Comparative Toxic Effects of Manufactured Nanoparticles and Atmospheric Particulate Matter in Human Lung Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Substances
2.2. Cell Culture and Exposure Conditions
2.3. Cell Viability Assay
2.4. ROS Measurement
2.5. ATP Measurement
2.6. Statistical Analysis
3. Results
3.1. Characterization of Test Particles
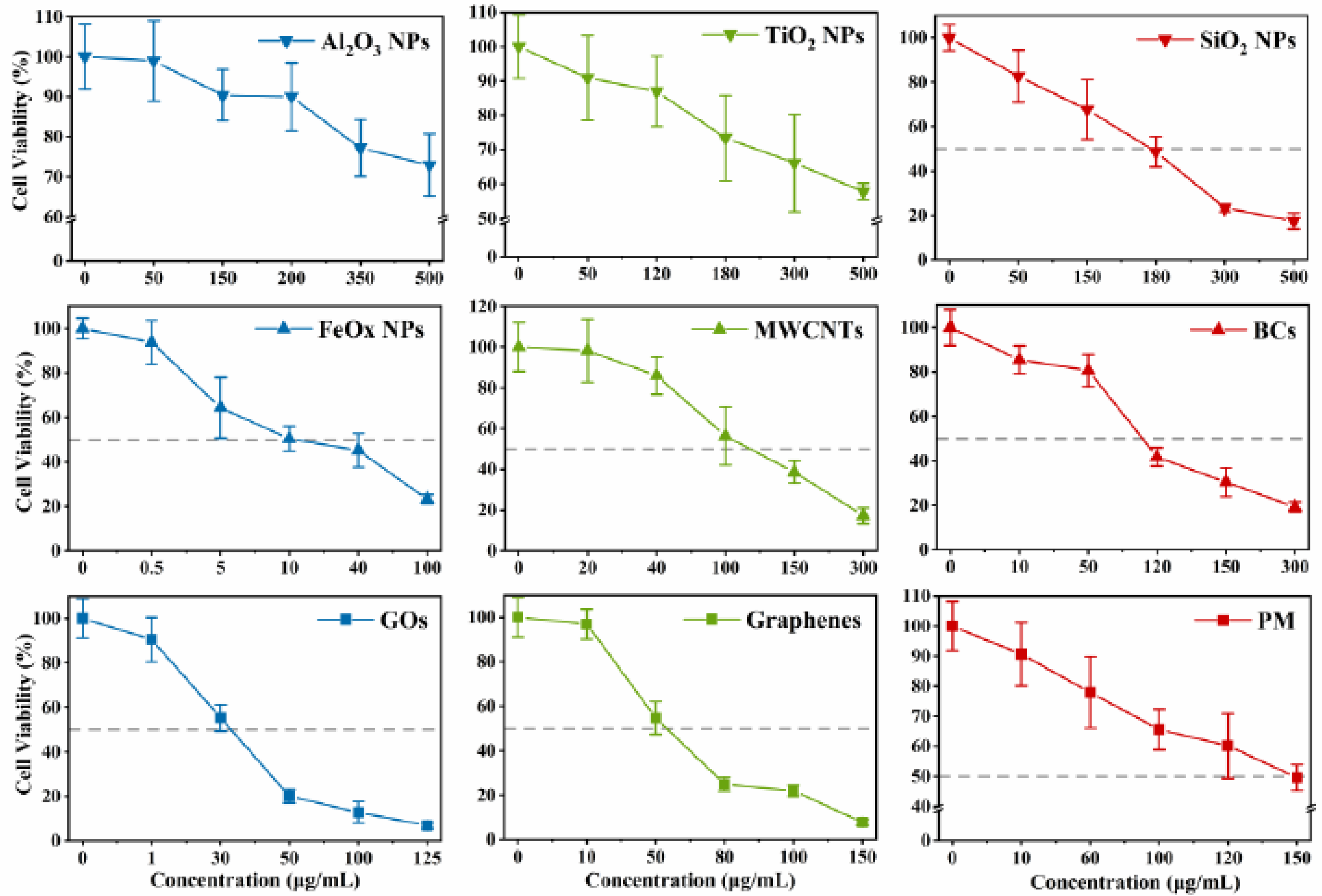
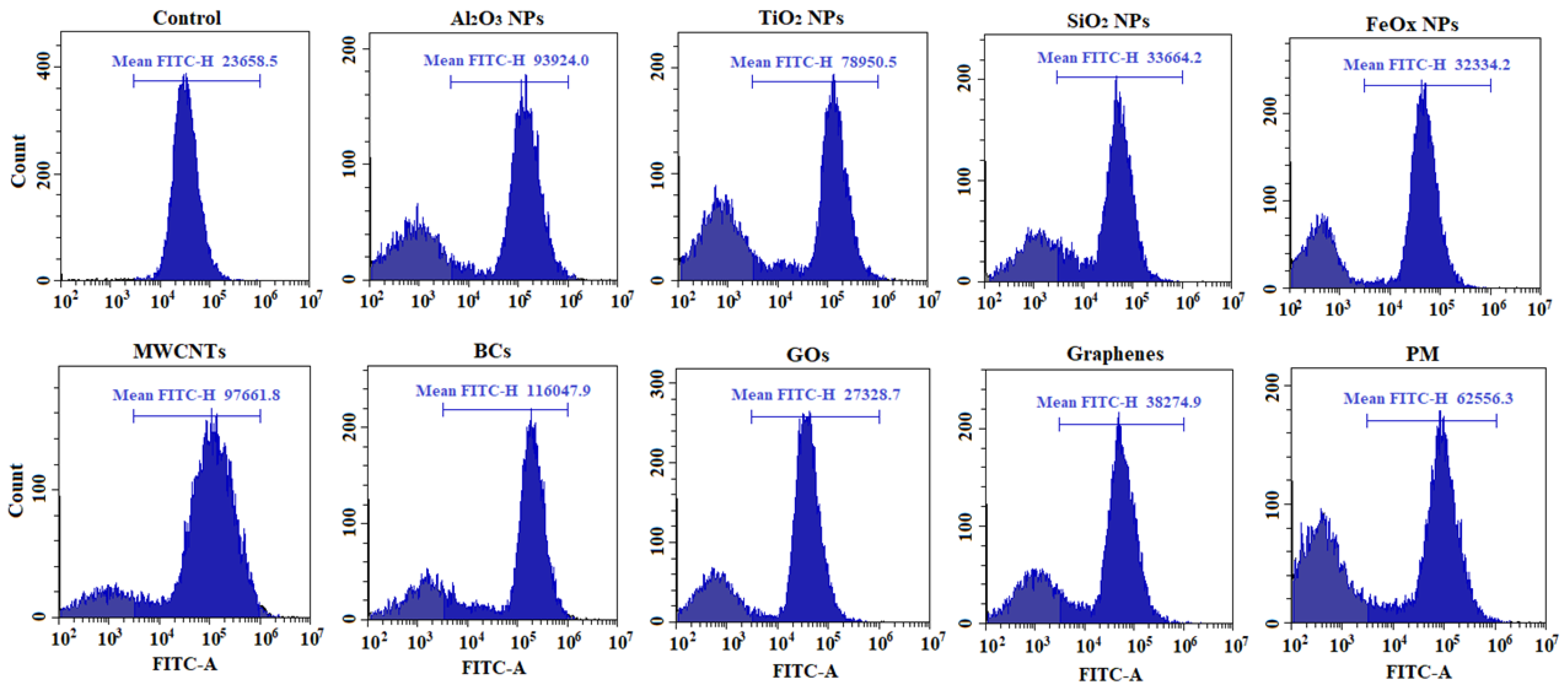
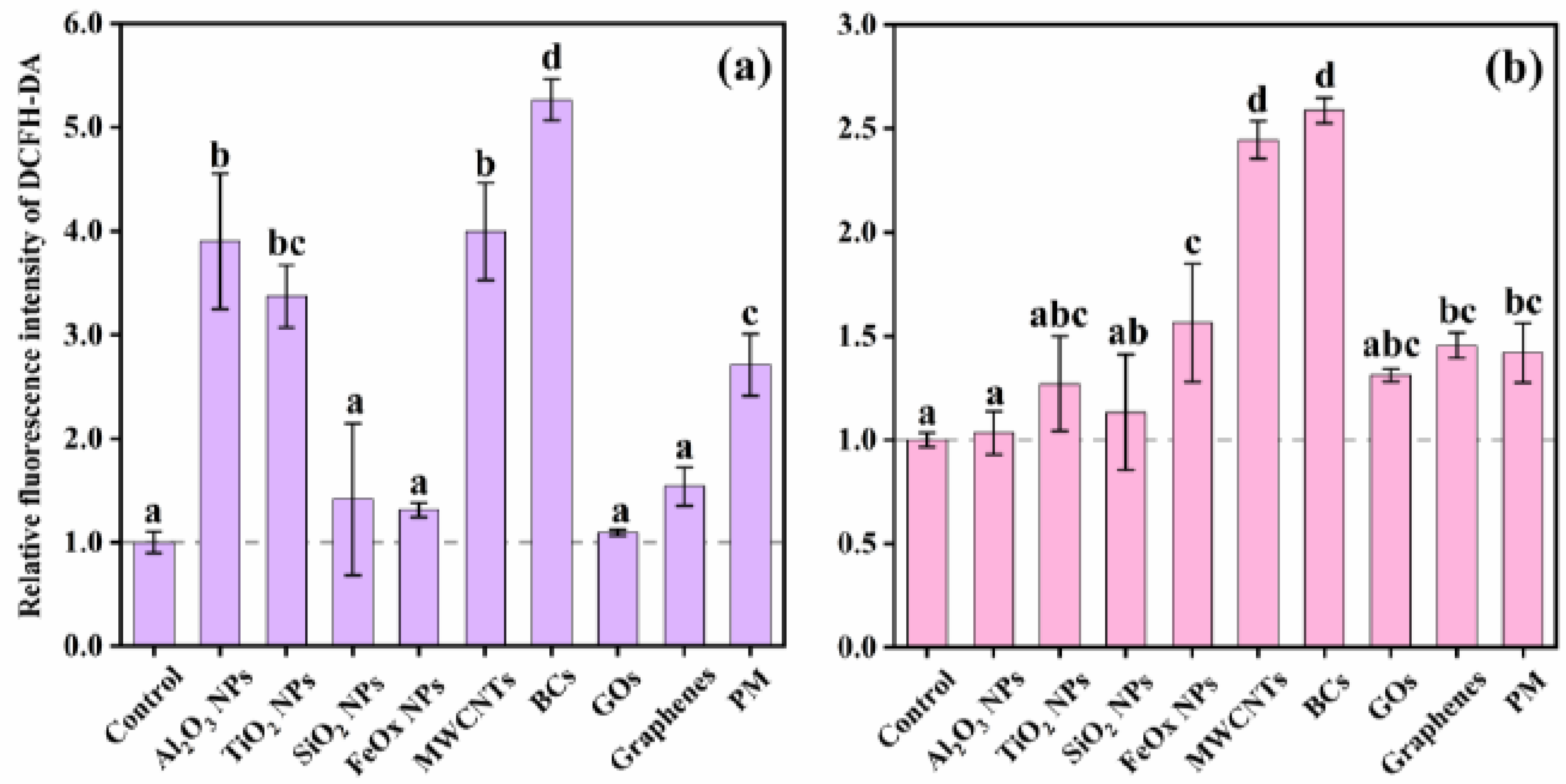
3.2. Cytotoxicity and Intracellular Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seaton, A.; Godden, D.; MacNee, W.; Donaldson, K. Particulate air pollution and acute health effects. Lancet 1995, 345, 176–178. [Google Scholar] [CrossRef]
- Nemmar, A.; Vanbilloen, H.; Hoylaerts, M.F.; Hoet, P.H.M.; Verbruggen, A.; Nemery, B. Passage of Intratracheally Instilled Ultrafine Particles from the Lung into the Systemic Circulation in Hamster. Am. J. Respir. Crit. Care Med. 2001, 164, 1665–1668. [Google Scholar] [CrossRef] [PubMed]
- Nie, D.; Chen, M.; Wu, Y.; Ge, X.; Hu, J.; Zhang, K.; Ge, P. Characterization of Fine Particulate Matter and Associated Health Burden in Nanjing. Int. J. Environ. Res. Public Health 2018, 15, 602. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhang, Z.; Lau, A.K.H.; Lin, C.Q.; Chuang, Y.C.; Chan, J.; Jiang, W.K.; Tam, T.; Yeoh, E.-K.; Chan, T.-C.; et al. Effect of long-term exposure to fine particulate matter on lung function decline and risk of chronic obstructive pulmonary disease in Taiwan: A longitudinal, cohort study. Lancet Planet. Health 2018, 2, e114–e125. [Google Scholar] [CrossRef]
- Jia, J.; Yuan, X.; Peng, X.; Yan, B. Cr(VI)/Pb2+ are responsible for PM2.5-induced cytotoxicity in A549 cells while pulmonary surfactant alleviates such toxicity. Ecotoxicol. Environ. Saf. 2019, 172, 152–158. [Google Scholar] [CrossRef]
- Di, A.; Wu, Y.; Chen, M.; Nie, D.; Ge, X. Chemical Characterization of Seasonal PM2.5 Samples and Their Cytotoxicity in Human Lung Epithelial Cells (A549). Int. J. Environ. Res. Public Health 2020, 17, 4599. [Google Scholar] [CrossRef]
- Badaloni, C.; Cesaroni, G.; Cerza, F.; Davoli, M.; Brunekreef, B.; Forastiere, F. Effects of long-term exposure to particulate matter and metal components on mortality in the Rome longitudinal study. Environ. Int. 2017, 109, 146–154. [Google Scholar] [CrossRef]
- Nel, A.E.; Xia, T.; Mädler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Kong, B.; Seog, J.H.; Graham, L.M.; Lee, S.B. Experimental considerations on the cytotoxicity of nanoparticles. Nanomedcine 2011, 6, 929–941. [Google Scholar] [CrossRef]
- Li, J.J.; Muralikrishnan, S.; Ng, C.-T.; Yung, L.-Y.L.; Bay, B.-H. Nanoparticle-induced pulmonary toxicity. Exp. Biol. Med. 2010, 235, 1025–1033. [Google Scholar] [CrossRef]
- Khan, W.; Godugu, C. Cytotoxicity of Nanomaterials: Using Nanotoxicology to Address the Safety Concerns of Nanoparticles. Pharm. Nanotechnol. 2018, 6, 3–16. [Google Scholar] [CrossRef]
- Fanizza, C.; De Berardis, B.; Ietto, F.; Soggiu, M.E.; Schirò, R.; Inglessis, M.; Ferdinandi, M.; Incoronato, F. Analysis of major pollutants and physico-chemical characteristics of PM2.5 at an urban site in Rome. Sci. Total Environ. 2018, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Soto, K.; Garza, K.; Shi, Y.; Murr, L.E. Direct contact cytotoxicity assays for filter-collected, carbonaceous (soot) nanoparticulate material and observations of lung cell response. Atmos. Environ. 2008, 42, 1970–1982. [Google Scholar] [CrossRef]
- Lu, J.; Yang, S.; Ng, K.M.; Su, C.-H.; Yeh, C.-S.; Wu, Y.-N.; Shieh, D.-B. Solid-state synthesis of monocrystalline iron oxide nanoparticle based ferrofluid suitable for magnetic resonance imaging contrast application. Nanotechnology 2006, 17, 5812–5820. [Google Scholar] [CrossRef]
- Gong, C.; Tao, G.; Yang, L.; Liu, J.; He, H.; Zhuang, Z. The role of reactive oxygen species in silicon dioxide nanoparticle-induced cytotoxicity and DNA damage in HaCaT cells. Mol. Biol. Rep. 2011, 39, 4915–4925. [Google Scholar] [CrossRef] [PubMed]
- John, J. Determination of ATP in chlorella with the luciferin-luciferase enzyme system. Anal. Biochem. 1970, 37, 409–416. [Google Scholar] [CrossRef]
- Kouassi, K.S.; Billet, S.; Garã§on, G.; Verdin, A.; Diouf, A.; Cazier, F.; Djaman, J.; Courcot, D.; Shirali, P. Oxidative damage induced in A549 cells by physically and chemically characterized air particulate matter (PM2.5) collected in Abidjan, CÃ’te d’Ivoire. J. Appl. Toxicol. 2009, 30, 310–320. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, Y.; Ge, X.; Nie, D.; Wang, M.; Zhou, H.; Chen, M. In vitro toxicity evaluation of heavy metals in urban air particulate matter on human lung epithelial cells. Sci. Total. Environ. 2019, 678, 301–308. [Google Scholar] [CrossRef]
- Guan, L.; Rui, W.; Bai, R.; Zhang, W.; Zhang, F.; Ding, W. Effects of Size-Fractionated Particulate Matter on Cellular Oxidant Radical Generation in Human Bronchial Epithelial BEAS-2B Cells. Int. J. Environ. Res. Public Health 2016, 13, 483. [Google Scholar] [CrossRef]
- Zhang, Y.; Darland, D.C.; He, Y.; Yang, L.; Dong, X.; Chang, Y. Reduction of PM2.5 toxicity on human alveolar epithelial cells A549 by tea polyphenols. J. Food Biochem. 2018, 42, e12496. [Google Scholar] [CrossRef]
- Baulig, A.; Poirault, J.-J.; Ausset, P.; Schins, R.; Shi, T.; Baralle, D.; Dorlhene, P.; Meyer, M.; Lefevre, R.; Baeza-Squiban, A.; et al. Physicochemical Characteristics and Biological Activities of Seasonal Atmospheric Particulate Matter Sampling in Two Locations of Paris. Environ. Sci. Technol. 2004, 38, 5985–5992. [Google Scholar] [CrossRef] [PubMed]
- Matassoni, L.; Pratesi, G.; Centioli, D.; Cadoni, F.; Lucarelli, F.; Nava, S.; Malesani, P. Saharan dust contribution to PM10, PM2.5 and PM1 in urban and suburban areas of Rome: A comparison between single-particle SEM-EDS analysis and whole-sample PIXE analysis. J. Environ. Monit. 2011, 13, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Ward, M.; Lin, R.; Brydson, R.; Dall’Osto, M.; Harrison, R.M. Comparative study of single particle characterisation by Transmission Electron Microscopy and time-of-flight aerosol mass spectrometry in the London atmosphere. Atmos. Environ. 2012, 62, 400–407. [Google Scholar] [CrossRef]
- Jin, L.; Xie, J.; Wong, C.K.-C.; Chan, S.K.Y.; Abbaszade, G.; Schnelle-Kreis, J.; Zimmermann, R.; Li, J.; Zhang, G.; Fu, P.; et al. Contributions of City-Specific Fine Particulate Matter (PM2.5) to Differential In Vitro Oxidative Stress and Toxicity Implications between Beijing and Guangzhou of China. Environ. Sci. Technol. 2019, 53, 2881–2891. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Jin, C.; Su, Y.; Li, J.; Zhu, B. Water soluble and insoluble components of urban PM2.5 and their cytotoxic effects on epithelial cells(A549) in vitro. Environ. Pollut. 2016, 212, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Buchman, J.T.; Hudson-Smith, N.V.; Landy, K.M.; Haynes, C.L. Understanding Nanoparticle Toxicity Mechanisms To Inform Redesign Strategies To Reduce Environmental Impact. Accounts Chem. Res. 2019, 52, 1632–1642. [Google Scholar] [CrossRef]
- Kim, W.; Jeong, S.-C.; Shin, C.-Y.; Song, M.-K.; Cho, Y.; Lim, J.-H.; Gye, M.C.; Ryu, J.-C. A study of cytotoxicity and genotoxicity of particulate matter (PM2.5) in human lung epithelial cells (A549). Mol. Cell. Toxicol. 2018, 14, 163–172. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the Abilities of Ambient and Manufactured Nanoparticles to Induce Cellular Toxicity According to an Oxidative Stress Paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef]
- Li, N.; Xia, T.; Nel, A.E. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free. Radic. Biol. Med. 2008, 44, 1689–1699. [Google Scholar] [CrossRef]
- Simon, H.-U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Asharani, P.V.; Mun, G.L.K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, Y.; Liu, X.; Jin, M.; Zhang, L.; Du, Z.; Guo, C.; Huang, P.; Sun, Z. Cytotoxicity and mitochondrial damage caused by silica nanoparticles. Toxicol. Vitr. 2011, 25, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Su, S.; Jin, W.; Wang, B.; Li, N.; Shen, H.; Li, W.; Huang, Y.; Chen, H.; Zhang, Y.; et al. Characteristics and cellular effects of ambient particulate matter from Beijing. Environ. Pollut. 2014, 191, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Carnovale, C.; Bryant, G.; Shukla, R.; Bansal, V. Identifying Trends in Gold Nanoparticle Toxicity and Uptake: Size, Shape, Capping Ligand, and Biological Corona. ACS Omega 2019, 4, 242–256. [Google Scholar] [CrossRef]
- Duch, M.C.; Budinger, G.R.S.; Liang, Y.T.; Soberanes, S.; Urich, D.; Chiarella, S.E.; Campochiaro, L.A.; Gonzalez, A.; Chandel, N.S.; Hersam, M.C.; et al. Minimizing Oxidation and Stable Nanoscale Dispersion Improves the Biocompatibility of Graphene in the Lung. Nano Lett. 2011, 11, 5201–5207. [Google Scholar] [CrossRef]
- Karakoti, A.S.; Hench, L.L.; Seal, S. The potential toxicity of nanomaterials—The role of surfaces. JOM 2006, 58, 77–82. [Google Scholar] [CrossRef]
- Tedesco, S.; Doyle, H.; Blasco, J.; Redmond, G.; Sheehan, D. Exposure of the blue mussel, Mytilus edulis, to gold nanoparticles and the pro-oxidant menadione. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 151, 167–174. [Google Scholar] [CrossRef]
- Van Dong, P.; Ha, C.H.; Binh, L.T.; Kasbohm, J. Chemical synthesis and antibacterial activity of novel-shaped silver nanoparticles. Int. Nano Lett. 2012, 2, 9. [Google Scholar] [CrossRef]
- Saha, S.; Malik, M.; Qureshi, M. Study of Synergistic Effects of Antibiotics And Triangular Shaped Silver Nanoparticles, Synthesized Using UV-Light Irradiation, on S. Aureus and P. Aeruginosa. Mater. Today Proc. 2019, 18, 920–927. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, L.; Cao, Z.; Zhou, X.; Yang, F.; Fu, P.; Wang, Z.; Hu, J.; Ding, L.; Jiang, W. Dispersion of atmospheric fine particulate matters in simulated lung fluid and their effects on model cell membranes. Sci. Total. Environ. 2016, 542, 36–43. [Google Scholar] [CrossRef] [PubMed]
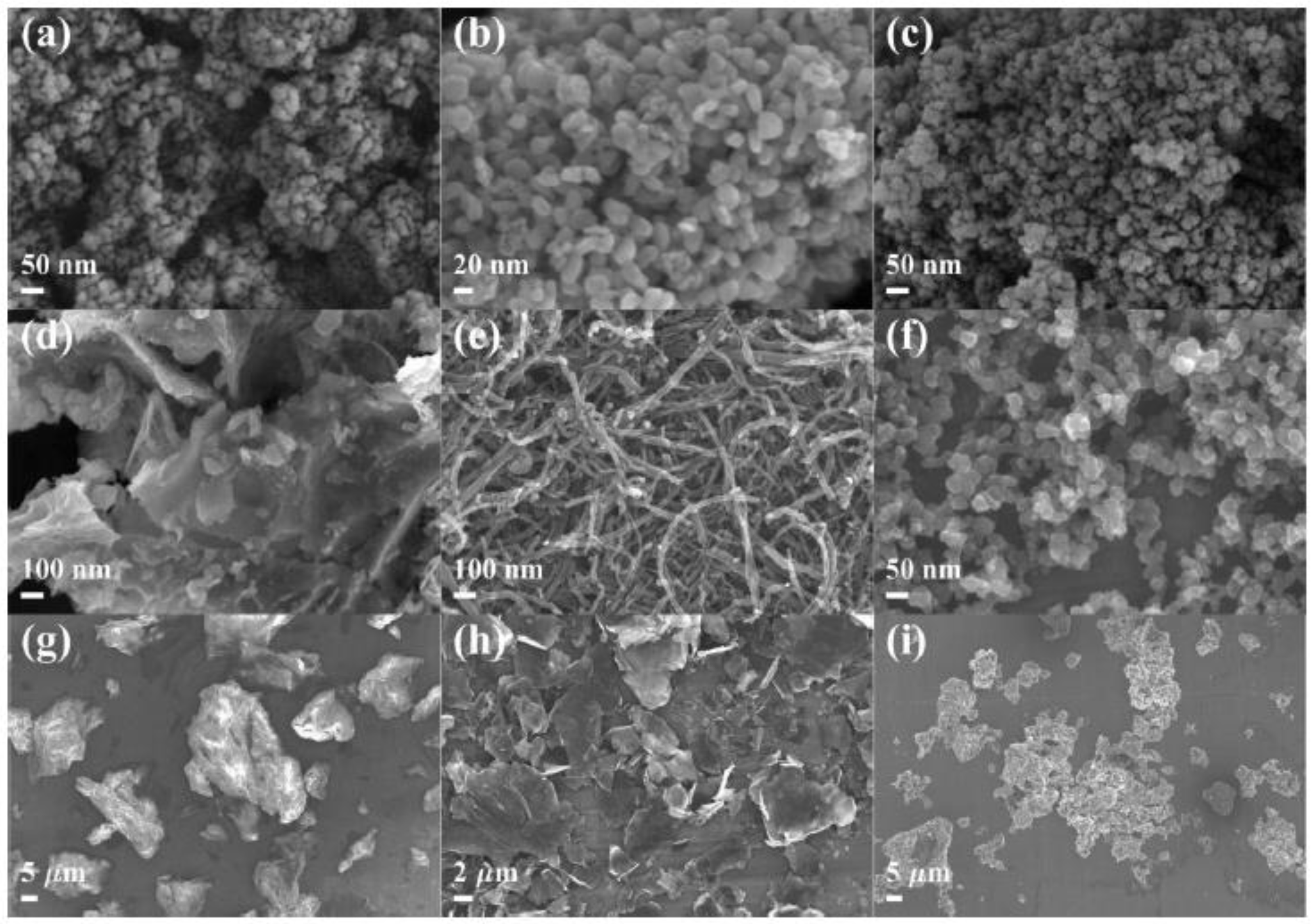


| Particles | Primary Particle Size | Hydrodynamic Diameter (μm) | Zeta Potential (mV) pH = 7.4 | ||
|---|---|---|---|---|---|
| Provided by the Manufacturers | FESEM Images | 0 h | 48 h | ||
| Al2O3 NPs | 10–15 nm | 14–60 nm | 2.2 ± 0.2 | 2.4 ± 0.2 | −19.5 ± 2.7 |
| TiO2 NPs | 15–25 nm | 15–45 nm | 1.4 ± 0.1 | 4.7 ± 1.2 | −23.9 ± 1.4 |
| SiO2 NPs | 20 nm | 13–47 nm | 0.8 ± 0.1 | 1.4 ± 0.1 | −21.3 ± 0.5 |
| FeOx NPs | — | 0.2–7.6 μm | 2.0 ± 0.1 | 1.9 ± 0.0 | −22.5 ± 1.3 |
| MWCNTs | 30–50 nm in diameter; 0.5–2.0 μm in length | 10–50 nm in diameter | 8.5 ± 1.3 | 8.2 ± 2.1 | −21.4 ± 1.2 |
| BCs | 30–45 nm | 10–60 nm | 1.0 ± 0.1 | 1.1 ± 0.1 | −26.6 ± 1.0 |
| GOs | — | 2–55 μm | 11.9 ± 0.4 | 28.3 ± 0.7 | −18.9 ± 1.3 |
| Graphenes | 6–8 nm in thickness; 5 μm in width | 1–12 μm | 13.2 ± 0.1 | 11.7 ± 3.0 | −27.6 ± 1.6 |
| PM | 5.85 μm | 1–26 μm | 12.6 ± 0.2 | 14.7 ± 3.1 | −18.8 ± 2.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Wang, M.; Luo, S.; Gu, Y.; Nie, D.; Xu, Z.; Wu, Y.; Chen, M.; Ge, X. Comparative Toxic Effects of Manufactured Nanoparticles and Atmospheric Particulate Matter in Human Lung Epithelial Cells. Int. J. Environ. Res. Public Health 2021, 18, 22. https://doi.org/10.3390/ijerph18010022
Wu Y, Wang M, Luo S, Gu Y, Nie D, Xu Z, Wu Y, Chen M, Ge X. Comparative Toxic Effects of Manufactured Nanoparticles and Atmospheric Particulate Matter in Human Lung Epithelial Cells. International Journal of Environmental Research and Public Health. 2021; 18(1):22. https://doi.org/10.3390/ijerph18010022
Chicago/Turabian StyleWu, Yun, Mei Wang, Shaojuan Luo, Yunfeng Gu, Dongyang Nie, Zhiyang Xu, Yue Wu, Mindong Chen, and Xinlei Ge. 2021. "Comparative Toxic Effects of Manufactured Nanoparticles and Atmospheric Particulate Matter in Human Lung Epithelial Cells" International Journal of Environmental Research and Public Health 18, no. 1: 22. https://doi.org/10.3390/ijerph18010022
APA StyleWu, Y., Wang, M., Luo, S., Gu, Y., Nie, D., Xu, Z., Wu, Y., Chen, M., & Ge, X. (2021). Comparative Toxic Effects of Manufactured Nanoparticles and Atmospheric Particulate Matter in Human Lung Epithelial Cells. International Journal of Environmental Research and Public Health, 18(1), 22. https://doi.org/10.3390/ijerph18010022









