Proper Management of the Clinical Exposure Index Based on Body Thickness Using Dose Optimization Tools in Digital Chest Radiography: A Phantom Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Equipment
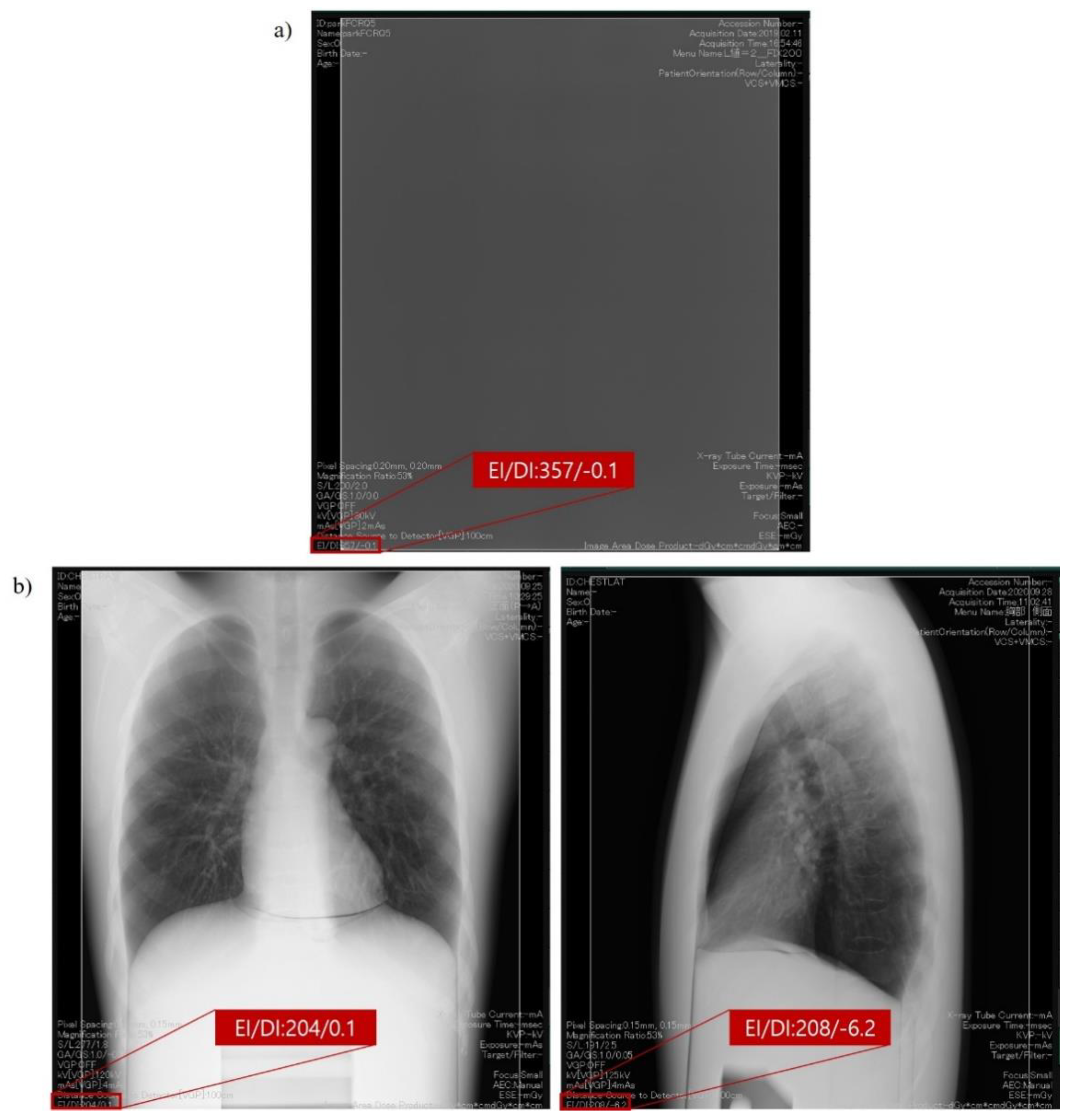
2.2. Exposure Index as per the International Electrotechnical Commission Standard Framework for Quality Assurance and Control of Digital Radiography Systems Used in This Study
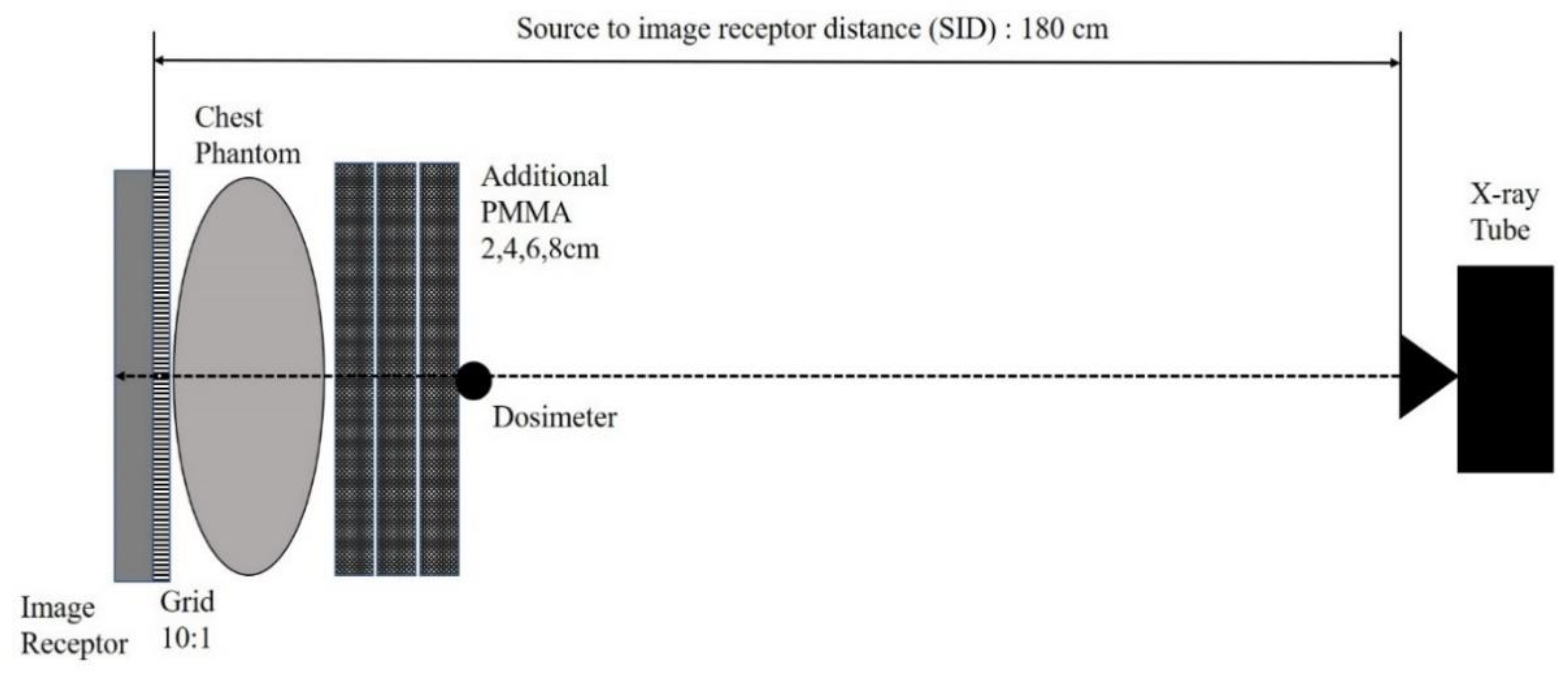
2.3. Exposure Condition and Geometry for Obtaining Clinical Exposure Index with Various Thickness of Phantom
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doi, K. Diagnostic imaging over the last 50 years: Research and development in medical imaging science and technology. Phys. Med. Biol. 2006, 51, R5–R27. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.; Jang, S.; Kim, J.; Kim, J.; Sung, D.; Kim, H.; Yoon, Y. A comparative assessment of entrance surface doses in analogue and digital radiography during common radiographic examinations. Radiat. Prot. Dosim. 2014, 158, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Körner, M.; Weber, C.H.; Wirth, S.; Pfeifer, K.J.; Reiser, M.F.; Treitl, M. Advances in digital radiography: Physical principles and system overview. RadioGraphics 2007, 27, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.D.; Cooper, M.L.; Piersall, K.; Apgar, B.K. Quality assurance: Using the exposure index and the deviation index to monitor radiation exposure for portable chest radiographs in neonates. Pediatric Radiol. 2011, 41, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Kim, H.; Park, M.; Kim, J.; Seo, D.; Choi, I.; Jeong, H.; Kim, J. Monte Carlo simulation-based feasibility study of a dose-area product meter built into a collimator for diagnostic X-ray. Radiat. Prot. Dosim. 2014, 162, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Vano, E. ICRP recommendations on ‘Managing patient dose in digital radiology’. Radiat. Prot. Dosim. 2005, 114, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Gale, M.E.; Gale, D.R. DICOM modality worklist: An essential component in a PACS environment. J. Digit. Imaging 2000, 13, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Han, S.G.; Roh, Y.; Lim, H.J.; Kim, J.M.; Kim, J.U.; Chae, H.S.; Yoon, Y.S. Performance evaluation of domestic prototype dose area product meter SFT-1. J. Radiol. Sci. Technol. 2016, 39, 435–441. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Jung, H.; Kim, J.; Cho, B. Patient radiation dose values during interventional cardiology examinations in University Hospital, Korea. J. Radiol. Sci. Technol. 2016, 39, 27–33. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, Y.; Seo, D.; Kwon, S.; Shim, J.; Kim, J. Real-time patient radiation dose monitoring system used in a large university hospital. J. Digit. Imaging 2016, 29, 627–634. [Google Scholar] [CrossRef] [PubMed]
- International Electrotechnical Commission IEC 62494-1, Ed 1.0. In Medical Electrical Equipment- Exposure Index of Digital X-ray Imaging System-Part 1: Definitions and Requirements for General Radiography; International Electrotechnical Commission: Geneva, Switzerland, 2008.
- American Association of Physicists in Medicine. An Exposure Indicator for Digital Radiography; AAPM REPORT NO. 116; American Association of Physicists in Medicine: Alexandria, VA, USA, 2009; Available online: https://www.aapm.org/pubs/reports/rpt_116.pdf (accessed on 20 September 2020).
- Park, H.; Yoon, Y.; Kim, J.; Kim, J.; Joeng, H.; Tanaka, N.; Morishita, J. Use of clinical exposure index and deviation index based on national diagnostic reference level as dose-optimization tools for general radiography in Korea. Radiat. Prot. Dosim. 2020, 191, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Yoon, Y.; Tanaka, N.; Kim, J.; Kim, J.; Morishita, J. Feasibility of displayed exposure index in IEC standard framework as a dose optimisation tool for digital radiography systems. Radiat. Prot. Dosim. 2020, 189, 384–394. [Google Scholar] [CrossRef]
- Seibert, J.A.; Morin, R.L. The standardized exposure index for digital radiography: An opportunity for optimization of radiation dose to the pediatric population. Pediatric Radiol. 2011, 41, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Takaki, T.; Fujibushi, T.; Murakami, S.; Aoki, T.; Ohki, M. The clinical significance of modifying X-ray tube current-time product based on prior image deviation index for digital radiography. Phys. Med. 2019, 63, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Takaki, T.; Murakami, S.; Watanabe, R.; Aoki, T.; Fujibuchi, T. Calculating the target exposure index using a deep convolutional neural network and a rule base. Phys. Med. 2020, 71, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Martin, C. Optimization in general radiography. Biomed. Imaging. Inter. J. 2007, 3, e18. [Google Scholar] [CrossRef]
- International Commission on Radiological Protection. Diagnostic Reference Levels in Medical Imaging; ICRP Publication 135; International Commission on Radiological Protection: Stockholm, Sweden, 2017; Available online: https://journals.sagepub.com/doi/pdf/10.1177/ANIB_46_1 (accessed on 20 November 2020).
- Kim, J.; Lee, S.; Kim, S.; Yoo, S.; Kim, J.; Yoon, S. National Diagnostic Reference Levels and Achievable Doses for 13 Adult CT Protocols and a Paediatric Head CT Protocol: National Survey of Korean Hospitals. Radiat. Prot. Dosim. 2019, 187, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Korea Centers for Disease Control and Prevention. Guideline for Diagnostic Reference Levels. In KCDC Medical Radiation Series No. 16; Korea Centers for Disease Control and Prevention: Osong, Korea, 2019. [Google Scholar]
- Do, K. Development of the Diagnostic Reference Level of General Radiography: Twelve Area Including Brain, Chest, Pelvis, etc.; Research Report No.11-1352159-000916-01; Korea Centers for Disease Control and Prevention: Osong, Korea, 2017.
- Kyoto Kagaku CO., LTD. Multipurpose Chest Phantom N1 “LUNGMAN”. Available online: https://www.kyotokagaku.com/en/products_introduction/ph-1/ (accessed on 13 November 2020).
- Seeram, E. Optimization of the Exposure Indicator of a Computed Radiography Imaging System as a Radiation Dose Management Strategy. Ph.D. Thesis, Charles Sturt University, Wagga Wagga, Australia, 2012. [Google Scholar]
- Yasumatsu, S.; Tanaka, N.; Iwase, K.; Shimizu, Y.; Morishita, J. Effect of X-ray beam quality on determination of exposure index. Radiol. Phys. Technol. 2016, 9, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Takaki, T.; Takeda, K.; Murakami, S.; Ogawa, H.; Ogawa, M.; Sakamoto, M. Evaluation of the effects of subject thickness on the exposure index in digital radiography. Radiol. Phys. Technol. 2016, 9, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.A.; Reiser, I.; Baxter, T.; Zhang, Y.; Finkle, J.H.; Lu, Z.F.; Feinstein, K.A. Portable abdomen radiography: Moving to thickness-based protocols. Pediatric Radiol. 2018, 48, 210–215. [Google Scholar] [CrossRef] [PubMed]
- International Electrotechnical Commission IEC 60601-2-43, Ed 2.0. In Medical Electrical Equipment-Part 2-43: Particular Requirements for the Basic Safety and Essential Performance of X-ray Equipment for Interventional Procedures; International Electrotechnical Commission: Geneva, Switzerland, 2010.



| Examination | Tube Voltage (kVp) | Tube Current Time Product (mAs) | SID (cm) | Field Size (cm × cm) |
|---|---|---|---|---|
| Chest PA | 120 | 4 (2.8–7.4) | 180 | 42 × 43 |
| Chest LAT | 120 | 10.6 (8–16) | 180 | 42 × 43 |
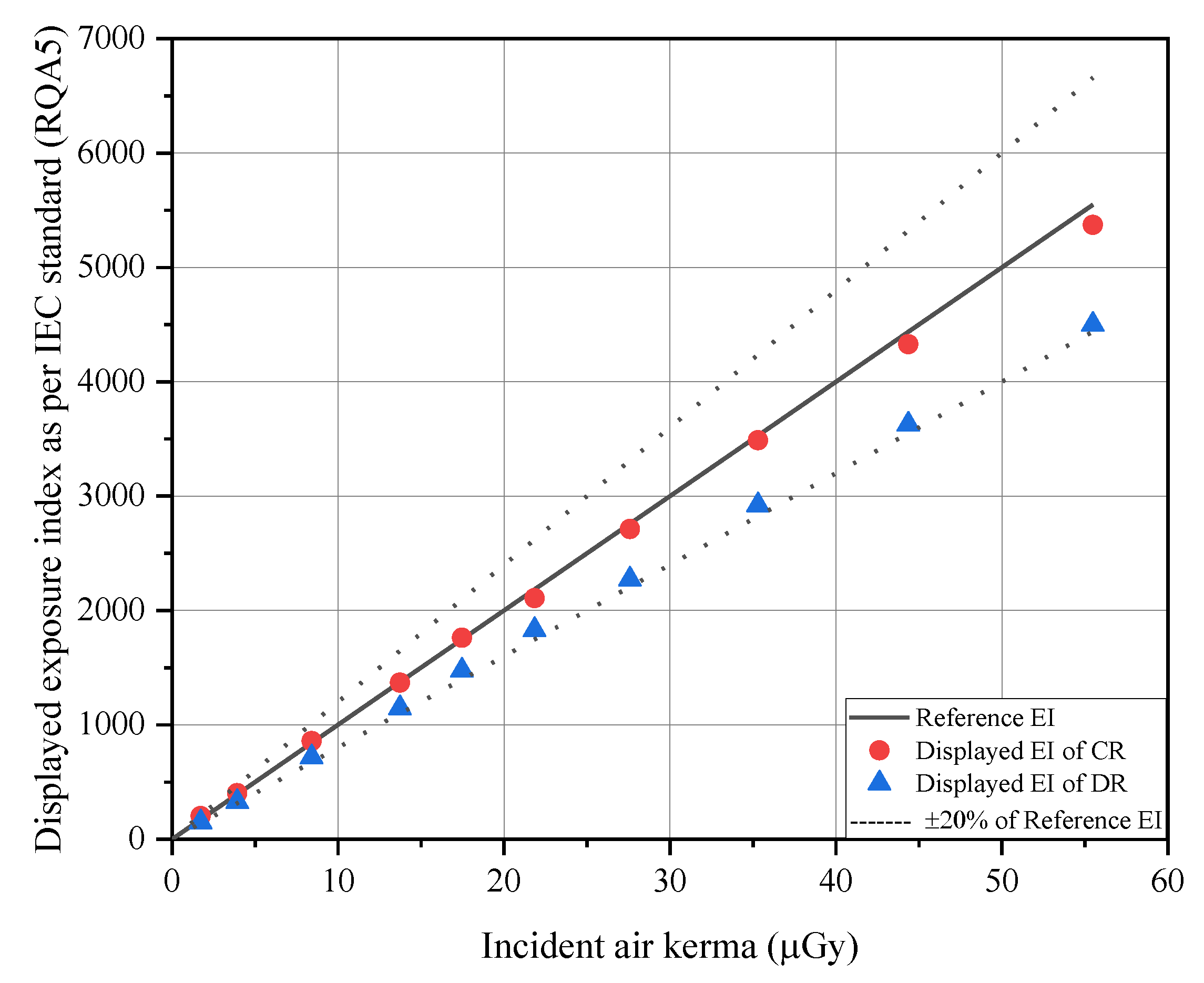
| mAs | Incident Air-Kerma (μGy) | Reference EI (1) | Displayed EI (Uncertainty %) | |
|---|---|---|---|---|
| CR | DR | |||
| 2 | 1.73 | 173 | 203 (+17.59) | 148 (−14.27) |
| 4 | 3.93 | 393 | 403 (+2.65) | 326 (−16.96) |
| 8 | 8.42 | 842 | 857 (+1.84) | 718 (−14.68) |
| 12.6 | 13.74 | 1374 | 1369 (−0.34) | 1147 (−16.50) |
| 16 | 17.47 | 1747 | 1761 (+0.82) | 1475 (−15.55) |
| 20 | 21.84 | 2184 | 2108 (−3.49) | 1831 (−16.18) |
| 25 | 27.59 | 2759 | 2711 (−1.75) | 2272 (−17.66) |
| 32 | 35.30 | 3530 | 3488 (−1.20) | 2922 (−17.23) |
| 40 | 44.36 | 4436 | 4328 (−2.43) | 3627 (−18.24) |
| 50 | 55.49 | 5549 | 5371 (−3.20) | 4500 (−18.90) |
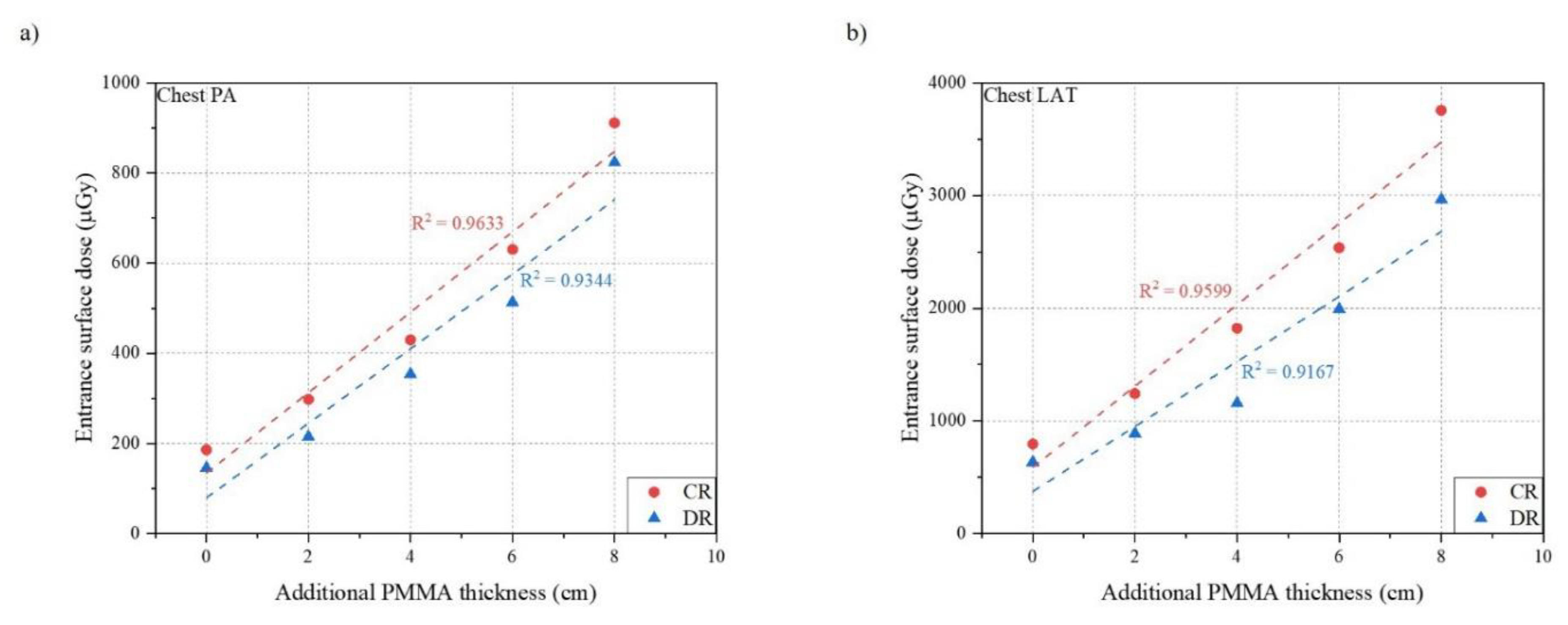
| Body Thickness | mAs | S Value | ESD (μGy) | Clinical EI | ||||
|---|---|---|---|---|---|---|---|---|
| CR | DR | CR | DR | CR | DR | CR | DR | |
| Only Phantom | 3.6 | 2.8 | 210 | 205 | 186.1 | 145.8 | 449 | 277 |
| +2 cm PMMA | 5.6 | 4 | 200 | 220 | 298.2 | 215.2 | 474 | 277 |
| +4 cm PMMA | 8 | 6.4 | 215 | 200 | 430.2 | 353.8 | 457 | 272 |
| +6 cm PMMA | 11.2 | 9 | 210 | 215 | 630.5 | 513.0 | 457 | 267 |
| +8 cm PMMA | 16 | 14.4 | 196 | 191 | 911.0 | 823.5 | 509 | 292 |
| Body Thickness | mAs | S Value | ESD (μGy) | Clinical EI | ||||
|---|---|---|---|---|---|---|---|---|
| CR | DR | CR | DR | CR | DR | CR | DR | |
| Only Phantom | 12.8 | 10 | 191 | 205 | 796.1 | 635.4 | 349 | 183 |
| +2 cm PMMA | 17.9 | 12.6 | 183 | 191 | 1243.0 | 888.3 | 368 | 208 |
| +4 cm PMMA | 25.6 | 16 | 183 | 210 | 1822.7 | 1158.7 | 375 | 193 |
| +6 cm PMMA | 35.2 | 27.5 | 196 | 215 | 2537.3 | 1991.7 | 362 | 183 |
| +8 cm PMMA | 51.2 | 40 | 191 | 205 | 3757.0 | 2965.7 | 368 | 193 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, Y.; Park, H.; Kim, J.; Kim, J.; Roh, Y.; Tanaka, N.; Morishita, J. Proper Management of the Clinical Exposure Index Based on Body Thickness Using Dose Optimization Tools in Digital Chest Radiography: A Phantom Study. Int. J. Environ. Res. Public Health 2021, 18, 5203. https://doi.org/10.3390/ijerph18105203
Yoon Y, Park H, Kim J, Kim J, Roh Y, Tanaka N, Morishita J. Proper Management of the Clinical Exposure Index Based on Body Thickness Using Dose Optimization Tools in Digital Chest Radiography: A Phantom Study. International Journal of Environmental Research and Public Health. 2021; 18(10):5203. https://doi.org/10.3390/ijerph18105203
Chicago/Turabian StyleYoon, Yongsu, Hyemin Park, Jungmin Kim, Jungsu Kim, Younghoon Roh, Nobukazu Tanaka, and Junji Morishita. 2021. "Proper Management of the Clinical Exposure Index Based on Body Thickness Using Dose Optimization Tools in Digital Chest Radiography: A Phantom Study" International Journal of Environmental Research and Public Health 18, no. 10: 5203. https://doi.org/10.3390/ijerph18105203
APA StyleYoon, Y., Park, H., Kim, J., Kim, J., Roh, Y., Tanaka, N., & Morishita, J. (2021). Proper Management of the Clinical Exposure Index Based on Body Thickness Using Dose Optimization Tools in Digital Chest Radiography: A Phantom Study. International Journal of Environmental Research and Public Health, 18(10), 5203. https://doi.org/10.3390/ijerph18105203






