Interrupted Time Series Analysis of Changes in Zolpidem Use Due to Media Broadcasts
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jang, Y.; Song, I.; Oh, I.-S.; Shin, J.-Y. Twelve-year trend in the use of zolpidem and physicians’ non-compliance with recommended duration: A Korean national health insurance database study. Eur. J. Clin. Pharmacol. 2018, 75, 109–117. [Google Scholar] [CrossRef]
- Schroeck, J.L.; Ford, J.; Conway, E.L.; Kurtzhalts, K.E.; Gee, M.E.; Vollmer, K.A.; Mergenhagen, K.A. Review of Safety and Efficacy of Sleep Medicines in Older Adults. Clin. Ther. 2016, 38, 2340–2372. [Google Scholar] [CrossRef]
- Darcourt, G.; Pringuey, D.; Sallière, D.; Lavoisy, J. The safety and tolerability of zolpidem—An update. J. Psychopharmacol. 1999, 13, 81–93. [Google Scholar] [CrossRef]
- Qaseem, A.; Kansagara, D.; Forciea, M.A.; Cooke, M.; Denberg, T.D. For the Clinical Guidelines Committee of the American College of Physicians Management of Chronic Insomnia Disorder in Adults: A Clinical Practice Guideline From the American College of Physicians. Ann. Intern. Med. 2016, 165, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.; Bracchi, R.; Hewitt, J.; Routledge, P.A.; Carter, B. Benzodiazepines, Z-drugs and the risk of hip fracture: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0174730. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.R.; Kim, Y.-J.; Kim, M.-S.; Jung, S.-Y.; Choi, N.-K.; Hwang, B.; Park, B.-J.; Lee, J. Prescription of Zolpidem and the Risk of Fatal Motor Vehicle Collisions: A Population-Based, Case-Crossover Study from South Korea. CNS Drugs 2018, 32, 593–600. [Google Scholar] [CrossRef]
- Stranks, E.K.; Crowe, S.F. The acute cognitive effects of zopiclone, zolpidem, zaleplon, and eszopiclone: A systematic review and meta-analysis. J. Clin. Exp. Neuropsychol. 2014, 36, 691–700. [Google Scholar] [CrossRef]
- Ram, D.; Eiman, N.; Gowdappa, A.B. Multimodal Hallucination (Audio-visual, Kinaesthetic and Scenic) Associated with the Use of Zolpidem. Clin. Psychopharmacol. Neurosci. 2015, 13, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lin, C.-C.; Lu, C.-J.; Hsu, C.-Y.; Kao, C.-H. Association Between Zolpidem and Suicide: A Nationwide Population-Based Case-Control Study. Mayo Clin. Proc. 2016, 91, 308–315. [Google Scholar] [CrossRef]
- McCall, W.V.; Benca, R.M.; Rosenquist, P.B.; Riley, M.A.; McCloud, L.; Newman, J.C.; Case, D.; Rumble, M.; Krystal, A.D. Hypnotic Medications and Suicide: Risk, Mechanisms, Mitigation, and the FDA. Am. J. Psychiatry 2017, 174, 18–25. [Google Scholar] [CrossRef]
- Choi, B.; Sung, H.G.; Nam, J.H.; Shin, J. Zolpidem Use and Suicide Death in South Korea: A Population-Based Case–Control Study. Suicide Life-Threat. Behav. 2018, 49, 1653–1667. [Google Scholar] [CrossRef]
- SBS. I Want to Know That Episode Devil’s Whispher-Who Is the Killer of the Serial Death Case. Available online: https://programs.sbs.co.kr/culture/unansweredquestions/vod/55075/22000184573 (accessed on 3 January 2021).
- Kim, J.W.; Jung, H.Y.; Won, D.Y.; Noh, J.H.; Shin, Y.S.; Kang, T.I. Suicide Trends According to Age, Gender, and Marital Status in South Korea. Omega-J. Death Dying 2017, 79, 90–105. [Google Scholar] [CrossRef] [PubMed]
- EMA. Guideline on Good Pharmacovigilance Practices (GVP). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-module-xvi-risk-minimisation-measures-selection-tools_en-2.pdf (accessed on 1 March 2021).
- Norman, J.L.; Fixen, D.R.; Saseen, J.J.; Saba, L.M.; Linnebur, S.A. Zolpidem prescribing practices before and after Food and Drug Administration required product labeling changes. SAGE Open Med. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Kesselheim, A.S.; Donneyong, M.; Pan, G.J.D.; Zhou, E.H.; Avorn, J.; Schneeweiss, S.; Seeger, J.D. Changes in prescribing and healthcare resource utilization after FDA Drug Safety Communications involving zolpidem-containing medications. Pharmacoepidemiol. Drug Saf. 2017, 26, 712–721. [Google Scholar] [CrossRef]
- Touchard, J.; Sabatier, P.; Airagnes, G.; Berdot, S. Consequences of the new zolpidem prescription regulations: A cohort study from the French national healthcare database. Eur. J. Clin. Pharmacol. 2020, 76, 89–95. [Google Scholar] [CrossRef]
- Park, M.-J.; Kim, M.-H.; Shin, S.M.; Chung, S.Y. Effect of providing drug utilization review information on tricyclic antidepressant prescription in the elderly. J. Med. Syst. 2018, 42, 1–10. [Google Scholar] [CrossRef]
- Jung, S.; Jang, E.J.; Choi, S.; Im, S.G.; Kim, D.; Cho, S.; Kim, H.; Sung, Y. Effect of a Nationwide Real-Time Drug Utilization Review System on Duplicated Nonsteroidal Antiinflammatory Drug Prescriptions in Korea. Arthritis Rheum. 2020, 72, 1374–1382. [Google Scholar] [CrossRef]
- Barry, C.L.; Busch, S.H. News Coverage of FDA Warnings on Pediatric Antidepressant Use and Suicidality. Pediatrics 2009, 125, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Metlay, J.P.; Cohen, A.; Polsky, D.; Kimmel, S.E.; Koppel, R.; Hennessy, S. Medication Safety in Older Adults: Home-Based Practice Patterns. J. Am. Geriatr. Soc. 2005, 53, 976–982. [Google Scholar] [CrossRef]
- Grilli, R.; Ramsay, C.; Minozzi, S. Mass media interventions: Effects on health services utilisation. Cochrane Database Syst. Rev. 2002, CD000389. [Google Scholar] [CrossRef]
- De Jesus, M. The Impact of Mass Media Health Communication on Health Decision-Making and Medical Advice-Seeking Behavior of U.S. Hispanic Population. Health Commun. 2012, 28, 525–529. [Google Scholar] [CrossRef]
- Waller, P.C.; Evans, S.J.W.; Beard, K. Drug safety and the media. Br. J. Clin. Pharmacol. 2006, 61, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Rabi, D.M.; Lewin, A.M.; Brown, G.E.; Edwards, A.L.; Johnson, J.A.; Ghali, W.A. Lay media reporting of rosiglitazone risk: Extent, messaging and quality of reporting. Cardiovasc. Diabetol. 2009, 8, 40. [Google Scholar] [CrossRef]
- Im, H.; Huh, J. Does Health Information in Mass Media Help or Hurt Patients? Investigation of Potential Negative Influence of Mass Media Health Information on Patients’ Beliefs and Medication Regimen Adherence. J. Health Commun. 2017, 22, 1–9. [Google Scholar] [CrossRef]
- Owens, S.R. Injection of confidence. EMBO Rep. 2002, 3, 406–409. [Google Scholar] [CrossRef][Green Version]
- Sloane, R.; Osanlou, O.; Lewis, D.A.; Bollegala, D.; Maskell, S.; Pirmohamed, M. Social media and pharmacovigilance: A review of the opportunities and challenges. Br. J. Clin. Pharmacol. 2015, 80, 910–920. [Google Scholar] [CrossRef]
- Viale, P.H. Direct-to-Consumer Advertising of Prescription Medications: Implications for Patients with Cancer. Oncol. Nurs. Forum 2002, 29, 505–513. [Google Scholar] [CrossRef]
- Fogel, J.; King, K. Perceived Realism and Twitter Use Are Associated with Increased Acceptance of Cosmetic Surgery among Those Watching Reality Television Cosmetic Surgery Programs. Plast. Reconstr. Surg. 2014, 134, 233–238. [Google Scholar] [CrossRef]
- Kim, L.; Kim, J.-A.; Kim, S. A guide for the utilization of Health Insurance Review and Assessment Service National Patient Samples. Epidemiol. Health 2014, 36, e2014008. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.-C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Sobel, R.E.; Bate, A.; Marshall, J.; Haynes, K.; Selvam, N.; Nair, V.; Daniel, G.; Brown, J.S.; Reynolds, R.F. Do FDA label changes work? Assessment of the 2010 class label change for proton pump inhibitors using the Sentinel System’s analytic tools. Pharmacoepidemiol. Drug Saf. 2018, 27, 332–339. [Google Scholar] [CrossRef]
- Kim, H.M.; Gerlach, L.B.; Yosef, M.; Stano, C.; Conroy, D.A.; Valenstein, M.; Pfeiffer, P.N.; Sales, A.E.; Zivin, K. Responsiveness of Veterans Affairs Health Care System to Zolpidem Safety Warnings. J. Clin. Sleep Med. 2018, 14, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Kesselheim, A.S.; Sinha, M.S.; Rausch, P.; Lu, Z.; Tessema, F.A.; Lappin, B.M.; Zhou, E.H.; Pan, G.J.D.; Zwanziger, L.; Ramanadham, A.; et al. Patients’ Knowledge of Key Messaging in Drug Safety Communications for Zolpidem and Eszopiclone: A National Survey. J. Law Med. Ethic 2019, 47, 430–441. [Google Scholar] [CrossRef]
- Fogel, J.; Shlivko, A. Reality Television Programs Are Associated with Illegal Drug Use and Prescription Drug Misuse Among College Students. Subst. Use Misuse 2015, 51, 62–72. [Google Scholar] [CrossRef]
- Dopheide, J.A. Insomnia overview: Epidemiology, pathophysiology, diagnosis and monitoring, and nonpharmacologic therapy. Am. J. Manag. Care 2020, 26, S76–S84. [Google Scholar] [CrossRef]
- Kesselheim, A.S.; McGraw, S.A.; Dejene, S.Z.; Rausch, P.; Pan, G.J.D.; Lappin, B.M.; Zhou, E.H.; Avorn, J.; Campbell, E.G. Patient and Physician Perceptions of Drug Safety Information for Sleep Aids: A Qualitative Study. Drug Saf. 2017, 40, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Dusetzina, S.B.; Higashi, A.S.; Dorsey, E.R.; Conti, R.; Huskamp, H.A.; Zhu, S.; Garfield, C.F.; Alexander, G.C. Impact of FDA Drug Risk Communications on Health Care Utilization and Health Behaviors. Med. Care 2012, 50, 466–478. [Google Scholar] [CrossRef]
- Woloshin, S.; Schwartz, L.M.; Dejene, S.; Rausch, P.; Pan, G.J.D.; Zhou, E.H.; Kesselheim, A.S. Media Coverage of FDA Drug Safety Communications about Zolpidem: A Quantitative and Qualitative Analysis. J. Health Commun. 2017, 22, 365–372. [Google Scholar] [CrossRef]
| Zolpidem (n = 129,787) | Other Hypnotics (n = 241,048) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2015 | 2016 | 2017 | |||||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| Number of user | 42,921 | (3.73) | 43,505 | (3.72) | 43,361 | (3.69) | 77,828 | (6.77) | 81,028 | (6.92) | 82,192 | (7.00) |
| Age a,b,c | ||||||||||||
| 20–39 | 5864 | (13.66) | 5766 | (13.25) | 5311 | (12.25) | 12,204 | (15.68) | 13,096 | (16.16) | 13,133 | (15.98) |
| 40–64 | 19,793 | (46.11) | 20,232 | (46.50) | 20,964 | (48.35) | 35,871 | (46.09) | 37,121 | (45.81) | 38,867 | (47.29) |
| 65+ | 17,264 | (40.22) | 17,507 | (40.24) | 17,086 | (39.40) | 29,753 | (38.23) | 30,811 | (38.03) | 30,192 | (36.73) |
| Sex, Female a,b,c | 27,132 | (63.21) | 27,359 | (62.89) | 27,278 | (62.91) | 50,707 | (65.15) | 52,316 | (64.57) | 53,130 | (64.64) |
| Type of health insurance a,b,c | ||||||||||||
| Health Insurance | 38,666 | (90.09) | 39,088 | (89.85) | 38,891 | (89.69) | 70,364 | (90.41) | 73,370 | (90.55) | 74,195 | (90.27) |
| Medical Aid | 3892 | (9.07) | 4063 | (9.34) | 4138 | (9.54) | 6986 | (8.98) | 7176 | (8.86) | 7478 | (9.10) |
| National Meritorious service | 363 | (0.85) | 354 | (0.81) | 332 | (0.77) | 478 | (0.61) | 482 | (0.59) | 519 | (0.63) |
| Comorbidities | ||||||||||||
| Depression a,b,c | 13,678 | (31.87) | 13,739 | (31.58) | 13,279 | (30.62) | 31,181 | (40.06) | 32,513 | (40.13) | 33,547 | (40.82) |
| Bipolar disorder | 2932 | (6.83) | 2874 | (6.61) | 3086 | (7.12) | 5215 | (6.70) | 5484 | (6.77) | 6181 | (7.52) |
| Anxiety disorder a,b,c | 16,804 | (39.15) | 16,773 | (38.55) | 16,714 | (38.55) | 47,206 | (60.65) | 49,058 | (60.54) | 50,174 | (61.04) |
| Schizophrenia b,c | 1827 | (4.26) | 1718 | (3.95) | 1766 | (4.07) | 3482 | (4.47) | 3536 | (4.36) | 3957 | (4.81) |
| Substance Use Disorder a | 1314 | (3.06) | 1188 | (2.73) | 1328 | (3.06) | 2210 | (2.84) | 2161 | (2.67) | 2542 | (3.09) |
| Headache a,b,c | 13,409 | (31.24) | 12,900 | (29.65) | 12,977 | (29.93) | 27,796 | (35.71) | 28,184 | (34.78) | 28,711 | (34.93) |
| Dementia a,b,c | 2993 | (6.97) | 3092 | (7.11) | 3030 | (6.99) | 5720 | (7.35) | 6106 | (7.54) | 6304 | (7.67) |
| CCI score, mean ± SD a,b,c | 2.33 | 2.36 | 2.42 | 2.40 | 2.26 | 2.23 | 2.18 | 2.20 | 2.27 | 2.27 | 2.10 | 2.11 |
| 0 | 9672 | (22.53) | 9231 | (21.22) | 9723 | (22.42) | 18,210 | (23.40) | 18,062 | (22.29) | 19,701 | (23.97) |
| 1 or 2 | 17,584 | (40.97) | 17,776 | (40.86) | 18,177 | (41.92) | 33,086 | (42.51) | 34,104 | (42.09) | 35,316 | (42.97) |
| ≥ 3 | 15,665 | (36.50) | 16,498 | (37.92) | 15,461 | (35.66) | 26,532 | (34.09) | 28,862 | (35.62) | 27,175 | (33.06) |
| Subgroup | Zolpidem Estimate (95% CI; Lower, Upper) | Other Hypnotics Estimate (95% CI; Lower, Upper) | ||||||
|---|---|---|---|---|---|---|---|---|
| Intercept | Baseline Trend | Broadcasting | Time after Broadcasting | Intercept | Baseline Trend | Broadcasting | Time after Broadcasting | |
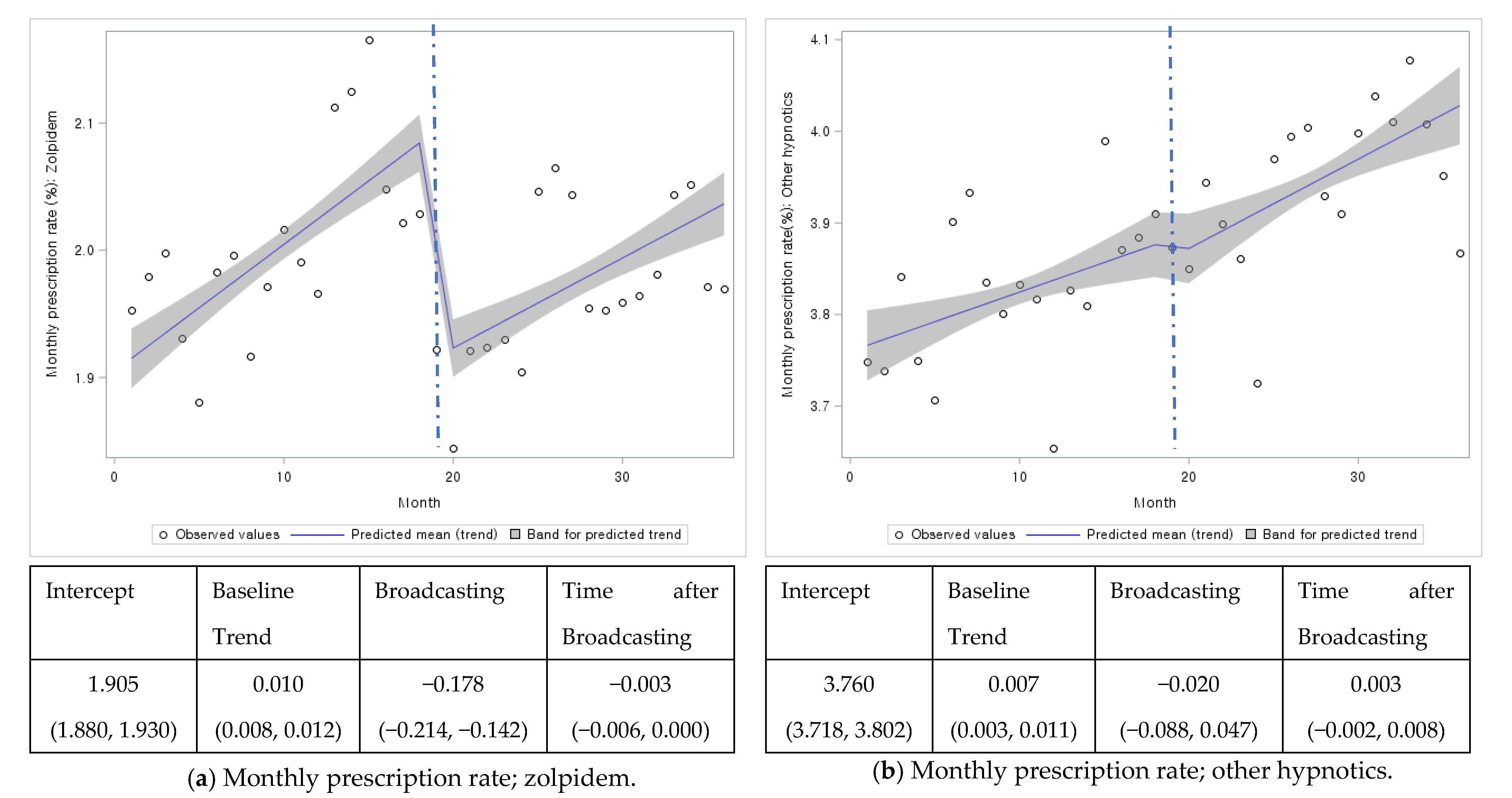
| Overall | 1.905 (1.880, 1.930) | 0.010 (0.008, 0.012) | −0.178 (−0.214, −0.142) | −0.003 (−0.006, <0.001) | 3.760 (3.718, 3.802) | 0.007 (0.003, 0.011) | −0.020 (−0.088, 0.047) | 0.003 (−0.002, 0.008) |
| Male | 1.649 (1.616, 1.682) | 0.01 (0.008, 0.012) | −0.172 (−0.202, −0.142) | −0.004 (−0.007, −0.001) | 3.067 (2.996, 3.137) | 0.006 (0.000, 0.013) | −0.022 (−0.123, 0.080) | −0.001 (−0.010, 0.009) |
| Female | 2.119 (2.017, 2.221) | 0.008 (0.001, 0.015) | −0.170 (−0.266, −0.074) | 0.000 (−0.010, 0.010) | 4.280 (4.191, 4.369) | 0.009 (0.000, 0.017) | −0.033 (−0.164, 0.098) | 0.000 (−0.012, 0.012) |
| Age group | ||||||||
| 20–39 | 0.820 (0.801, 0.839) | 0.008 (0.006, 0.009) | −0.133 (−0.162, −0.104) | −0.003 (−0.006, −0.001) | 1.615 (1.568, 1.662) | 0.012 (0.008, 0.017) | 0.027 (−0.045, 0.100) | 0.001 (−0.006, 0.006) |
| 40–64 | 1.741 (1.715, 1.768) | 0.010 (0.008, 0.012) | −0.170 (−0.201, −0.14) | −0.003 (−0.006, 0.000) | 3.450 (3.392, 3.508) | 0.004 (0.000, 0.008) | −0.051 (−0.112, 0.009) | 0.004 (−0.002, 0.010) |
| 65+ | 3.382 (3.302, 3.462) | 0.009 (0.001, 0.017) | −0.239 (−0.367, −0.112) | 0.003 (−0.006, 0.013) | 6.553 (6.471, 6.635) | 0.009 (0.004, 0.014) | −0.047 (−0.118, 0.023) | 0.000 (−0.007, 0.008) |
| Health insurance | 1.733 (1.706, 1.761) | 0.008 (0.006, 0.011) | −0.164 (−0.201, −0.127) | −0.003 (−0.006, 0.001) | 3.393 (3.319, 3.467) | 0.006 (−0.001, 0.013) | −0.008 (−0.115, 0.098) | 0.000 (−0.011, 0.010) |
| Medical Aid | 5.329 (5.238, 5.421) | 0.045 (0.037, 0.054) | −0.391 (−0.538, −0.244) | −0.003 (−0.014, 0.009) | 11.542 (11.387, 11.697) | 0.019 (0.005, 0.034) | −0.192 (−0.415, 0.031) | 0.052 (0.031, 0.074) |
| National Meritorious service | 4.866 (4.581, 5.151) | 0.012 (−0.016, 0.039) | −0.715 (−1.175, −0.255) | 0.029 (−0.008, 0.065) | 6.580 (6.231, 6.928) | 0.003 (−0.027, 0.032) | −0.157 (−0.568, 0.253) | 0.069 (0.024, 0.114) |
| Psychiatric disorder (−) | 0.844 (0.819, 0.870) | 0.007 (0.005, 0.009) | −0.102 (−0.137, −0.067) | −0.002 (−0.006, 0.001) | 0.670 (0.651, 0.690) | 0.002 (0.000, 0.003) | −0.014 (−0.033, 0.005) | 0.001 (−0.001, 0.003) |
| Psychiatric disorder (1) (+) | 6.451 (6.343, 6.559) | 0.038 (0.029, 0.047) | −0.528 (−0.663, −0.394) | −0.022 (−0.035, −0.009) | 17.024 (16.759, 17.290) | 0.071 (0.046, 0.095) | −0.086 (−0.468, 0.296) | −0.029 (−0.065, 0.007) |
| CCI score | ||||||||
| 0 | 0.984 (0.958, 1.010) | 0.007 (0.004, 0.009) | −0.100 (−0.143, −0.057) | −0.001 (−0.004, 0.002) | 2.004 (1.960, 2.047) | 0.005 (0.001, 0.009) | 0.009 (−0.061, 0.079) | 0.010 (0.005, 0.016) |
| 1 and 2 | 1.873 (1.814, 1.933) | 0.005 (0.001, 0.01) | −0.166 (−0.235, −0.097) | 0.003 (−0.004, 0.01) | 3.776 (3.671, 3.881) | −0.004 (−0.013, 0.006) | −0.015 (−0.162, 0.133) | 0.020 (0.005, 0.034) |
| ≥3 | 3.862 (3.781, 3.942) | 0.003 (−0.004, 0.011) | −0.250 (−0.377, −0.123) | 0.015 (0.005, 0.025) | 7.162 (7.052, 7.273) | 0.006 (−0.005, 0.016) | −0.021 (−0.180, 0.138) | −0.001 (−0.016, 0.015) |
| Subgroup | Zolpidem Estimate (95% CI; Lower, Upper); | Other Hypnotics Estimate (95% CI; Lower, Upper) | ||||||
|---|---|---|---|---|---|---|---|---|
| Intercept | Baseline Trend | Broadcasting | Time after Broadcasting | Intercept | Baseline Trend | Broadcasting | Time after Broadcasting | |
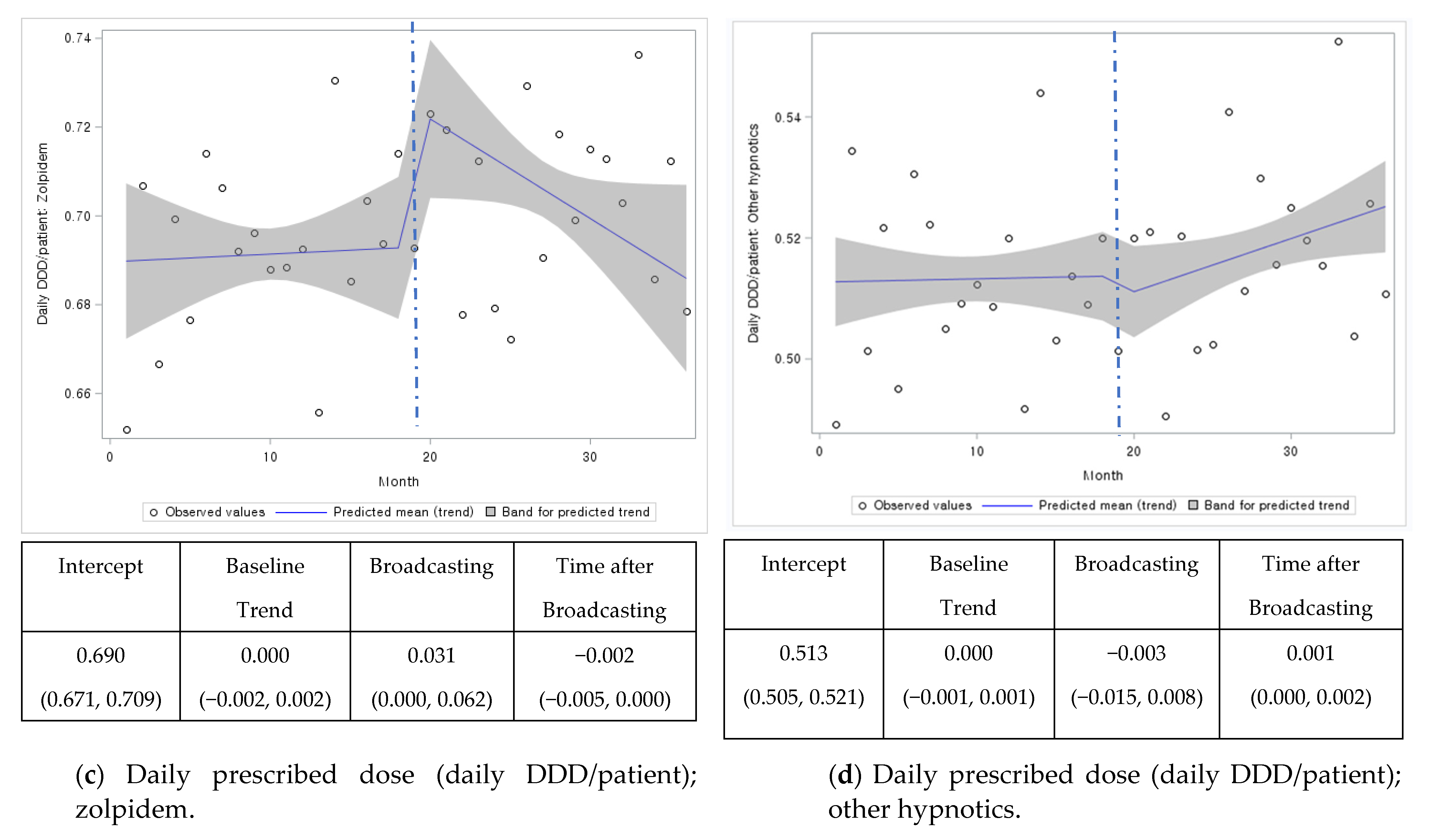
| Overall | 0.690 (0.671, 0.709) | 0.000 (−0.002, 0.002) | 0.031 (0.000, 0.062) | −0.002 (−0.005, 0.000) | 0.513. (0.505, 0.521) | 0.000 (−0.001, 0.001) | −0.003 (−0.015, 0.008) | 0.001 (0.000, 0.002) |
| Male | 0.703 (0.700, 0.706) | 0.001 (0.001, 0.001) | 0.003 (−0.001, 0.007) | −0.001 (−0.001, −0.001) | 0.550 (0.54, 0.560) | 0.000 (−0.001, 0.001) | 0.002 (−0.012, 0.016) | 0.001 (0.002, 0.003) |
| Female | 0.669 (0.653, 0.686) | 0.001 (0.000, 0.003) | 0.000 (−0.023, 0.023) | −0.001 (−0.003, 0.001) | 0.492 (0.4, 0.494) | 0.000 (0.000, 0.001) | −0.006 (−0.009, −0.002) | 0.001 (0.000, 0.001) |
| Age group | ||||||||
| 20–39 | 0.654 (0.635, 0.672) | 0.002 (0.001, 0.004) | 0.004 (−0.023, 0.031) | −0.003 (−0.005, 0.000) | 0.491 (0.475, 0.508) | 0.002 (0.000, 0.003) | −0.009 (−0.033, 0.015) | −0.001 (−0.004, 0.001) |
| 40–64 | 0.670 (0.659, 0.681) | 0.001 (0.000, 0.002) | 0.004 (−0.012, 0.019) | −0.001 (−0.002, 0.001) | 0.534 (0.526, 0.542) | 0.001 (0.000, 0.002) | −0.001 (−0.013, 0.011) | −0.001 (−0.002, 0.001) |
| 65+ | 0.707 (0.691, 0.722) | 0.000 (−0.001, 0.001) | 0.000 (−0.012, 0.013) | 0.000 (−0.001, 0.001) | 0.496 (0.487, 0.506) | −0.001 (−0.002, 0.000) | −0.004 (−0.018, 0.009) | 0.002 (0.001, 0.003) |
| Health insurance | 0.668 (0.659, 0.678) | 0.001 (0.000, 0.001) | 0.002 (−0.008, 0.012) | −0.001 (−0.002, 0.000) | 0.481 (0.473, 0.488) | 0.000 (0.000, 0.001) | −0.006 (−0.016, 0.005) | 0.001 (0.000, 0.002) |
| Medical Aid | 0.7782 (0.7642, 0.7922) | 0.0009 (−0.0004, 0.0023) | 0.0055 (−0.0154, 0.0264) | −0.0011 (−0.003, 0.0009) | 0.69 (0.686, 0.693) | 0.000 (0.000, 0.000) | −0.001 (−0.006, 0.003) | 0.000 (−0.001, 0.001) |
| National Meritorious service | 0.8831 (0.7576, 1.0086) | 0.011 (−0.001, 0.023) | 0.0544 (−0.1496, 0.2583) | −0.0226 (−0.038, −0.0073) | 0.737 (0.555, 0.919) | −0.001 (−0.006, 0.003) | 0.137 (0.077, 0.197) | 0.001 (−0.005, 0.007) |
| Psychiatric disorder (−) | 0.572 (0.554, 0.590) | 0.002 (0.000, 0.004) | 0.000 (−0.026, 0.025) | −0.002 (−0.005, 0.000) | 0.319 (0.309, 0.328) | 0.001 (0.000, 0.001) | −0.007 (−0.015, 0.001) | 0.001 (0.000, 0.002) |
| Psychiatric disorder (1) (+) | 0.746 (0.734, 0.758) | 0.001 (−0.001, 0.002) | −0.001 (−0.018, 0.016) | 0.000 (−0.002, 0.002) | 0.546 (0.543, 0.549) | 0.000 (0.000, 0.000) | −0.002 (−0.006, 0.003) | 0.001 (0.000, 0.001) |
| CCI score | ||||||||
| 0 | 0.650 (0.631, 0.669) | 0.002 (0.000, 0.004) | 0.005 (−0.022, 0.032) | −0.003 (−0.005, 0.000) | 0.514 (0.505, 0.524) | 0.000 (−0.001, 0.001) | 0.003 (−0.010, 0.016) | 0.000 (−0.001, 0.002) |
| 1 and 2 | 0.669 (0.659, 0.679) | 0.000 (0.000, 0.001) | 0.004 (−0.006, 0.015) | 0.000 (−0.001, 0.001) | 0.499 (0.492, 0.506) | 0.000 (−0.001, 0.001) | −0.004 (−0.014, 0.006) | 0.001 (0.000, 0.002) |
| ≥3 | 0.717 (0.702, 0.732) | 0.001 (0.000, 0.002) | 0.001 (−0.013, 0.015) | −0.001 (−0.002, 0.001) | 0.526 (0.523, 0.529) | 0.000 (0.000, 0.000) | −0.006 (−0.009, −0.002) | 0.001 (0.001, 0.001) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.-R.; Heo, K.-N.; Yu, Y.M.; Yeom, G.-B.; Choi, H.D.; Lee, J.-Y.; Ah, Y.-M. Interrupted Time Series Analysis of Changes in Zolpidem Use Due to Media Broadcasts. Int. J. Environ. Res. Public Health 2021, 18, 5114. https://doi.org/10.3390/ijerph18105114
Yang B-R, Heo K-N, Yu YM, Yeom G-B, Choi HD, Lee J-Y, Ah Y-M. Interrupted Time Series Analysis of Changes in Zolpidem Use Due to Media Broadcasts. International Journal of Environmental Research and Public Health. 2021; 18(10):5114. https://doi.org/10.3390/ijerph18105114
Chicago/Turabian StyleYang, Bo-Ram, Kyu-Nam Heo, Yun Mi Yu, Ga-Bin Yeom, Hye Duck Choi, Ju-Yeun Lee, and Young-Mi Ah. 2021. "Interrupted Time Series Analysis of Changes in Zolpidem Use Due to Media Broadcasts" International Journal of Environmental Research and Public Health 18, no. 10: 5114. https://doi.org/10.3390/ijerph18105114
APA StyleYang, B.-R., Heo, K.-N., Yu, Y. M., Yeom, G.-B., Choi, H. D., Lee, J.-Y., & Ah, Y.-M. (2021). Interrupted Time Series Analysis of Changes in Zolpidem Use Due to Media Broadcasts. International Journal of Environmental Research and Public Health, 18(10), 5114. https://doi.org/10.3390/ijerph18105114






