Comorbidities and Cofactors of Anaphylaxis in Patients with Moderate to Severe Anaphylaxis. Analysis of Data from the Anaphylaxis Registry for West Pomerania Province, Poland
Abstract
1. Introduction
- Non-modifiable/stable/independent/intrinsic
- Modifiable/unstable/dependent/extrinsic
2. Objective
3. Material and Methods
3.1. Study Design and Data Collection
3.2. Statistical Analysis
4. Results
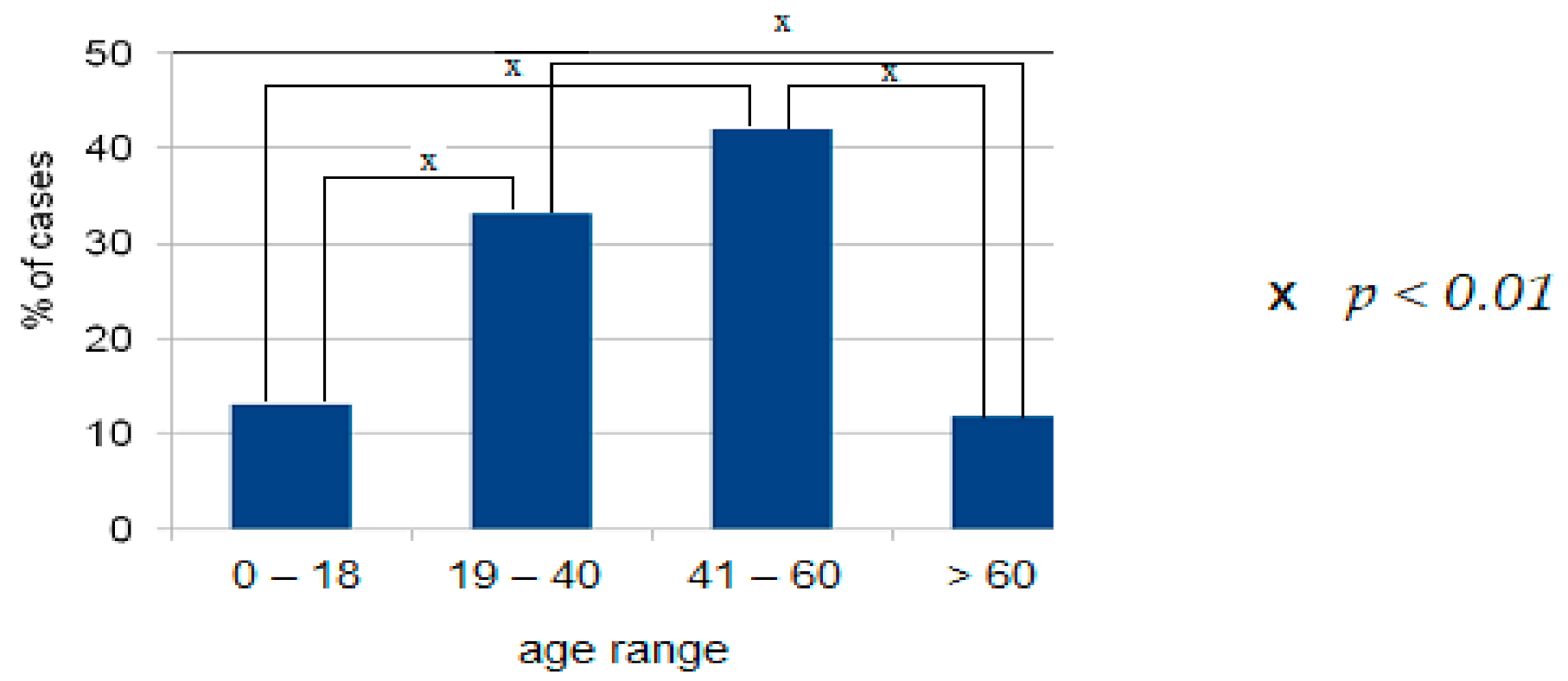
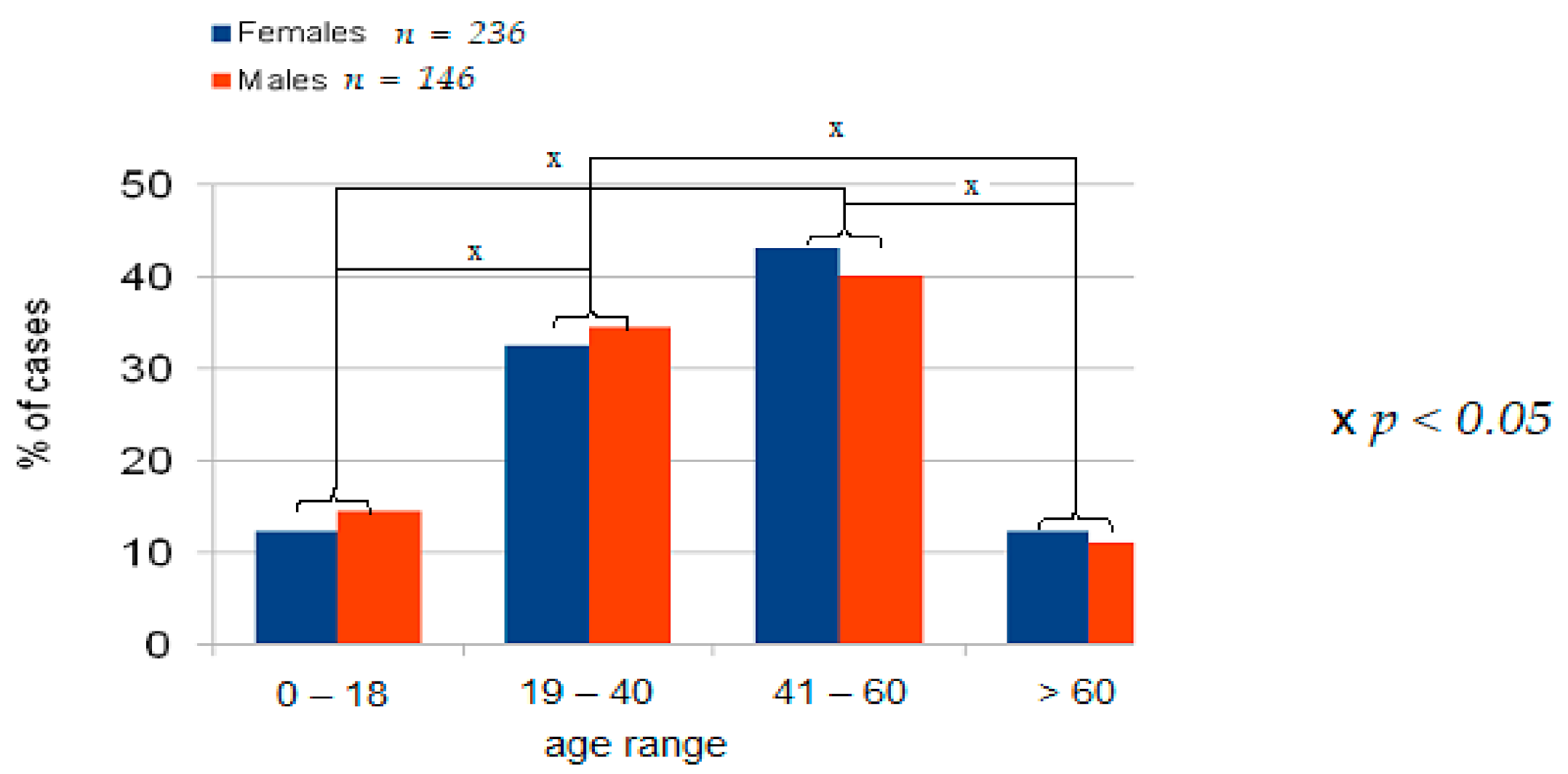
4.1. Demographic Data
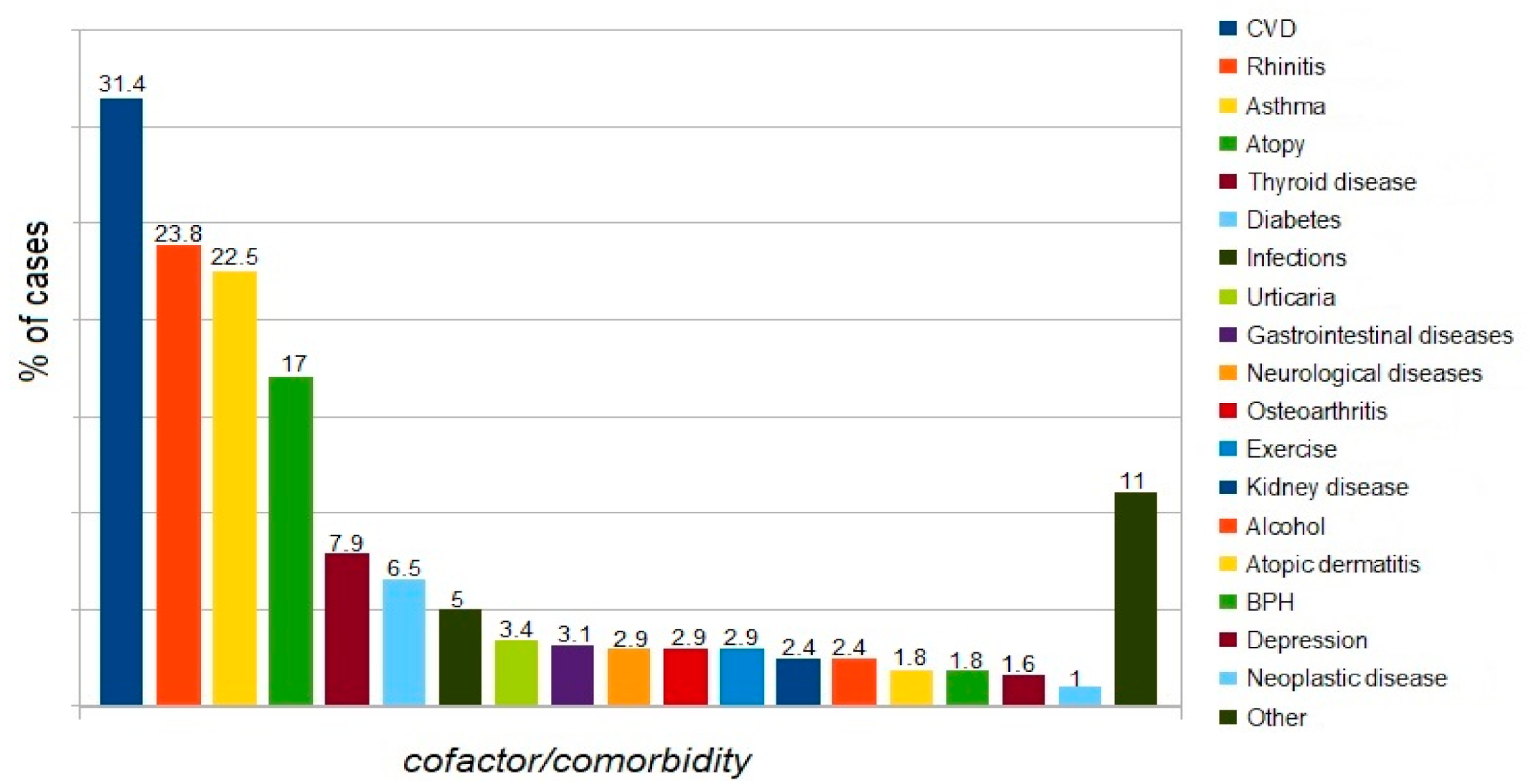
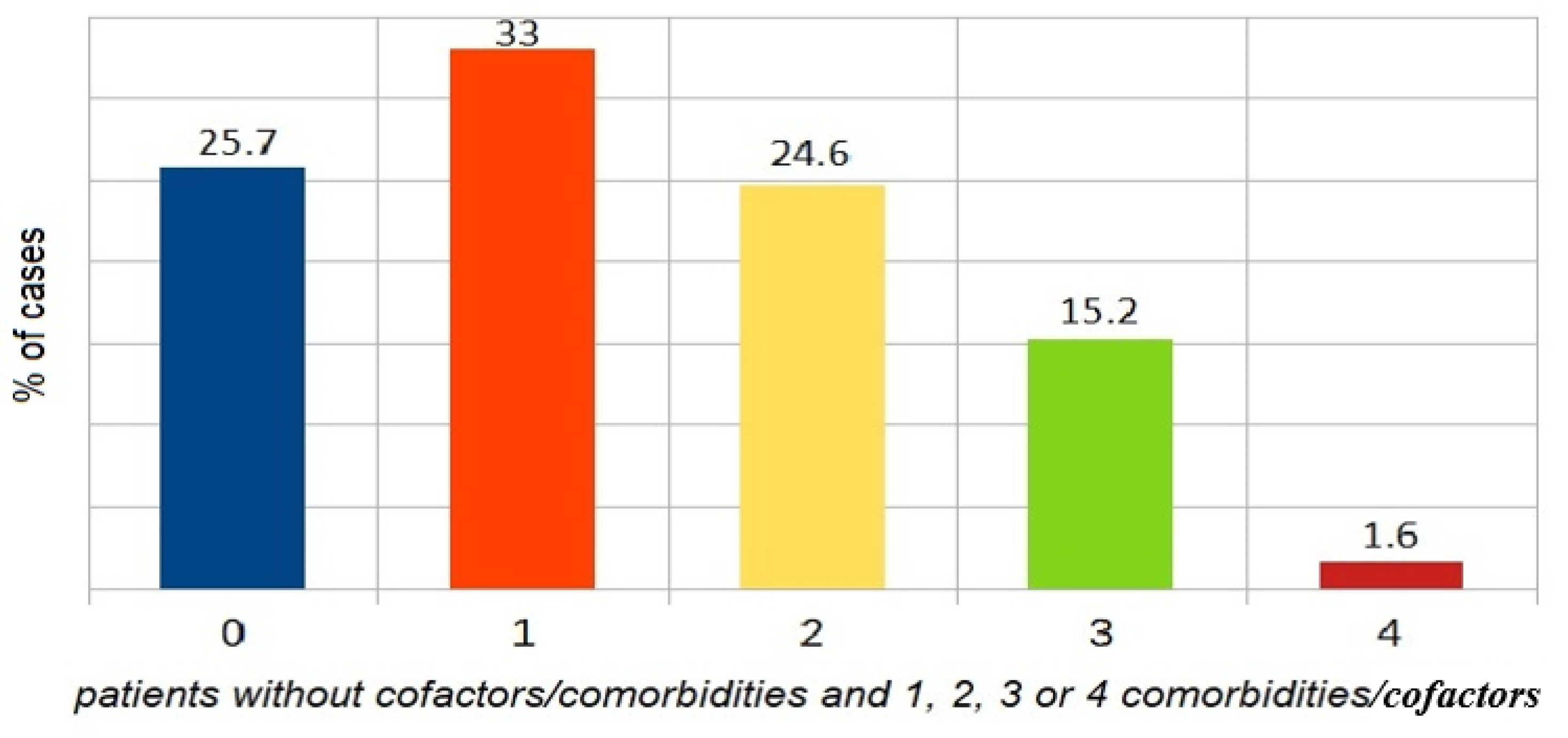
4.2. Comorbidities
4.3. Tryptase
4.4. Drugs
5. Analysis of Results and Discussion
5.1. Additional Material
5.2. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- Did Your Patients, after contact with any factor, or spontaneously experience: difficulty breathing, wheezing, hypotension, cramping abdominal pain, diarrhea, vomiting, loss of consciousness? If so, complete the data
- Year of the patient’ birth
- Date of anaphylaxis onset
- Place of reaction
- Gender
- Mark the organ systems involved
- 6.1.
- Cutaneous: angioedema, flush, generalized erythema, generalized itching, generalized urticaria
- 6.2.
- Respiratory: apnoea, dyspnoea, stridor
- 6.3.
- Gastrointestinal: abdominal pain, diarrhoea, nausea, vomiting, incontinence
- 6.4.
- Cardiovascular: loss of consciousness, drop in blood pressure, collapse, cardiac arrest, dizziness, tachycardia, disorientation
- Mark the diagnostic tests usedMedical Interview, skin tests, sIgE, tryptase, provocation, other
- Was the reaction the first time?
- Is the trigger factor known?—is the identified trigger of anaphylaxis?Is this medicine? substanceIs this food? which oneIs this venom? which oneOther
- Prevention
- Please write other important details about this episode
References
- Hepner, D.L.; Castells, M.C. Anaphylaxis during the Perioperative Period. Anesth. Analg. 2003, 97, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.J.; Worm, M.; Ansotegui, I.J.; El-Gamal, Y.; Rivas, M.F.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; Tanno, L.K.; et al. Time to revisit the definition and clinical criteria for anaphylaxis? World Allergy Organ. J. 2019, 12, 100066. [Google Scholar] [CrossRef] [PubMed]
- Simons, F.E.R.; Ardusso, L.R.F.; Bilò, M.B. World allergy organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ. J. 2011, 4, 13–37. [Google Scholar] [CrossRef]
- Panesar, S.S.; Javad, S.; De Silva, D.; Nwaru, B.I.; Hickstein, L.; Muraro, A.; Roberts, G.; Worm, M.; Bilò, M.B.; Cardona, V.; et al. The epidemiology of anaphylaxis in Europe: A systematic review. Allergy 2013, 68, 1353–1361. [Google Scholar] [CrossRef]
- Lieberman, P.; Nicklas, R.A.; Oppenheimer, J. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J. Allergy Clin. Immunol. 2010, 126, 477–480. [Google Scholar] [CrossRef]
- Brown, S.G.; Mullins, R.J.; Gold, M.S. Anaphylaxis: Diagnosis and management. Med. J. Aust. 2006, 185, 283–289. [Google Scholar] [CrossRef]
- Wood, R.A.; Camargo, C.A.; Lieberman, P.; Sampson, H.A.; Schwartz, L.B.; Zitt, M.; Collins, C.; Tringale, M.; Wilkinson, M.; Boyle, J.; et al. Anaphylaxis in America: The prevalence and characteristics of anaphylaxis in the United States. J. Allergy Clin. Immunol. 2014, 133, 461–467. [Google Scholar] [CrossRef]
- Tanno, L.K.; Bierrenbach, A.L.; Simons, F.E.R.; Cardona, V.; Bernard, T.Y.H.; Molinari, N.; Calderón, M.; Worm, M.; Chang, Y.-S.; Papadopoulos, N.G.; et al. Critical view of anaphylaxis epidemiology: Open questions and new perspectives. Allergy Asthma Clin. Immunol. 2018, 14, 1–11. [Google Scholar] [CrossRef]
- Jimenez-Rodriguez, T.W.; Garcia-Neuer, M.; Alenazy, L.A.; Castells, M. Anaphylaxis in the 21st century: Phenotypes, endotypes, and biomarkers. J. Asthma Allergy 2018, 11, 121–142. [Google Scholar] [CrossRef]
- Sampson, H.A.; Muñoz-Furlong, A.; Campbell, R.L.; Adkinson, N.F.; Bock, S.A.; Branum, A.; Brown, S.G.; Camargo, C.A.; Cydulka, R.; Galli, S.J.; et al. Second symposium on the definition and management of anaphylaxis: Summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J. Allergy Clin. Immunol. 2006, 117, 391–397. [Google Scholar] [CrossRef]
- Simons, F.E.R.; Ardusso, L.R.; Bilò, M.B.; Cardona, V.; Ebisawa, M.; El-Gamal, Y.M.; Lieberman, P.; Lockey, R.F.; Muraro, A.; Roberts, G.; et al. International consensus on (ICON) anaphylaxis. World Allergy Organ. J. 2014, 7, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Roberts, G.; Worm, M.; Bilò, M.B.; Brockow, K.; Rivas, M.F.; Santos, A.F.; Zolkipli, Z.Q.; Bellou, A.; Beyer, K.; et al. Anaphylaxis: Guidelines from the European Academy of Allergy and Clinical Immunology. Allergy 2014, 69, 1026–1045. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Cano, R.; Pascal, M.; Araujo, G.; Goikoetxea, M.J.; Valero, A.L.; Picado, C.; Bartra, J. Mechanisms, Cofactors, and Augmenting Factors Involved in Anaphylaxis. Front. Immunol. 2017, 8, 1193. [Google Scholar] [CrossRef]
- Ring, J.; Messmer, K. Incidence and severity of anaphylactoid reactions to colloid volume substituted. Lancet 1977, 1, 466–469. [Google Scholar] [CrossRef]
- Muller, H.L. Diagnosis and treatment of insect sensitivity. J. Asthma Res. 1966, 3, 331–333. [Google Scholar] [CrossRef]
- Brown, S.G. Clinical features and severity gradning of anaphylaxis. J. Allergy Clin. Immunol. 2004, 114, 371–376. [Google Scholar] [CrossRef]
- Muraro, A.; Roberts, G.; Clark, A.; Eigenmann, P.A.; Halken, S.; Lack, G.; Moneret-Vautrin, A.; Niggemann, B.; Rancé, F.; EAACI Task Force on Anaphylaxis in Children. The management of anaphylaxis in childhood: Position paper of the European academy of allergology and clinical immunology. Allergy 2007, 62, 857–871. [Google Scholar] [CrossRef]
- Mehl, A.; Wahn, U.; Niggermann, B. Anaphylactic reactions in children—A questionnaire- based survey in Germany. Allergy 2005, 60, 1440–1445. [Google Scholar] [CrossRef]
- Simons, F.E.R.; Ebisawa, M.; Sanchez-Borges, M.; Thong, B.Y.; Worm, M.; Tanno, L.K.; Lockey, R.F.; El-Gamal, Y.M.; Brown, S.G.A.; Park, H.-S.; et al. 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ. J. 2015, 8, 32. [Google Scholar] [CrossRef]
- Clark, S.; Wei, W.; Rudders, S.A.; Camargo, C.A., Jr. Risk factors for severe anaphylaxis in patients receiving anaphylaxis treatment in US emergency departments and hospitals. J. Allergy Clin. Immunol. 2014, 134, 1125–1130. [Google Scholar] [CrossRef]
- Ye, Y.-M.; Kim, M.K.; Kang, H.-R.; Kim, T.-B.; Sohn, S.-W.; Koh, Y.-I.; Park, H.-K.; Jang, G.C.; Kim, C.-W.; Jee, Y.K.; et al. Predictors of the severity and serious outcomes of anaphylaxis in Korean adults: A multicenter retrospective case study. Allergy Asthma Immunol. Res. 2015, 7, 22–29. [Google Scholar] [CrossRef]
- Vazquez-Ortiz, M.; Álvaro, M.; Piquer, M.; Giner, M.T.; Domínguez, O.; Lozano, J.; Jiménez-Feijoo, R.; Cambra, F.J.; Plaza, A.M. Life-threatening anaphylaxis to egg and milk oral immunotherapy in asthmatic teenagers. Ann. Allergy Asthma Immunol. 2014, 113, 482–484. [Google Scholar] [CrossRef]
- Triggiani, M.; Montagni, M.; Parente, R.; Ridolo, E. Anaphylaxis and cardiovascular diseases: A dangerous liaison. Curr. Opin. Allergy Clin. Immunol. 2014, 14, 309–315. [Google Scholar] [CrossRef]
- Rueff, F.; Vos, B.; Elberink, J.O.; Bender, A.; Chatelain, R.; Dugas-Breit, S.; Horny, H.-P.; Kuechenhoff, H.; Linhardt, A.; Mastnik, S.; et al. Predictors of clinical effectiveness of Hymenoptera venom immunotherapy. Clin. Exp. Allergy 2014, 44, 736–746. [Google Scholar] [CrossRef]
- Valent, P. Risk factors and management of severe life-threatening anaphylaxis in patients with clonal mast cell disorders. Clin. Exp. Allergy 2014, 44, 914–920. [Google Scholar] [CrossRef]
- Gülen, T.; Hägglund, H.; Sander, B.; Dahlén, B.; Nilsson, G. The presence of mast cell clonality in patients with unexplained anaphylaxis. Clin. Exp. Allergy 2014, 44, 1179–1187. [Google Scholar] [CrossRef]
- Álvarez-Twose, I.; Zanotti, R.; González-De-Olano, D.; Bonadonna, P.; Vega, A.; Matito, A.; Sánchez-Muñoz, L.; Morgado, J.M.; Perbellini, O.; Montero, A.G.; et al. Nonaggressive systemic mastocytosis (SM) without skin lesions associated with insect-induced anaphylaxis shows unique features versus other indolent SM. J. Allergy Clin. Immunol. 2014, 133, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.T.; Worm, M.; Bilo, M.B. Editorial: Anaphylaxis—A distinct immunological syndrome, but how much do we really understand? Front. Immunol. 2019, 10, 2943. [Google Scholar] [CrossRef]
- Skypala, I.J. Food-induced anaphylaxis: Role of hidden allergens and cofactors. Front. Immunol. 2019, 10, 673. [Google Scholar] [CrossRef]
- Brown, S.G.A.; Stone, S.F.; Fatovich, D.M.; Burrows, S.; Holdgate, A.; Celenza, A.; Coulson, A.; Hartnett, L.; Nagree, Y.; Cotterell, C.; et al. Anaphylaxis: Clinical patterns, mediator release, and severity. J. Allergy Clin. Immunol. 2013, 132, 1141–1149.e5. [Google Scholar] [CrossRef]
- Wölbing, F.; Fischer, J.; Köberle, M.; Kaesler, S.; Biedermann, T. About the role and underlying mechanisms of cofactors in anaphylaxis. Allergy 2013, 68, 1085–1092. [Google Scholar] [CrossRef]
- Fischer, J.; Hebsaker, J.; Caponetto, P.; Platts-Mills, T.A.; Biedermann, T. Galactose-alpha-1,3-galactose sensitization is a prerequisite for pork-kidney allergy and cofactor-related mammalian meat anaphylaxis. J. Allergy Clin. Immunol. 2014, 134, 755–759.e1. [Google Scholar] [CrossRef]
- Hox, V.; Desai, A.; Bandara, G.; Gilfillan, A.M.; Metcalfe, D.D.; Olivera, A. Estrogen increases the severity of anaphylaxis in female mice through enhanced endothelial nitric oxide synthase expression and nitric oxide production. J. Allergy Clin. Immunol. 2015, 135, 729–736.e5. [Google Scholar] [CrossRef]
- Shadick, N.A.; Liang, M.H.; Partridge, A.J.; Bingham, C.; Wright, E.; Fossel, A.H.; Sheffer, A.L. The natural history of exercise-induced anaphylaxis: Survey results from a 10-year follow-up study. J. Allergy Clin. Immunol. 1999, 104, 123–127. [Google Scholar] [CrossRef]
- Worm, M.; Francuzik, W.; Renaudin, J.-M.; Bilo, M.B.; Cardona, V.; Hofmeier, K.S.; Köhli, A.; Bauer, A.; Christoff, G.; Cichocka-Jarosz, E.; et al. Factors increasing the risk for a severe reaction in anaphylaxis: An analysis of data from The European Anaphylaxis Registry. Allergy 2018, 73, 1322–1330. [Google Scholar] [CrossRef]
- Turner, P.J.; Gowland, M.H.; Sharma, V.; Ierodiakonou, D.; Harper, N.; Garcez, T.; Pumphrey, R.; Boyle, R.J. Increase in anaphylaxis-related hospitalizations but no increase in fatalities: An analysis of United Kingdom national anaphylaxis data, 1992-2012. J. Allergy Clin. Immunol. 2015, 135, 956–963.e1. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, P.; Simons, F.E.R. Anaphylaxis and cardiovascular disease: Therapeutic dilemmas. Clin. Exp. Allergy 2015, 45, 1288–1295. [Google Scholar] [CrossRef]
- Valencia, B.; Inés, M. Perioperative anaphylaxis. Rev. Bras. Anestesiol. 2015, 65, 292–297. [Google Scholar]
- Asaumi, T.; Ebisawa, M. How to manage food dependent exercise induced anaphylaxis (FDEIA). Curr. Opin. Allergy Clin. Immunol. 2018, 18, 243–247. [Google Scholar] [CrossRef]
- Sampson, H.A. Anaphylaxis and emergency treatment. Pediatrics 2003, 111, 1601–1608. [Google Scholar]
- Worm, M.; Dölle, S.; Francuzik, W. Data from the anaphylaxis registry of the German-speaking countries. Revue Française d’Allergologie 2015, 55, 452–455. [Google Scholar] [CrossRef]
- Poziomkowska-Gesicka, I.; Kurek, M. Clinical manifestations and causes of anaphylaxis. Analysis of 382 cases from the anaphylaxis registry in West Pomerania Province in Poland. Int. J. Environ. Res. Public Health 2020, 17, 2787. [Google Scholar] [CrossRef]
- Przybilla, B.; Rueff, F.; Walker, A.I.; Räwer, H.-C.; Aberer, W.; Bauer, C.P.; Berdel, D.; Biedermann, T.; Brockow, K.; Forster, J.; et al. Diagnose und Therapie der Bienen- und Wespengiftallergie. Allergo J. 2011, 20, 318–339. [Google Scholar] [CrossRef]
- Sturm, G.J.; Varga, E.M.; Roberts, G.; Mosbech, H.; Bilò, M.B.; Akdis, C.A.; Antolín-Amérigo, D.; Cichocka-Jarosz, E.; Gawlik, R.; Jakob, T.; et al. EAACI guidelines on allergen immunotherapy: Hymenoptera venom allergy. Allergy 2018, 73, 744–764. [Google Scholar] [CrossRef] [PubMed]
- Koschel, D. Impaired quality of life in patients with insect venom allergy. Allergo J. Int. 2017, 26, 88–92. [Google Scholar] [CrossRef]
- Manmohan, M.; Müller, S.; Rauber, M.M.; Koberne, F.; Reisch, H.; Koster, J.; Böhm, R.; Messelken, M.; Fischer, M.; Jakob, T. Current state of follow-up care for patients with Hymenoptera venom anaphylaxis in southwest Germany. Allergo J. Int. 2018, 27, 4–14. [Google Scholar] [CrossRef]
- Fehr, D.; Micaletto, S.; Moehr, T.; Schmid-Grendelmeier, P. Risk factors for severe systemic sting reactions in wasp (Vespula spp.) and honeybee (Apis mellifera) venom allergic patients. Clin. Transl. Allergy 2019, 9, 54. [Google Scholar] [CrossRef]
- Oropeza, A.R.; Bindslev-Jensen, C.; Broesby-Olsen, S.; Kristensen, T.K.; Møller, M.B.; Vestergaard, H.; Kjaer, H.F.; Halken, S.; Lassen, A.; Mortz, C.G. Patterns of anaphylaxis after diagnostic workup: A follow-up study of 226 patients with suspected anaphylaxis. Allergy 2017, 72, 1944–1952. [Google Scholar] [CrossRef]
- Jahnz-Rozyk, K.; Raciborski, F.; Śliwczyński, A.M.; Kłak, A.; Pinkas, J. Anaphylaxis in Poland the epidemiology and direct costs. ADV Dermatol. Alergol. 2017, 34, 573–579. [Google Scholar] [CrossRef]
- Tejedor-Alonso, M.A.; Moro-Moro, M.; Múgica-García, M.V. Epidemiology of anaphylaxis: Contributions from the last 10 years. J. Investig. Allergol. Clin. Immunol. 2015, 25, 163–175. [Google Scholar]
- Ewan, P.; Brathwaite, N.; Leech, S.; Luyt, D.; Till, S.; Nasser, S.M.; Powell, R.; Clark, A. Prescribing an adrenaline auto-injector—Personalized care recommended. Clin. Exp. Allergy 2016, 46, 1621–1622. [Google Scholar] [CrossRef] [PubMed]
- Rueff, F.; Przybilla, B.; Biló, M.B.; Müller, U.; Scheipl, F.; Aberer, W.; Birnbaum, J.; Bodzenta-Lukaszyk, A.; Bonifazi, F.; Bucher, C.; et al. Predictors of severe systemic anaphylactic reactions in patients with Hymenoptera venom allergy: Importance of baseline serum tryptase—A study of the European Academy of Allergology and Clinical Immunology Interest Group on Insect Venom Hypersensitivity. J. Allergy Clin. Immunol. 2009, 124, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Mempel, M.; Schober, W.; Behrendt, H.; Ring, J. Gender difference, sex hormones, and immediate type hypersensitivity reactions. Allergy 2008, 63, 1418–1427. [Google Scholar] [CrossRef]
- Shaker, M.; Wallace, D.V.; Golden, D.B.; Oppenheimer, J.; Bernstein, J.A.; Campbell, R.C.; Dinakar, C.; Ellis, A.; Greenhawt, M.; Khan, D.A.; et al. Anaphylaxis—A 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J. Allergy Clin. Immunol. 2020, 145, 1082–1123. [Google Scholar] [CrossRef]
- Kucharewicz, I.; Bodzenta-Lukaszyk, A.; Szymanski, W.; Mroczko, B.; Szmitkowski, M. Basal serum tryptase level correlates with severity of hymenoptera sting and age. J. Investig. Allergol. Clin. Immunol. 2007, 17, 65–69. [Google Scholar]
- Haeberli, G.; Brönnimann, M.; Hunziker, T.; Müller, U. Elevated basal serum tryptase and hymenoptera venom allergy: Relation to severity of sting reactions and to safety and efficacy of venom immunotherapy. Clin. Exp. Allergy 2003, 33, 1216–1220. [Google Scholar] [CrossRef]
- Ludolph-Hauser, D.; Ruëff, F.; Fries, C.; Schöpf, P.; Przybilla, B. Constitutively raised serum concentrations of mast-cell tryptase and severe anaphylactic reactions to Hymenoptera stings. Lancet 2001, 357, 361–362. [Google Scholar] [CrossRef]
- Greenhawt, M.; Akin, C. Mastocytosis and allergy. Curr. Opin. Allergy Clin. Immunol. 2007, 7, 387–392. [Google Scholar] [CrossRef]
- Lieberman, P.; Nicklas, R.A.; Randolph, C.; Oppenheimer, J.; Bernstein, D.I.; Bernstein, J.A.; Ellis, A.K.; Golden, D.B.; Greenberger, P.A.; Kemp, S.; et al. Anaphylaxis—A practice parameter update 2015. Ann. Allergy Asthma Immunol. 2015, 115, 341–384. [Google Scholar] [CrossRef]
- Bilo, B.M.; Rueff, F.; Mosbech, H.; Bonifazi, F.; Oude-Elberink, J.N.G.; The EAACI Interest Group on Insect Venom Hypersensitivity. Diagnosis of Hymenoptera venom allergy. Allergy 2005, 60, 1339–1349. [Google Scholar] [CrossRef]
- Aurich, S.; Dölle-Bierke, S.; Francuzik, W.; Bilo, M.B.; Christoff, G.; Fernandez-Rivas, M.; Hawranek, T.; Pföhler, C.; Poziomkowska-Gȩsicka, I.; Renaudin, J.-M.; et al. Anaphylaxis in elderly patients—Data from the European Anaphylaxis Registry. Front. Immunol. 2019, 10, 750. [Google Scholar] [CrossRef] [PubMed]
- Versluis, A.; Van Os-Medendorp, H.; Kruizinga, A.G.; Blom, W.M.; Houben, G.F.; Knulst, A.C. Cofactors in allergic reactions to food: Physical exercise and alcohol are the most important. Immun. Inflamm. Dis. 2016, 4, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Helbling, A.; Hurni, T.; Mueller, U.R.; Pichler, W.J. Incidence of anaphylaxis with circulatory symptoms: A study over a 3-year period comprising 940,000 inhabitants of the Swiss Canton Bern. Clin. Exp. Allergy 2004, 34, 285–290. [Google Scholar] [CrossRef]
- Alvarez-Perea, A.; Tomás-Pérez, M.; Martínez-Lezcano, P.; Marco, G.; Pérez, D.; Zubeldia, J.M.; Baeza, M.L. Anaphylaxis in adolescent/adult patients treated in the emergency department: Differences between initial impressions and the definitive diagnosis. J. Investig. Allergol. Clin. Immunol. 2015, 25, 288–294. [Google Scholar]
- Worm, M.; Edenharter, G.; Rueff, F.; Scherer, K.; Pföhler, C.; Mahler, V.; Treudler, V.; Lang, R.; Nemat, K.; Koehli, A. Symptom profile and risk factors of anaphylaxis in Central Europe. Allergy 2012, 67, 691–698. [Google Scholar] [CrossRef]
- De Silva, R.; Dasanayake, W.M.D.K.; Karunatilleke, C.; Malavige, G.N. Food dependant exercise induced anaphylaxis a retrospective study from 2 allergy clinics in Colombo, Sri Lanka. Allergy Asthma Clin. Immunol. 2015, 11, 22. [Google Scholar] [CrossRef]
- Scherf, K.A.; Lindenau, A.-C.; Valentini, L.; Collado, M.C.; García-Mantrana, I.; Christensen, M.; Tomsitz, D.; Kugler, C.; Biedermann, T.; Brockow, K. Cofactors of wheat-dependent exercise-induced anaphylaxis do not increase highly individual gliadin absorption in healthy volunteers. Clin. Transl. Allergy 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Hompes, S.; Dölle, S.; Grünhagen, J.; Grabenhenrich, L.B.; Worm, M. Elicitors and co-factors in food-induced anaphylaxis in adults. Clin. Transl. Allergy 2013, 3, 38. [Google Scholar] [CrossRef]
- Aihara, M.; Miyazawa, M.; Osuna, H.; Tsubaki, K.; Ikebe, T.; Aihara, Y.; Ikezawa, Z. Food-dependent exercise-induced anaphylaxis: Influence of concurrent aspirin administration on skin testing and provocation. Br. J. Dermatol. 2002, 146, 466–472. [Google Scholar] [CrossRef]
- Christensen, M.; Eller, E.; Mortz, C.G.; Brockow, K.; Bindslev-Jensen, C. Exercise lowers threshold and increases severity, but wheat-dependent, exercise-induced anaphylaxis can be elicited at rest. J. Allergy Clin. Immunol. Pract. 2018, 6, 514–520. [Google Scholar] [CrossRef]
- Zogaj, D.; Ibranji, A.; Hoxha, M. Exercise–induced anaphylaxis: The role of cofactors. Mater. Socio Med. 2014, 26, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Borrega, M.; Garcia, B.; Larramendi, C.H.; Azofra, J.; González-Mancebo, E.; Alvarado, M.; De Durana, A.D.; Núñez-Orjales, R.; Dieguez, M.C.; Guilarte, M.; et al. IgE-mediated sensitization to Galactose-α-1,3- Galactose (α-Gal) in urticaria and anaphylaxis in Spain: Geographical variations and risk factors. J. Investig. Allergol. Clin. Immunol. 2019, 29, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Heaps, A.; Carter, S.; Selwood, C.; Moody, M.; Unsworth, J.; Deacock, S.; Sumar, N.; Bansal, A.; Hayman, G.; El-Shanawany, T.; et al. The utility of the ISAC allergen array in the investigation of idiopathic anaphylaxis. Clin. Exp. Immunol. 2014, 177, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Cardona, V.; Guilarte, M.; Labrador-Horrillo, M. Molecular diagnosis usefulness for idiopathic anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 248–252. [Google Scholar] [CrossRef]
- Fiedler, E.-M.; Zuberbier, T.; Worm, M. A combination of wheat flour, ethanol and food additives inducing FDEIA. Allergy 2002, 57, 1090–1091. [Google Scholar] [CrossRef]
- Romano, A.; Scala, E.; Rumi, G.; Gaeta, F.; Caruso, C.M.R.; Alonzi, C.; Maggioletti, M.; Ferrara, R.; Palazzo, P.; Palmieri, V.; et al. Lipid transfer proteins: The most frequent sensitizer in Italian subjects with food-dependent exercise-induced anaphylaxis. Clin. Exp. Allergy 2012, 42, 1643–1653. [Google Scholar] [CrossRef]
- Pascal, M.; Muñoz-Cano, R.; Reina, Z.; Palacín, A.; Vilella, R.; Picado, C.; Juan, M.; Sánchez-López, J.; Rueda, M.; Salcedo, G.; et al. Lipid transfer protein syndrome: Clinical pattern, cofactor effect and profile of molecular sensitization to plant-foods and pollens. Clin. Exp. Allergy 2012, 42, 1529–1539. [Google Scholar] [CrossRef]
- Solley, G.O. Stinging and biting insect allergy: An Australian experience. Ann. Allergy Asthma Immunol. 2004, 93, 532–537. [Google Scholar] [CrossRef]
- Lockey, R.F.; Turkeltaub, P.C.; Baird-Warren, I.A.; Olive, C.A.; Olive, E.S.; Peppe, B.C.; Bukantz, S.C. The Hymenoptera venom study I, 1979–1982: Demographics and history-sting data. J. Allergy Clin. Immunol. 1988, 82, 370–381. [Google Scholar] [CrossRef]
- Schuberth, K.C.; Lichtenstein, L.M.; Kagey-Sobatka, A.; Szklo, M.; Kwiterovich, K.A.; Valentine, M.D. Epidemiologic study of insect allergy in children. II. Effect of accidental stings in allergic children. J. Pediatr. 1983, 102, 361–365. [Google Scholar] [CrossRef]
- Graft, D.F.; Schuberth, K.C.; Kagey-Sobotka, A.; Kwiterovich, K.A.; Niv, Y.; Lichtenstein, L.M.; Valentine, M.D. A prospective study of the natural history of large local reactions after Hymenoptera stings in children. J. Pediatr. 1984, 104, 664–668. [Google Scholar] [CrossRef]
- Mauriello, P.; Barde, S.; Georgitis, J.; Reisman, R. Natural history of large local reactions from stinging insects. J. Allergy Clin. Immunol. 1984, 74, 494–498. [Google Scholar] [CrossRef]
- Bonifazi, F.; Jutel, M.; Bilo, B.M.; Birnbaum, J.; Muller, U.; The EAACI Interest Group on Insect Venom Hypersensitivity. Prevention and treatment of hymenoptera venom allergy: Guidelines for clinical practice. Allergy 2005, 60, 1459–1470. [Google Scholar] [CrossRef]
- Pucci, S.; Incorvaia, C.; Romano, A. Large local reaction to Hymenoptera stings: Sound studies are needed to change a shared concept. Immun. Inflamm. Dis. 2019, 7, 258–259. [Google Scholar] [CrossRef]
- Severino, M.; Bonadonna, P.; Passalacqua, G. Large local reactions from stinging insects: From epidemiology to management. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 334–337. [Google Scholar] [CrossRef]
- Golden, D.B.; Demain, J.; Freeman, T.; Graft, D.; Tankersley, M.; Tracy, J.; Blessing-Moore, J.; Bernstein, D.; Dinakar, C.; Greenhawt, M.; et al. Stinging insect hypersensitivity. Ann. Allergy Asthma Immunol. 2017, 118, 28–54. [Google Scholar] [CrossRef]
- Bilò, M.B.; Martini, M.; Pravettoni, V.; Bignardi, D.; Bonadonna, P.; Cortellini, G.; Kosinska, M.; Macchia, D.; Mauro, M.; Meucci, E.; et al. Large local reactions to Hymenoptera stings: Outcome of restings in real life. Allergy 2019, 74, 1969–1976. [Google Scholar] [CrossRef]
- Golden, D.B. Large local reactions to insect stings. J. Allergy Clin. Immunol. Pract. 2015, 3, 331–334. [Google Scholar] [CrossRef]
- Severino, M.G.; Cortellini, G.; Bonadonna, P.; Francescato, E.; Panzini, I.; Macchia, D.; Campi, P.; Spadolini, I.; Canonica, W.G.; Passalacqua, G. Sublingual immunotherapy for large local reactions caused by honeybee sting: A double-blind, placebo-controlled trial. J. Allergy Clin. Immunol. 2008, 122, 44–48. [Google Scholar] [CrossRef]
- Mirakian, R.; Ewan, P.W.; Durham, S.R.; Youlten, L.J.; Dugué, P.; Friedmann, P.S.; English, J.S.; Huber, P.A.J.; Nasser, S.M. BSACI guidelines for themanagement of drug allergy. Clin. Exp. Allergy 2009, 39, 43–46. [Google Scholar] [CrossRef]
- Warrington, R.; Silviu-Dan, F. Drug allergy. Allergy Asthma Clin. Immunol. 2011, 7 (Suppl. S1), S10. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.J.; Jerschow, E.; Umasunthar, T.; Elina, J.; Campbell, D.E.; Boyle, R. Fatal anaphylaxis: Mortality rate and risk factors. J. Allergy Clin. Immunol. Pract. 2017, 5, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Mayorga, C.; Fernandez, T.D.; Montañez, M.I.; Moreno, E.; Torres, M.J. Recent developments and highlights in drug hypersensitivity. Allergy 2019, 74, 2368–2381. [Google Scholar] [CrossRef] [PubMed]
- Brockow, K.; Christiansen, C.; Kanny, G.; Clément, O.; Barbaud, A.; Bircher, A.; Dewachter, P.; Guéant, J.-L.; Rodriguez Guéant, R.-M.; Mouton-Faivre, C.; et al. EAACI interest group on drug hypersensitivity: Management of hypersensitivity reactions to iodinated contrast media. Allergy 2005, 60, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.A.W.; Stacul, F.; Thomsen, H.S.; Morcos, S.K.; Members of the Contrast Media Safety Committee of the European Society of Urogenital Radiology. Late adverse reactions to intravascular iodinated contrast media. Eur. Radiol. 2003, 13, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Lim, K.-W.; Chang, Y.-S. Radiocontrast media hypersensitivity in the Asia Pacific region. Asia Pac. Allergy 2014, 4, 119–125. [Google Scholar] [CrossRef]
- Sánchez-Borges, M.; Capriles-Hulett, A. Atopy is a risk factor for non-steroidal anti-inflammatory drug sensitivity. Ann. Allergy Asthma Immunol. 2000, 84, 101–106. [Google Scholar] [CrossRef]
- Pastorello, E.A.; Zara, C.; Riario-Sforza, G.G.; Pravettoni, V.; Incorvaia, C. Atopy and intolerance of antimicrobial drugs increase the risk of reactions to acetaminophen and nimesulide in patients allergic to nonsteroidal anti-inflammatory drugs. Allergy 1998, 53, 880–884. [Google Scholar] [CrossRef]
- Kurt, E.; Demir, A.; Cadirci, O.; Yildirim, H.; Eser, T.P. Immediate-type drug hypersensitivity and associated factors in a general population. Allergol. Immunopathol. 2011, 39, 27–31. [Google Scholar] [CrossRef]
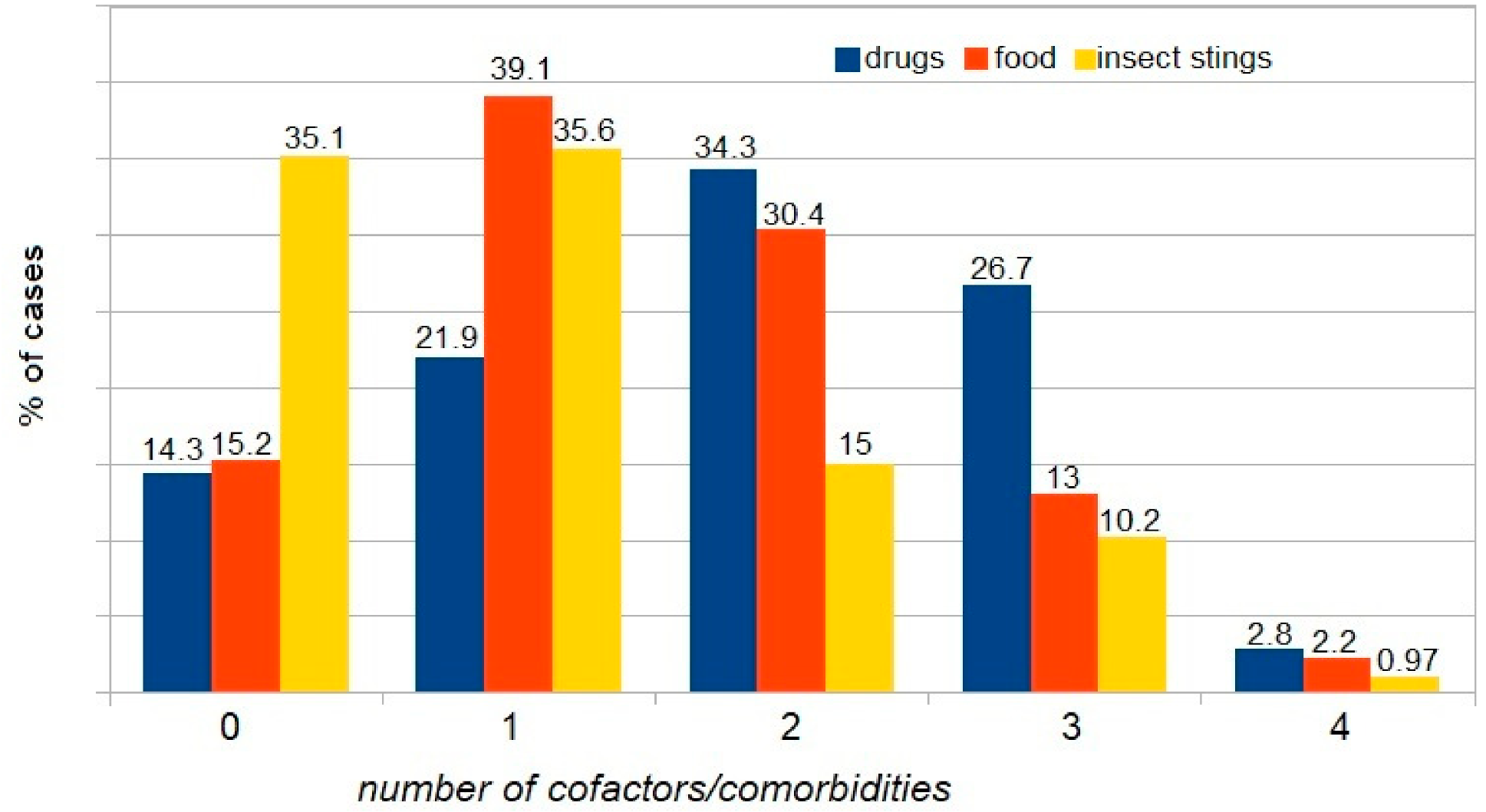
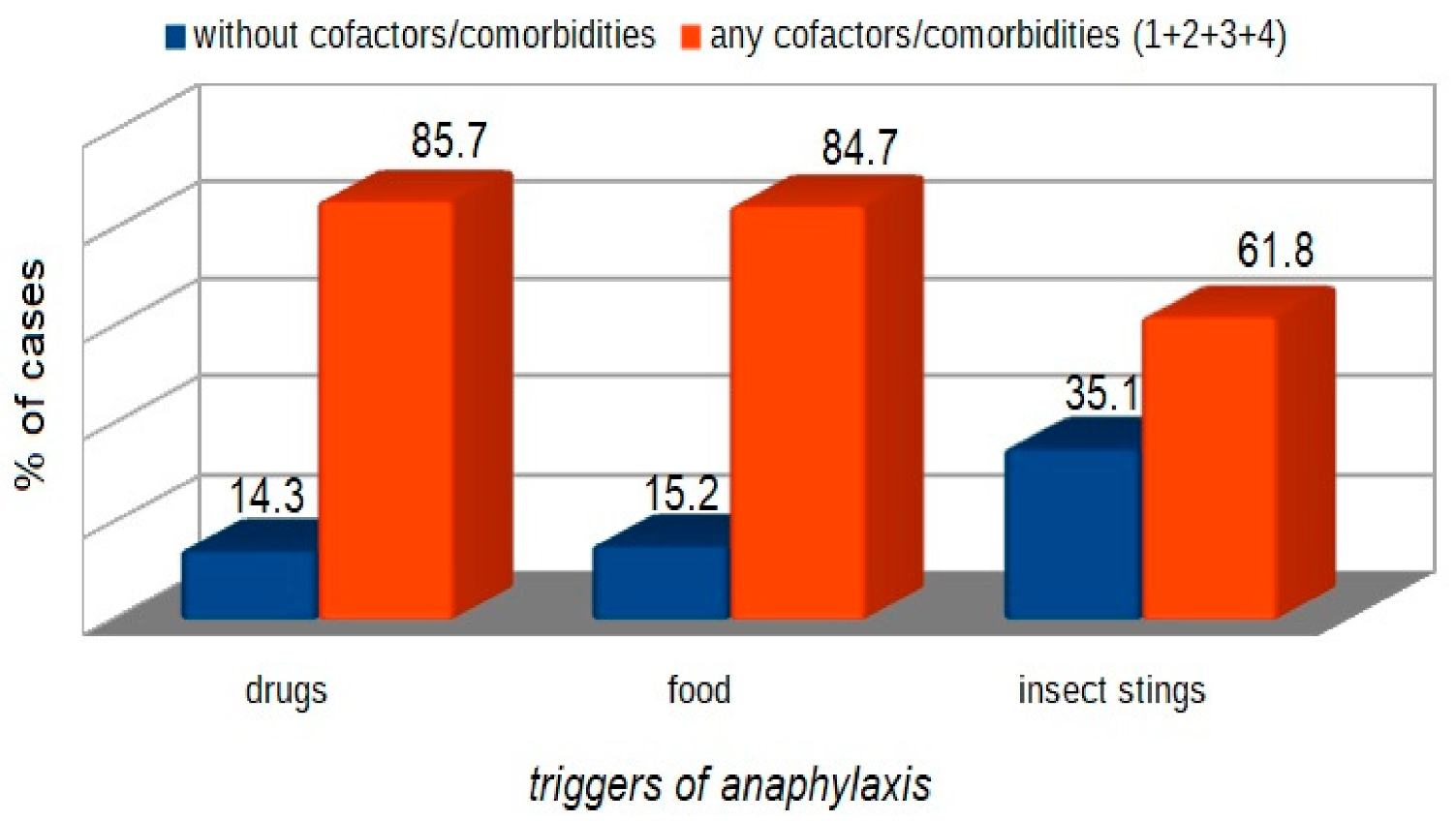
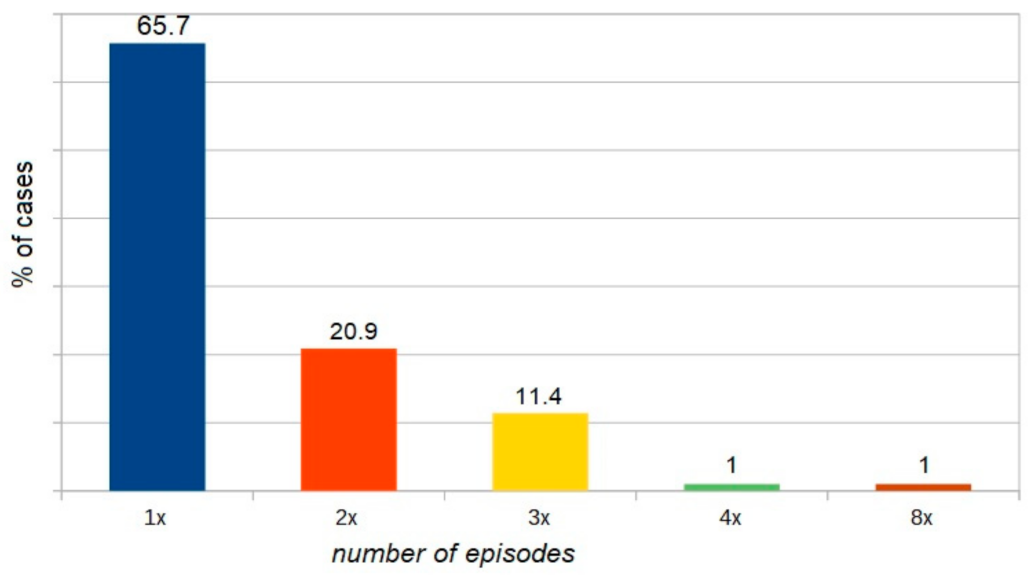
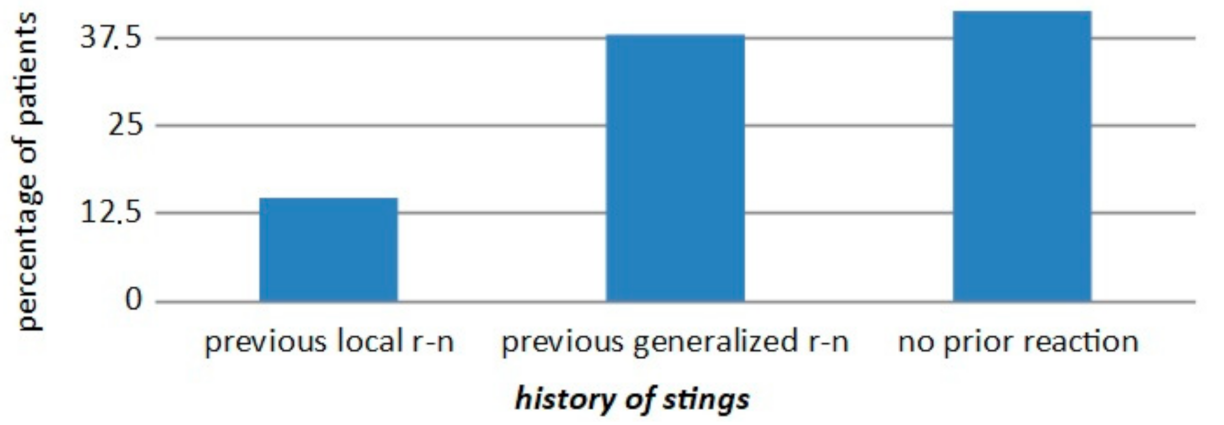
| WAO [3] | A serious life-threatening generalized or systemic hypersensitivity reaction. A serious allergic reaction that is rapid in onset and might cause death |
| EAACI [4] | A severe life-threatening generalized or systemic hypersensitivity reaction. An acute, potentially fatal, multi-organ system, allergic reaction. |
| AAAAI/ACAAI [5] | An acute life-threatening systemic reaction with varied mechanisms, clinical presentations, and severity that results from the sudden release of mediators from mast cells and basophils. |
| ASCIA [6] | Anaphylaxis is a serious, rapid-onset, allergic reaction that may cause death. Severe anaphylaxis is characterized by life-threatening upper airway obstruction, bronchospasm and/or hypotension. |
| Classification by Ring and Messmer | |
|---|---|
| Grade I | Generalized skin symptoms (e.g., flush, generalized urticaria, angioedema) |
| Grade II | Mild to moderate pulmonary, cardiovascular, and/or gastrointestinal symptoms |
| Grade III | Anaphylactic shock, loss of consciousness |
| Grade IV | Cardiac arrest, apnea |
| Age Range | n Patients | % of Whole Group n = 382 | % of Tested n = 148 | % of Age Group |
|---|---|---|---|---|
| 0–18 | 0 | 0 | 0 | 0 |
| 19–40 | 1 | 0.26 | 0.68 | 0.79 |
| 41–60 | 11 | 2.88 | 7.43 | 6.88 |
| >60 | 3 | 0.79 | 2.03 | 6.67 |
| Total | 15 | 3.93 | 10.14 | 3.93 |
| Allergen | Cofactor | Patient Age | Recurrence |
|---|---|---|---|
| Omega 5 gliadin | Exercise | 24 years | 8× |
| Omega 5 gliadin | Exercise + asthma | 28 years | 5× |
| Omega 5 gliadin | Exercise + asthma + alcohol | 28 years | 4× |
| Omega 5 gliadin | Exercise | 27 years | 2× |
| LPT | Exercise + asthma | 31 years | 4× |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poziomkowska-Gęsicka, I.; Kostrzewska, M.; Kurek, M. Comorbidities and Cofactors of Anaphylaxis in Patients with Moderate to Severe Anaphylaxis. Analysis of Data from the Anaphylaxis Registry for West Pomerania Province, Poland. Int. J. Environ. Res. Public Health 2021, 18, 333. https://doi.org/10.3390/ijerph18010333
Poziomkowska-Gęsicka I, Kostrzewska M, Kurek M. Comorbidities and Cofactors of Anaphylaxis in Patients with Moderate to Severe Anaphylaxis. Analysis of Data from the Anaphylaxis Registry for West Pomerania Province, Poland. International Journal of Environmental Research and Public Health. 2021; 18(1):333. https://doi.org/10.3390/ijerph18010333
Chicago/Turabian StylePoziomkowska-Gęsicka, Iwona, Magdalena Kostrzewska, and Michał Kurek. 2021. "Comorbidities and Cofactors of Anaphylaxis in Patients with Moderate to Severe Anaphylaxis. Analysis of Data from the Anaphylaxis Registry for West Pomerania Province, Poland" International Journal of Environmental Research and Public Health 18, no. 1: 333. https://doi.org/10.3390/ijerph18010333
APA StylePoziomkowska-Gęsicka, I., Kostrzewska, M., & Kurek, M. (2021). Comorbidities and Cofactors of Anaphylaxis in Patients with Moderate to Severe Anaphylaxis. Analysis of Data from the Anaphylaxis Registry for West Pomerania Province, Poland. International Journal of Environmental Research and Public Health, 18(1), 333. https://doi.org/10.3390/ijerph18010333





