K.; Sadigh-Eteghad, S.; Mansoori, B.; Mokhtarzadeh, A.; Shanehbandi, D.; Doustvandi, M.A.; Asadzadeh, Z.; Baradaran, B. Alpha7 nicotinic acetylcholine receptors in lung inflammation and carcinogenesis: Friends or foes? J. Cell Physiol. 2019, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Park, K.I.; Park, H.S.; Kang, S.R.; Nagappan, A.; Lee, D.H.; Kim, J.A.; Han, D.Y.; Kim, G.S. Korean Scutellariabaicalensis water extract inhibits cell cycle G1/S transition by suppressing cyclin D1 expression and matrix-metalloproteinase-2 activity in human lung cancer cells. J. Ethnopharmacol. 2011, 133, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Hong, S.H.; Ku, J.O.; Lim, Y.S.; Lee, S.J.; Song, J.; Kim, T.Y.; Cheon, C.; Ko, S. Scutellaria radix promotes apoptosis in non-small cell lung cancer cells via induction of AMPK-dependent autophagy. Am. J. Chin. Med. 2019, 47, 691–705. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, H.-J.; Sun, S.-J.; Dai, J.-Y.; Fang, J.-W.; Li, Q.-H.; Yan, C.; Mao, W.-W.; Zhang, Y.-Y. Total flavonoid aglycones extract in Radix Scutellariae, inhibits lung carcinoma and lung metastasis by affecting cell cycle and DNA synthesis. J. Ethnopharmacol. 2016, 194, 269–279. [Google Scholar] [CrossRef]
- Gao, J.; Morgan, W.A.; Sanchez-Medina, A.; Corcoran, O. The ethanol extract of Scutellariabaicalensis and the active compounds induce cell cycle arrest and apoptosis including upregulation of p53 and Bax in human lung cancer cells. Toxicol. Appl. Pharmacol. 2011, 254, 221–228. [Google Scholar] [CrossRef]
- Gong, W.; Wu, J.; Liu, B.; Zhang, H.; Cao, Y.; Sun, J.; Lv, Y.; Wu, X.; Dong, J. Flavonoid components in Scutellariabaicalensis inhibit nicotine-induced proliferation, metastasis and lung cancer-associated inflammation in vitro. Int. J. Oncol. 2014, 44, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wang, H.; Chen, S.; Yang, R.; Li, H.; Zhang, G. Baicalein inhibits cell growth and increases cisplatin sensitivity of A549 and H460 cells via miR-424-3p and targeting PTEN/PI3K/Akt pathway. J. Cell. Mol. Med. 2018, 22, 2478–2487. [Google Scholar] [CrossRef]
- Zhang, Z.; Nong, L.; Chen, M.; Gu, X.; Zhao, W.; Liu, M.; Cheng, W. Baicalein suppresses vasculogenic mimicry through inhibiting RhoA/ROCK expression in lung cancer A549 cell line. Acta Biochim. Biophys. Sin. 2020, 52, 1007–1015. [Google Scholar] [CrossRef]
- Li, J.; Yan, L.; Luo, J.; Tong, L.; Gao, Y.; Feng, W.; Wang, F.; Cui, W.; Li, S.; Sun, Z. Baicalein suppresses growth of non-small cell lung carcinoma by targeting MAP4K3. Biomed. Pharmacother. 2021, 133, 110965. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ruan, Q.; Zhai, Y.; Lu, D.; Li, C.; Fu, Y.; Zheng, Z.; Song, Y.; Guo, J. Baicalein inhibits non-small-cell lung cancer invasion and metastasis by reducing ezrin tension in inflammation microenvironment. Cancer Sci. 2020, 111, 3802–3812. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Chen, H.; Sun, X. Baicalein suppresses non small cell lung cancer cell proliferation, invasion and Notch signaling pathway. Cancer Biomark. 2018, 22, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Wang, R.; Zheng, X.; Li, N.; Li, H.; Cao, X.; Zhou, B.; Lin, Y.; Yang, L. Baicalin potentiates TRAIL-induced apoptosis through p38 MAPK activation and intracellular reactive oxygen species production. Mol. Med. Rep. 2017, 16, 8549–8555. [Google Scholar] [CrossRef]
- Xu, Z.; Mei, J.; Tan, Y. Baicalin attenuates DDP (cisplatin) resistance in lung cancer by downregulating MARK2 and p-Akt. Int. J. Oncol. 2017, 50, 93–100. [Google Scholar] [CrossRef]
- Diao, X.; Yang, D.; Chen, Y.; Liu, W. Baicalin suppresses lung cancer growth by targeting PDZ-binding kinase/T-LAK cell-originated protein kinase. Biosci. Rep. 2019, 39, BSR20181692. [Google Scholar] [CrossRef]
- You, J.; Cheng, J.; Yu, B.; Duan, C.; Peng, J. Baicalin a Chinese herbal medicine, inhibits the proliferation and migration of human non-small cell lung carcinoma (NSCLC) cells, A549 and H1299, by activating the SIRT1/AMPK signaling pathway. Med. Sci. Monit. 2018, 24, 2126–2133. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Wang, Q.; Zheng, X.; Yan, J.; Yang, L.; Sun, H.; Hu, L.; Lin, Y.; Wang, X. Wogonin potentiates cisplatin-induced cancer cell apoptosis through accumulation of intracellular reactive oxygen species. Oncol. Rep. 2012, 28, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yao, J.; Wu, X.; Zhao, L.; Zhou, Y.; Zhang, Y.; You, Q.; Guo, Q.; Lu, N. Wogonin suppresses human alveolar adenocarcinoma cell A549 migration in inflammatory microenvironment by modulating the IL-6/STAT3 signaling pathway. Mol. Carcinog. 2015, 1, E81–E93. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, J.; Ren, S.; Sun, W.; Zhang, W.; Zhang, J. Wogonin affects proliferation and the energy metabolism of SGC-7901 and A549 cells. Exp. Ther. Med. 2019, 17, 911–918. [Google Scholar] [CrossRef]
- Wang, C.; Cui, C. Inhibition of lung cancer proliferation by wogonin is associated with activation of apoptosis and generation of reactive oxygen species. Balk. Med. J. 2019, 37, 29–33. [Google Scholar] [CrossRef]
- Chen, X.; Bai, Y.; Zhong, Y.; Xie, X.; Long, H.; Yang, Y.; Wu, S.; Jia, Q.; Wang, X. Wogonin has multiple anti-cancer effects by regulating c-Myc/SKP2/Fbw7α and HDAC1/HDAC2 pathways and inducing apoptosis in human lung adenocarcinoma cell line A549. PLoS ONE 2013, 8, e79201. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Wang, Q.; Zhou, X.; Li, J.; Liu, H.; Gu, J.; Wang, H.; Wu, Y.; Ding, L.; Ni, S.; et al. Response of human non-small-cell lung cancer cells to the influence of Wogonin with SGK1 dynamics. Acta Biochim. Biophys. Sin. 2017, 49, 302–310. [Google Scholar] [CrossRef]
- Luo, M.; Mo, J.; Yu, Q.; Zhou, S.; Ning, R.; Zhang, Y.; Su, C.; Wang, H.; Cui, J. Wogonoside induces apoptosis in human non-small cell lung cancer A549 cells by promoting mitochondria dysfunction. Biomed. Pharmacother. 2018, 106, 593–598. [Google Scholar] [CrossRef]
- Wang, C.; Wan, J.; Zhang, C.; Lu, F.; Chen, L.; Yuan, C. Deglycosylation of wogonoside enhances its anticancer potential. J. Cancer Res. Ther. 2018, 14, S594–S599. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, L.; Li, H.; Liu, X.; Yu, X.; Hu, P.; Hui, H.; Guo, Q.; Zhang, S. Oroxylin A inhibits the generation of Tregs in non-small cell lung cancer. Oncotarget 2017, 8, 49395–49408. [Google Scholar] [CrossRef]
- Wei, L.; Yao, Y.; Zhao, K.; Huang, Y.; Zhou, Y.; Zhao, L.; Guo, Q.; Lu, N. Oroxylin A inhibits invasion and migration through suppressing ERK/GSK-3β signaling in snail-expressing non-small-cell lung cancer cells. Mol. Carcinog. 2016, 55, 2121–2134. [Google Scholar] [CrossRef]
- Wei, L.; Dai, Q.; Zhou, Y.; Zou, M.; Li, Z.; Lu, N.; Guo, Q. Oroxylin A sensitizes non-small cell lung cancer cells to anoikis via glucose-deprivation-like mechanisms: C-Src and hexokinase II. Biochim. Biophys. Acta 2013, 1830, 3835–3845. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Li, W.; Xu, Y.; Zhuo, Y.; Li, M.; He, Y.; Wang, X.; Guo, Q.; Zhao, L.; et al. Oroxylin A reverses hypoxia-induced cisplatin resistance through inhibiting HIF-1α mediated XPC transcription. Oncogenesis 2020, 39, 6893–6905. [Google Scholar] [CrossRef] [PubMed]
- Wongtrakool, C.; Grooms, K.; Bijli, K.M.; Crothers, K.; Fitzpatrick, A.M.; Hart, C.M. Nicotine stimulates nerve growth factor in lung fibroblasts through an NFκB-dependent mechanism. PLoS ONE 2014, 9, e109602. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Peng, G.; Hao, B.; Liao, B.; Zhao, Z.; Zhou, Y.; Peng, F.; Ye, X.; Huang, L.; Zheng, M.; et al. Nicotine-induced airway smooth muscle cell proliferation involves TRPC6-dependent calcium influx via α7 nAChR. Cell. Physiol. Biochem. 2017, 43, 986–1002. [Google Scholar] [CrossRef]
- Wylam, M.E.; Sathish, V.; VanOosten, S.K.; Freeman, M.; Burkholder, D.; Thompson, M.A.; Pabelick, C.M.; Prakash, Y.S. Mechanisms of cigarette smoke effects on human airway smooth muscle. PLoS ONE 2015, 10, e0128778. [Google Scholar] [CrossRef]
- Siew, L.Q.C.; Wu, S.; Ying, S.; Corrigan, C.J. Cigarette smoking increases bronchial mucosal IL-17A expression in asthmatics, which acts in concert with environmental aeroallergens to engender neutrophilic inflammation. Clin. Exp. Allergy 2017, 47, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Evasovic, J.M.; Singer, C.A. Regulation of IL-17A and implications for TGF-β1 comodulation of airway smooth muscle remodeling in severe asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L843–L868. [Google Scholar] [CrossRef] [PubMed]
- Al-Alwan, L.A.; Chang, Y.; Baglole, C.J.; Risse, P.; Halayko, A.J.; Martin, J.G.; Eidelman, D.H.; Hamid, Q. Autocrine-regulated airway smooth muscle cell migration is dependent on IL-17-induced growth-related oncogenes. J. Allergy Clin. Immunol. 2012, 130, 977–985.e6. [Google Scholar] [CrossRef]
- Al-Alwan, L.A.; Chang, Y.; Mogas, A.; Halayko, A.J.; Baglole, C.J.; Martin, J.G.; Rousseau, S.; Eidelman, D.H.; Hamid, Q. Differential roles of CXCL2 and CXCL3 and their receptors in regulating normal and asthmatic airway smooth muscle cell migration. J. Immunol. 2013, 191, 2731–2741. [Google Scholar] [CrossRef]
- Ji, X.; Li, J.; Xu, L.; Wang, W.; Luo, M.; Luo, S.; Ma, L.; Li, K.; Gong, S.; He, L.; et al. IL4 and IL-17A provide a Th2/Th17-polarized inflammatory milieu in favor of TGF-β1 to induce bronchial epithelial-mesenchymal transition (EMT). Int. J. Clin. Exp. Pathol. 2013, 6, 1481–1492. [Google Scholar]
- Zhang, Y.; Jing, Y.; Qiao, J.; Luan, B.; Wang, X.; Wang, L.; Song, Z. Activation of the mTOR signaling pathway is required for asthma onset. Sci. Rep. 2017, 7, 4532. [Google Scholar] [CrossRef]
- Liu, A.; Wu, J.; Li, A.; Bi, W.; Liu, T.; Cao, L.; Liu, Y.; Dong, L. The inhibitory mechanism of Cordyceps sinensis on cigarette smoke extract-induced senescence in human bronchial epithelial cells. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 1721–1731. [Google Scholar] [PubMed]
- Wang, Z.; Su, R.; Yang, B.; Yang, K.; Yang, L.; Yan, Y.; Chen, Z. Potential role of cellular senescence in asthma. Front. Cell Dev. Biol. 2020, 8, 59. [Google Scholar] [CrossRef]
- Athari, S.S. Targeting cell signaling in allergic asthma. Signal. Transduct. Target. Ther. 2019, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Zou, Y.; Zhao, Z.; Li, B.; Ran, P. Nicotine-induced epithelial-mesenchymal transition via Wnt/β-catenin signaling in human airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 304, L199–L209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, X.; Xu, Y.; Liu, X.; Zhang, Z. The effect of cigarette smoke extract on the proliferation of human airway smooth muscle cells sensitized by serum from bronchial asthmatic patients. ZhonghuaJie He He Hu Xi Za Zhi 2011, 34, 604–608. [Google Scholar]
- Ijaz, T.; Pazdrak, K.; Kalita, M.; Konig, R.; Choudhary, S.; Tian, B.; Boldogh, I.; Brasier, A.R. Systems biology approaches to understanding Epithelial Mesenchymal Transition (EMT) in mucosal remodeling and signaling in asthma. World Allergy Org. J. 2014, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, L.; Li, S.; Wang, K.; Ke, R.; Shi, W.; Wang, J.; Yan, X.; Zhang, Q.; Wang, Q.; et al. Activation of AMPK inhibits TGF-β1-induced airway smooth muscle cells proliferation and its potential mechanisms. Sci. Rep. 2018, 8, 3624. [Google Scholar] [CrossRef]
- Zhou, X.; Tu, J.; Li, Q.; Kolosov, V.P.; Perelman, J.M. Hypoxia induces mucin expression and secretion in human bronchial epithelial cells. Transl. Res. 2012, 160, 419–427. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kwon, S.; Kim, K.H.; Moon, H.S.; Song, J.S.; Park, S.H.; Kim, Y.K. Expression of vascular endothelial growth factor and hypoxia-inducible factor in the airway of asthmatic patients. Ann. Allergy Asthma Immunol. 2006, 97, 794–799. [Google Scholar] [CrossRef]
- Wu, S.; Li, H.; Yu, L.; Wang, N.; Li, X.; Chen, W. IL-1β upregulates Muc5ac expression via NF-κB-induced HIF-1α in asthma. Immunol. Lett. 2017, 192, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Qin, S.; Qin, L.; Liu, L.; Sun, W.; Li, X.; Li, N.; Wu, R.; Wang, X. Cigarette smoke extract-induced p120-mediated NF-κB activation in human epithelial cells is dependent on the RhoA/ROCK pathway. Sci. Rep. 2016, 6, 23131. [Google Scholar] [CrossRef]
- Zhang, Y.; Saradna, A.; Ratan, R.; Ke, X.; Tu, W.; Do, D.C.; Hu, C.; Gao, P. RhoA/Rho-kinases in asthma: From pathogenesis to therapeutic targets. Clin. Transl. Immunol. 2020, 9, e1134. [Google Scholar] [CrossRef]
- Ji, M.; Zhang, Y.; Li, N.; Wang, C.; Xia, R.; Zhang, Z.; Wang, S. Nicotine component of cigarette smoke extract (CSE) decreases the cytotoxicity of CSE in BEAS-2B cells stably expressing human cytochrome P450 2A13. Int. J. Environ. Res. Public Health 2017, 14, 1221. [Google Scholar] [CrossRef] [PubMed]
- Schwalm, K.; Stevens, J.F.; Jiang, Z.; Schuyler, M.R.; Schrader, R.; Randell, S.H.; Green, F.H.; Tesfaigzi, Y. Expression of the pro-apoptotic protein bax is reduced in bronchial mucous cells of asthmatics. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L1102–L1109. [Google Scholar] [CrossRef]
- Liu, X.; Conner, H.; Kobayashi, T.; Kim, H.; Wen, F.; Abe, S.; Fang, Q.; Wang, X.; Hashimoto, M.; Bitterman, P.; et al. Cigarette smoke extract induces DNA damage but not apoptosis in human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 2005, 33, 121–129. [Google Scholar] [CrossRef]
- Zaffini, R.; Gotte, G.; Menegazzi, M. Asthma and poly(ADP-ribose) polymerase inhibition: A new therapeutic approach. Drug Des. Devel. Ther. 2018, 12, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Bucchieri, F.; Gammazza, A.M.; Pitruzzella, A.; Fucarino, A.; Farina, F.; Howarth, P.; Holgate, S.T.; Zummo, G.; Davies, D.E. Cigarette smoke causes caspase-independent apoptosis of bronchial epithelial cells from asthmatic donors. PLoS ONE 2015, 10, e0120510. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, C.; Boulet, L.; Page, N.; Laviolette, M.; Zimmermann, N.; Rothenberg, M.E.; Hamid, Q. Influence of cigarette smoke on the arginine pathway in asthmatic airways: Increased expression of arginase I. J. Allergy Clin. Immunol. 2007, 119, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Martins, M.A.; Tibério, I.F.L.C. Nitric oxide in asthma physiopathology. ISRN Allergy 2011, 2011, 832560. [Google Scholar] [CrossRef]
- Xu, T.; Ge, X.; Lu, C.; Dai, W.; Chen, H.; Xiao, Z.; Wu, L.; Liang, G.; Ying, S.; Zhang, Y.; et al. Baicalein attenuates OVA-induced allergic airway inflammation through the inhibition of the NF-κB signaling pathway. Aging 2019, 11, 9310–9327. [Google Scholar] [CrossRef]
- Dong, S.; Zhong, Y.; Lu, W.; Li, G.; Jiang, H.; Mao, B. Baicalin Inhibits Lipopolysaccharide-Induced Inflammation Through Signaling NF-κB Pathway in HBE16 Airway Epithelial Cells. Inflammation 2015, 38, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Al-Muhsen, S.; Johnson, J.R.; Hamid, Q. Remodeling in asthma. J. Allergy Clin. Immunol. 2011, 128, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Michalik, M.; Wójcik-Pszczoła, K.; Paw, M.; Wnuk, D.; Koczurkiewicz, P.; Sanak, M.; Pękala, E.; Madeja, Z. Fibroblast-to-myofibroblast transition in bronchial asthma. Cell. Mol. Life Sci. 2018, 75, 3943–3961. [Google Scholar] [CrossRef] [PubMed]
- Ryszawy, D.; Rolski, F.; Ryczek, K.; Catapano, J.; Wróbel, T.; Michalik, M.; Czyż, J. Invasive bronchial fibroblasts derived from asthmatic patients activate lung cancer A549 cells in vitro. Oncol. Lett. 2018, 16, 6582–6588. [Google Scholar] [CrossRef]
- Wong, A.W.; Ryerson, C.J.; Guler, S.A. Progression of fibrosing interstitial lung disease. Respir. Res. 2020, 21, 32. [Google Scholar] [CrossRef]
- Li, J.; Yang, M.; Li, P.; Su, Z.; Gao, P.; Zhang, J. Idiopathic pulmonary fibrosis will increase the risk of lung cancer. Chin. Med. J. 2014, 127, 3142–3149. [Google Scholar] [PubMed]
- Ballester, B.; Milara, J.; Cortijo, J. Idiopathic pulmonary fibrosis and lung cancer: Mechanisms and molecular targets. Int. J. Mol. Sci. 2019, 20, 593. [Google Scholar] [CrossRef] [PubMed]
| Functions | α7nAChR-Associated Signalling Pathways in NSCLC |
|---|---|
| Cell proliferation | α7nAChR enhances cell proliferation by activating retinoblastoma tumor suppressor protein-proto-oncogene, serine/threonine kinase (Rb-RAF-1) and Src pathways mediated by protein β-arrestin. α7nAChR causes activation of phosphatidylinositol-3 kinase/Akt (PI3K/Akt), Sp1 transcription factor/GATA binding protein (Sp1/GATA1) and the mammalian target of rapamycin (mTOR) signalling pathways, which enhance proliferation of NSCLC cells. α7nAChR activates signalling pathways of PI3K/Akt/Src, leading to upregulation of cyclinD1 expression in NSCLC cells. α7nAChR promotes cell proliferation by the activation of nicotine-induced vimentin and fibronectin expression through the Raf-1/extracellular signal-regulated kinase/mitogen-activated protein kinase (Raf-1/ERK/MAPK) signaling pathway. |
| Metastasis | α7nAChR stimulates migration and invasion of NSCLC cells by activating the ERK/MAPK, Src/protein kinase Cι/FAK (Src/PKCι/FAK), PI3K and Yes-associated protein-E2F transcription factors 1 (YAP-E2F1) signaling pathways. |
| Angiogenesis | Upregulation of α7nAChR in NSCLC cells promotes angiogenesis via activation of Ca2+ influx, which stimulates signaling pathways including VEGF-A, NF-κB, PI3K/AKT and FGFR2. |
| Anti-apoptosis | α7nAChR suppresses apoptosis in nicotine-induced NSCLC cells by activating the PI3K/Akt pathway, which inhibits proapoptotic Bcl-2-associated X protein (Bax) expression. Nicotine upregulates the protein expression of B-cell lymphoma-2 (Bcl-2) through α7nAChR-mediated activation of the Raf-1/MAPK signaling pathway, which leads to phosphorylation of transcription factor c-myc, resulting in significantly inhibited apoptosis in NSCLC cells. |
| S. baicalensis/S. radix/Flavones | Concentration | NSCLC Cell Lines Target | Activity | Molecular Mechanism Target | Reference |
|---|---|---|---|---|---|
| S. baicalensis | 250, 500 µg/mL | A549 | Inhibition of cell motility/proliferation, induction of G1 phase arrest | cyclinD1, CDK4, MMP2 ↓ | [51] |
| S. radix | 750 µg/mL | H2087, H358 | Induction of apoptosis and autophagy | caspase 3, PARP, LC3-II/LC3-I, AMPK ↑ mTOR ↓ | [52] |
| S. radix/baicalein/wogonin | 42.3 μg/mL | A549 | Inhibition of cell proliferation/invasion, induction of S phase arrest | cyclinD1 ↓, P53 ↑ | [53] |
| S. baicalensis/baicalein/baicalin/wogonin | 29.8, 27.5, 16.7 μg/mL | SK-LU-1, A549, SK-MES-1 | Induction of apoptosis and S phase arrest | Bax, P53 ↑ cyclinA ↓ | [54] |
| S. baicalensis/baicalein/baicalin/wogonin | 1, 10, 50 µM | A549, H1299 | Anti-metastatic, anti-inflammatory, induction of apoptosis | Bax ↑ MMP2,MMP9, caspase-3, bcl-2/bax, bcl-2, NF-κB p65, TNF-α, IL-6, IκB-α ↓ | [55] |
| S. baicalensis/baicalein/baicalin/wogonin | 10, 40, 200 μM | A549, H1299 | Inhibition of cell migration/invasion, Id1 inhibition, anti-angiogenesis | VEGF-A, N-cadherin, vimentin, a7nAChR, Akt/Src ↓ E-cadherin ↑ | [41] |
| Baicalein | 40 µmol/L | A549, H460 | Inhibition of cell proliferation, induction of apoptosis | PI3K/Akt, miR-424-3p ↓ | [56] |
| Baicalein | 40 µmol/L | A549 | Inhibition of cell motility/viability, anti-angiogenesis | PI3K, MMP2, MMP9 MMP14, VE-cadherin, RhoA/ROCK ↓ | [57] |
| Baicalein | 100, 200 µM | A549, H1299 | Induction of autophagy | MAP4K3, mTOR ↓ | [58] |
| Baicalein | 10, 40 µmol/L | A549, H1299 | Inhibition of cell invasion/metastasis | ezrin tension transduction, leader cells production, iNOS ↓ | [59] |
| Baicalein | 1, 10, 100 µM | A549, H460, SKMES1 | Anti-angiogenesis | VEGF-A, FGFR-2, RB-1 ↓ | [42] |
| Baicalein | 80 µmol/L | A549, H1299 | Inhibition of cell proliferation/invasion, Notch1 and hes-1 expression | cyclinD1, CDK1, N-cadherin, vimentin ↓ E-cadherin ↑ | [60] |
| Baicalein | 10 µM | A549 | Anti-proliferative | N-cadherin, vimentin, Src/Id1 ↓ E-cadherin ↑ | [43] |
| Baicalin | 100 µM | A549, H2009 | TRAIL-induced apoptosis | MAPK, ROS ↑ | [61] |
| Baicalin | 2, 4, 8 μg/mL | A549/DDP | Inhibition of cell proliferation/invasion | MARK2 mRNA, p-Akt ↓ | [62] |
| Baicalin | 25, 50, 100 µM | H441, H1975, H1299 | Inhibition of cell proliferation/invasion | PBK/TOPK ↓ | [63] |
| Baicalin | 20, 40, 80 µmol/L | A549, H1299 | Inhibition of cell proliferation/invasion induction of apoptosis | SIRT1/AMPK ↑ MMP2, MMP9 ↓ | [64] |
| Wogonin | 20 µM | A549 | Induction of apoptosis, cisplatin-induced cell death | caspase 3, PARP, ROS ↑ | [65] |
| Wogonin | 20 µM | A549 | Inhibition of cell migration/metastasis, anti-inflammatory | (IL-6)-induced EMT, N-cadherin, vimentin, Snail, Twist, STAT3 ↓ E-cadherin ↑ | [66] |
| Wogonin | 15 μg/mL | A549 | Inhibition of cell proliferation, glucose metabolism alteration | LDH, ATP synthesis ↓ | [67] |
| Wogonin | 50 µM | A549, A427 | Induction of apoptosis and autophagy | caspases 8/9/3,ROS, LC3II ↑ | [68] |
| Wogonin | 35 μg/mL | A549 | Inhibition of cell viability, induction of apoptosis | HDAC1/2, Skp2, Fbw7, GSK3b ↓ | [69] |
| Wogonin | 10 µM | A549 | Cell cycle arrest, senescence, apoptosis | APAF1, Bax, p21, PML, GADD45A, YPEL3 ↑ SGK1 ↓ | [70] |
| Wogonoside | 80 μM | A549 | Induction of apoptosis, cell cycle arrest | caspases 3/9, Bax, mitochondrial cytochrome c, AMPK ↑ Bcl-2, mTOR ↓ | [71] |
| Wogonin | 60, 100, 200 μM | DMEM | Anti-proliferative | caspases 3/9 ↑ | [72] |
| Oroxylin A | 40 μM | H460 | Anti-inflammatory | Tregs, TGFβ, Smad3, ERK1/2, JNK, P38, NF-κB/p65, IKKα, IKKβ ↓ | [73] |
| Oroxylin A | 16 μM | A549,95-D | Anti-invasive/migration | Snail, ERK/GSK-3β, CD44v6, MMP-9, vimentin ↓ E-cadherin ↑ | [74] |
| Oroxylin A | 120 μM | A549 | Anti-invasion, glucose metabolism alteration | Src/PI3K/AKT, ATP synthesis, lactic acid formation, HK II ↓ | [75] |
| Oroxylin A | 50 μmol/L | H460 | Suppression of XPC | HIF-1α ↓ | [76] |
| Activity | Mechanism of Action |
|---|---|
| Anti-inflammatory | Downregulate expression of pro-inflammatory cytokines (IL-4, IL-6, IL-8, IL-17A, TNF-α/IL-1) and chemokines (CXCR1, CXCR2) in hASM cells through inhibiting TGF-β1-induced EMT, p-Smad3, ERK1/2 and p38 MAPK, and noncanonical and NF-κB signaling pathways Suppress expression of TNF-α, IL-13, IL-4, IL-17A and CCL2 via inhibiting NF-κB, STAT6, PI3K and RhoA/ROCK signaling pathways activation, which may lead to downregulation of RhoA mRNA expression |
| Anti-proliferation | Inhibit α7nAChR-mediated PI3K/Akt signaling pathway activation and downregulate TRPC6 mRNA and protein expression, which may lead to decreased ROCE, SOCE and basal [Ca2+]i levels in HBSMCs Suppress Orai1, CD38, STIM1 expression and TRPC3-mediated SOCE in hASM cells Downregulate TGF-β1-induced cyclinD1, cyclinE, PCNA and HDAC4 and up-regulate miR-206 expression through inhibiting Smad2/3 signaling pathway activation |
| Anti-angiogenesis | Downregulate expression of VEGF and IL-1β-induced HIF-1α activation in hASM cells via suppressing NF-κB signaling pathway Suppress MMPs, snail, twist and fibronectin, and up-regulate E-cadherin expression through noncanonical signaling pathway inactivation |
| Anti-apoptosis | Inhibit NF-κB signaling pathway activation and ROS production, which may lead to decreased PARP and BAX expression in hASM/HBSMCs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsharairi, N.A. Scutellaria baicalensis and Their Natural Flavone Compounds as Potential Medicinal Drugs for the Treatment of Nicotine-Induced Non-Small-Cell Lung Cancer and Asthma. Int. J. Environ. Res. Public Health 2021, 18, 5243. https://doi.org/10.3390/ijerph18105243
Alsharairi NA. Scutellaria baicalensis and Their Natural Flavone Compounds as Potential Medicinal Drugs for the Treatment of Nicotine-Induced Non-Small-Cell Lung Cancer and Asthma. International Journal of Environmental Research and Public Health. 2021; 18(10):5243. https://doi.org/10.3390/ijerph18105243
Chicago/Turabian StyleAlsharairi, Naser A. 2021. "Scutellaria baicalensis and Their Natural Flavone Compounds as Potential Medicinal Drugs for the Treatment of Nicotine-Induced Non-Small-Cell Lung Cancer and Asthma" International Journal of Environmental Research and Public Health 18, no. 10: 5243. https://doi.org/10.3390/ijerph18105243
APA StyleAlsharairi, N. A. (2021). Scutellaria baicalensis and Their Natural Flavone Compounds as Potential Medicinal Drugs for the Treatment of Nicotine-Induced Non-Small-Cell Lung Cancer and Asthma. International Journal of Environmental Research and Public Health, 18(10), 5243. https://doi.org/10.3390/ijerph18105243






