Functional MRI (fMRI) Evaluation of Hyperbaric Oxygen Therapy (HBOT) Efficacy in Chronic Cerebral Stroke: A Small Retrospective Consecutive Case Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Pts Enrolment and Investigation Protocol
2.2. Ethical Committee and Written Consent
2.3. Patients
- Patient (Pt) 1. Male, 60 years old, right-handed. The patient had a cryptogenetic ischaemic stroke from a left middle cerebral artery (MCA) occlusion 14 months prior to our first visit. Clinically, he presented with a severe, non-fluent aphasia, associated with retained comprehension, dyslexia and dysgraphia, a right upper limb spastic paralysis and a right lower limb paresis. He suffered also from post-stroke depression, mainly due to the constrained absence of social life. At that time, he had just finished both physiotherapy and speech therapy, with minimal results. Speech therapy and physiotherapy were restarted and associated to HBOT during the whole period.
- Pt 2. Male, 68 years old, right-handed. He presented with a chronic multifocal encephalopathy from recurrent cerebral ischaemic strokes 5 years earlier. His clinical examination revealed moderate signs of dysarthria (he sounded clumsy in reading aloud short passages), an unsteady, wide-base gait and dysphagia.
- Pt 3. Male, 48 years old, right-handed. He had a haemorrhagic stroke, involving the right MCA territory 15 years earlier. His past medical history was characterized by recurrent seizures, initially controlled by oxcarbazepine, and ultimately by phenobarbital and carbamazepine. At a clinical examination he presented mild signs of dysarthria (denied by the Pt), upper left limb paralysis, lower left limb paresis, lack of coordination in the right limbs, severe in the lower right extremity and to a lesser extent in the upper right extremity.
- Pt 4. Male, 34 years old, right-handed. The patient suffered from a haemorrhagic transformation of an ischaemic stroke in the left MCA territory 17 months earlier. At a clinical examination he presented a global non fluent aphasia with some deficits in the reading comprehension, a complete spastic hemiplegia of the right upper limb with spastic hypertonia, a partial paresis of the right inferior limb (but he was able to walk with some walking aids) and right hemilateral hypoaesthesia.
2.4. HyperBaric Oxygen Therapy (HBOT)
2.5. MRI Acquisition Parameters
2.6. fMRI Tasks
- Receptive language (passive Test Listening, TL): a 5 min task consisting in the alternation of five 30 s ON periods of listening to one of five complete different 30 s Aesop’s fable stories, each one followed by five 30 s OFF periods of silence.It has been shown [4,5,6,7,8] that this paradigm generates eloquent areas in the following two main brain areas:
- The bilateral Heschl circumvolution (where the primary acoustic area is located) along with the bilateral surrounding so-called “acoustic belt”.
- Other bilateral eloquent acoustic areas, located in the posterior part of the superior and middle temporal gyrus, often extending unilaterally to the angular and marginal gyri, where the receptive auditory language function is located, the so-called Wernicke’s area. Wernicke’s area is more frequently located in the left hemisphere, where the language function is placed.
- Productive language (covert Words-Verbs Generation, cWVG): a 5 min task consisting in the alternation of five 30 s ON periods of listening to common nouns. Every noun is separated from the following one by 1 s, during which the Pt had to silently think and find the corresponding verb (e.g., bike, to pedal). As the previous task, each of the five 30 s ON period is followed by five 30 s OFF periods of silence.It has been shown [4,5,6,7,8] that this paradigm generates eloquent areas in the following brain regions:
- Bilateral Heschl circumvolutions and respective acoustic belts.
- Temporal acoustic and Wernicke’s areas.
- In the frontal operculus, unilaterally, more often on the left side, where the productive language area is located (the so-called Broca’s area).
- Left/Right Hand Movement (L/R HM): a 5 min task consisting in the alternation of five 30 s ON periods of continuous repetitive opposition of the thumb to each of the four remaining fingers in a sequential tapping (order 2-3-4-5-4-3-2), each one followed by five 30 s OFF periods of rest.It has been shown [4,8,9] that this paradigm generates eloquent areas in the following brain regions:
- The hand primary motor area (contralateral to the side of the hand moved), located in the so-called “central knob” in the precentral gyrus, as predicted by the somatomotor homunculus.
- The corresponding hand primary sensory area (contralateral to the side of the hand moved) in the post-central gyrus, as predicted by the somatotosensory homunculus. Activation of this area is evoked by muscles and osteotendinous proprioceptors as well as by cutaneous touch receptors, all stimulated by the hand movements.
- The mirror contralateral primary hand motor area (ipsilateral to the side of the hand moved), less frequent and less extended. This phenomenon is caused by bilateral descending neuronal discharges, due to the mental effort to perform the task correctly, resulting in an unintentional stiffening of the contralateral limb.
- The supplementary motor area (bilaterally), located antero-medially with respect to primary motor areas. This area comes into action when a complex and coordinate movement has to be carried out.
- Left/Right Foot Movement (L/R FM): a 5 min task consisting in the alternation of five 30 s ON periods of continuous alternating flexion/extension of the right or the left foot, each one followed by five 30 s OFF periods of rest.It has been shown [4,8,9] that this paradigm generates eloquent areas in the following brain regions:
- In the foot motor area (contralateral to the side of the foot moved), located in the superior and medial part of the precentral gyrus, as by the somatotopic motor homunculus. This area is usually much smaller with respect to the hand’s one, owing to the lesser innervation rate of the inferior limb, accomplishing less fine movements than the hand ones.
- The corresponding foot primary sensory area (contralateral to the side of the foot moved) in the post-central gyrus, as by the somatotopic sensory homunculus. Also in this case, foot movements activate muscles and osteotendinous proprioceptors as well as cutaneous touch receptors.
- The supplementary motor area (bilaterally), may be more or less activated, depending on the difficulty of the movement to be carried out. Foot primary and supplementary motor areas are often fused.
2.7. fMRI Motor Task Application to Our Pts
2.8. fMRI Data Analysis
3. Results
3.1. Pt’s Clinical Pictures
- Pt 1 resumed both speech therapy and motor rehabilitation during the period of HBOT. After HBOT, aphasia improved noticeably. The recovery of the ability to speak allowed Pt 1 to regain some social life. Accordingly, the negative attitude was replaced by positivity, good mood and interest. Right lower limb paresis improved with better gait and stance. However, the right hand paralysis did not improve, except for a decrease in spasticity.
- Pt 2 did not undergo physiotherapy nor speech therapy. After HBOT, Pt 2 showed a marked clinical improvement in the language fluency, such that he was able to read aloud a written text, without a hitch. Ataxia and dysphagia improved as well.
- Pt 3 refused speech therapy and rehabilitation, but decided autonomously to practice a sport (swimming). After HBOT, he dramatically improved his speech fluency. The left lower limb paresis improved together with an increase in walking autonomy, but he was still unable to flex and extend the left foot rhythmically. Also the right limbs coordination improved, allowing the Pt to perform the requested upper limb motor task correctly. On the contrary, the left upper limb paralysis did not change at all.
- Pt 4. Even without any speech therapy or rehabilitation, after HBOT, Pt4 showed a good improvement in the common everyday acts of his life, as speaking and understanding and also in walking, but not in his upper limb paralysis.
3.2. Lesion Morphology Before HBOT
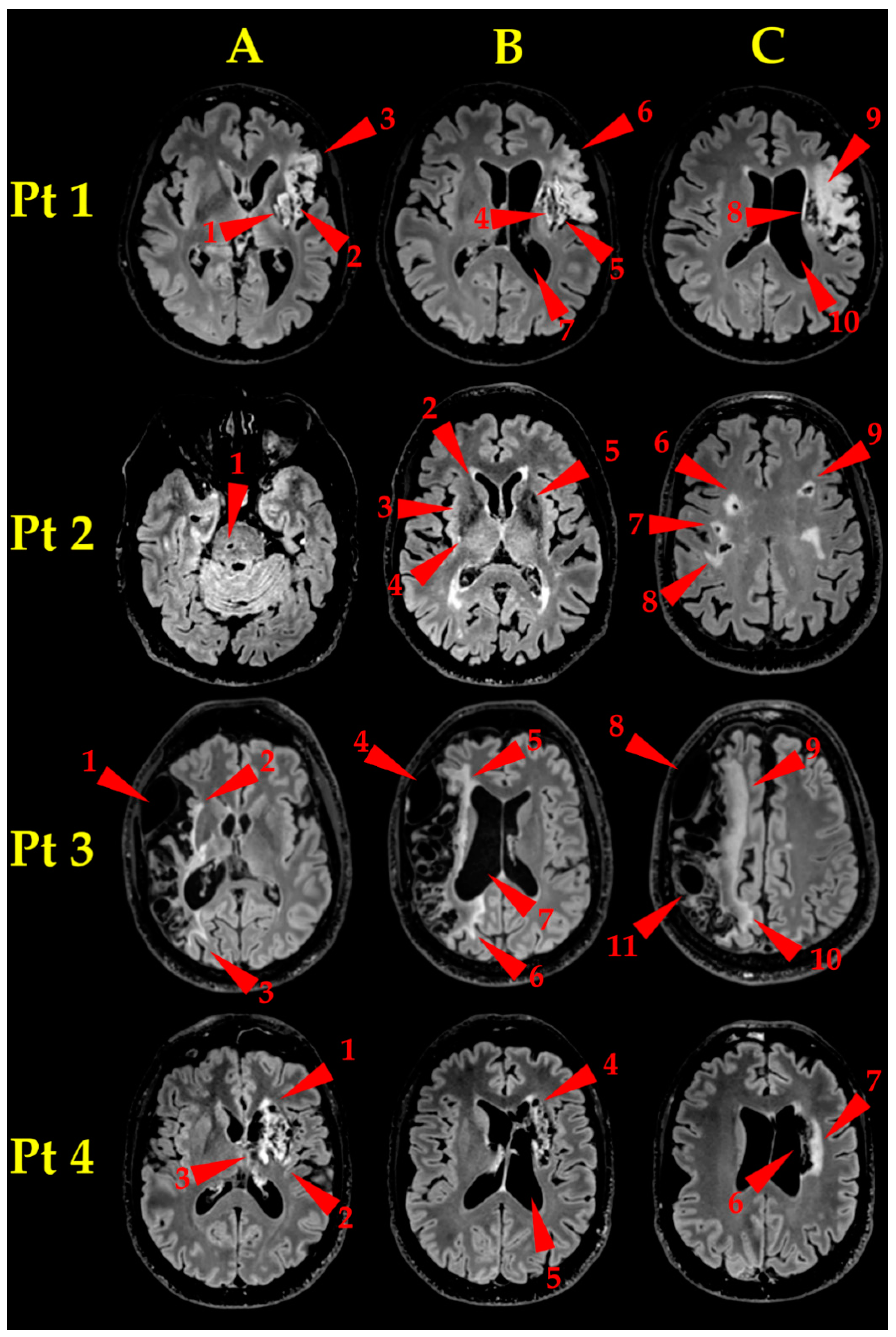
- Pt 1. Brain MR examination (Figure 2, Pt 1, A, B, C) showed an established ischaemic lesion in the deep and superficial territory of the left MCA. The lesion appeared as a wide malacic area (hypointense on T1-weighted, unhomogeneously hyperintense on T2-weighted FLAIR sequences) and showed signs of previous superficial haematic staining. A capsulo-lenticular cavitation (A and B, arrowheads 1 and 4, respectively) is present, with concomitant Wallerian degeneration of the bulb, extending laterally to the insula (A and B, arrowheads 2 and 5, respectively) and upward to the left corona radiata (C, arrowhead 8), which appeared also cavitated. The malacic area extends to the left frontal operculum (A and B, arrowheads 3 and 6, respectively), where the Broca’s area is located, and upward to the left fronto-lateral cortex (C, arrowhead 9). Finally, the massive tissue destruction caused an ex-vacuum enlargement of the adjacent segment of left lateral ventricle (B, C, arrowheads 7 and 10, respectively).
- Pt 2. Brain MR examination (Figure 2, Pt 2, A, B, C) showed many bilateral gliotic areas, prevalent on the right side, located mainly in the bi-hemispherical subcortical and para-ventricular deep white matter (C, arrowheads 6–9), extending downwards to the basal ganglia (B, arrowheads 2–5) and to the right side of the pons (A, arrowhead 1). They appeared as many small areas, hyperintense on T2-weighted images, consistent with chronic small vessels disease.
- Pt 3. Brain MR examination (Figure 2, Pt 3, A, B, C) showed a very wide right fronto-parieto-temporal malacic area (whose medial border is pointed to by arrowheads 2–3, 5–6 and 9–10, in A, B and C, respectively), involving the superficial territories of the right MCA, with ex-vacuo enlargement of the corresponding right lateral ventricle (B, arrowhead 7). It is also clearly visible a bulky arachnoid cyst, extending from the temporal pole to the anterior Sylvian region (A, B and C, arrowheads 1, 4 and 8, respectively) and a relatively smaller one, located in the parietal region (C, arrowhead 11).
- Pt 4. Brain MR examination (Figure 2, Pt 4, A, B, C) showed a gliotic-malacic area in the left capsulo-lenticular nucleus, partially involving the insula (A, arrowhead 1–3), extending upwards to the left caudate nucleus (B, arrowhead 4) and to the ipsilateral corona radiata (C, arrowhead 6, 7). Finally, the left lateral ventricle showed a moderate dilatation (C, arrowhead 5).
3.3. MRI
3.3.1. Language
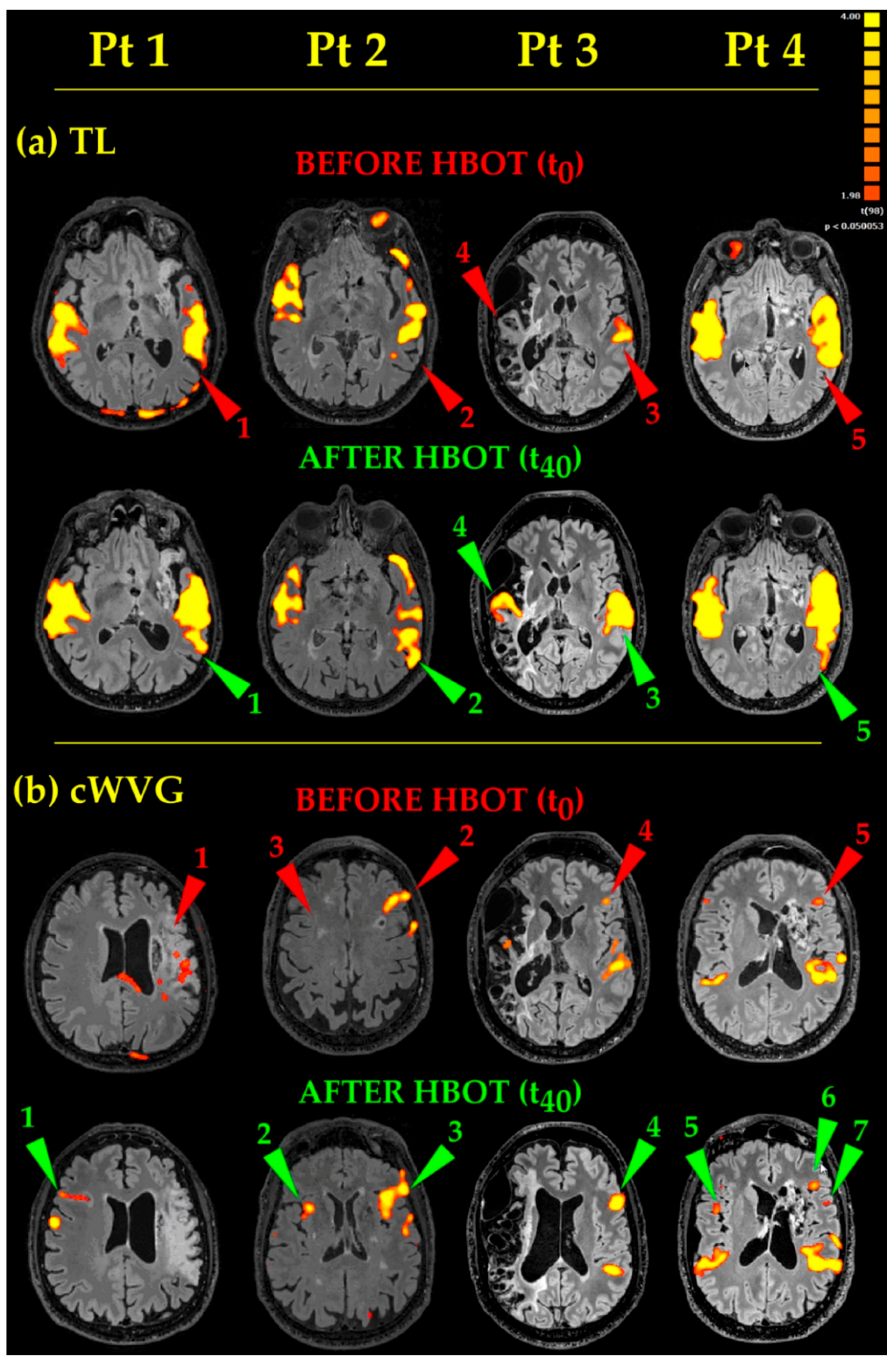
- As to Receptive Language (Figure 3, TL), the acoustic tasks evoked bilateral (except for Pt 3) temporal eloquent areas, located in the superior and middle temporal gyri (Figure 3, TL, Before HBOT, Pts 1, 2, 4, red arrowheads 1, 2, 5, respectively). After HBOT, the same acoustic tasks evoked temporal eloquent areas looking more extended antero-posteriorly and superiorly (involving the presumed Wernicke’s area) up to include the left angular gyrus too, in almost all Pts (Figure 3, TL, Pts 1, 2, 4, cp. red 1, 2, 5, Before HBOT, and green 1, 2, 5, After HBOT, arrowheads). In Pt 3, whose brain was one of the most damaged at the temporal lobe level, before HBOT, TL task generated only a unilateral, left sided, temporal eloquent acoustic area (Figure 3, TL, Before HBOT, Pt 3, red arrowhead 3). After HBOT, Pt 3′s eloquent areas became bilateral, with the appearance of a new activated area on the contralateral side (compare Figure 3, TL, After HBOT, Pt3, green arrowhead 4 with Figure 3, TL, Before HBOT, Pt 3, red arrowhead 4).
- Productive Language tasks (Figure 3, cWVG). Before HBOT, the eloquent areas in the left frontal operculum (where the Broca’s area, the structure at the basis of language fluency, is located) appeared small and barely visible in Pts 3 and 4 (Figure 3, cWVG, Before HBOT, Pts 3 and 4, red arrowheads 4 and 5, respectively). After HBOT, the same cWVG task evocated more extended and more significant (more yellow than orange-red) activated areas in Pts 2, 3 and 4 (Figure 3, cWVG, Pts 2, 3 and 4, compare red 2, 4, 5 and green 3, 4, 6 arrowheads, Before and After HBOT, respectively). A second small fronto-opercular activation appeared in Pt4 (note the green arrowhead 7 in Pt 4 in Figure 3, cWVG, After HBOT). In Pt 1, having a massive damage in the left hemisphere, no eloquent fronto-opercular areas appeared before HBOT (Figure 3, cWVG, Before HBOT, Pt1, red arrowhead 1). After HBOT, a clear-cut new frontal opercular eloquent area appeared on the contralateral right side (Figure 3, cWVG, After HBOT, Pt 1, green arrowhead 1), in a nearly mirror position (coherent with a new contralateral Broca’s area). Also in Pt 2 and Pt 4 a new eloquent area appeared on the right side, in between frontal operculum and insula (Figure 3, cWVG, After HBOT, Pt 2 and Pt 4, green arrowheads 2 and 5, respectively). Finally, the acoustic cWVG stimuli evoked the expected bilateral (except for Pt3) temporal Heschl’s and Wernicke’s activations, which were more extended and more statistically significant in all Pts, after HBOT).
3.3.2. Movements
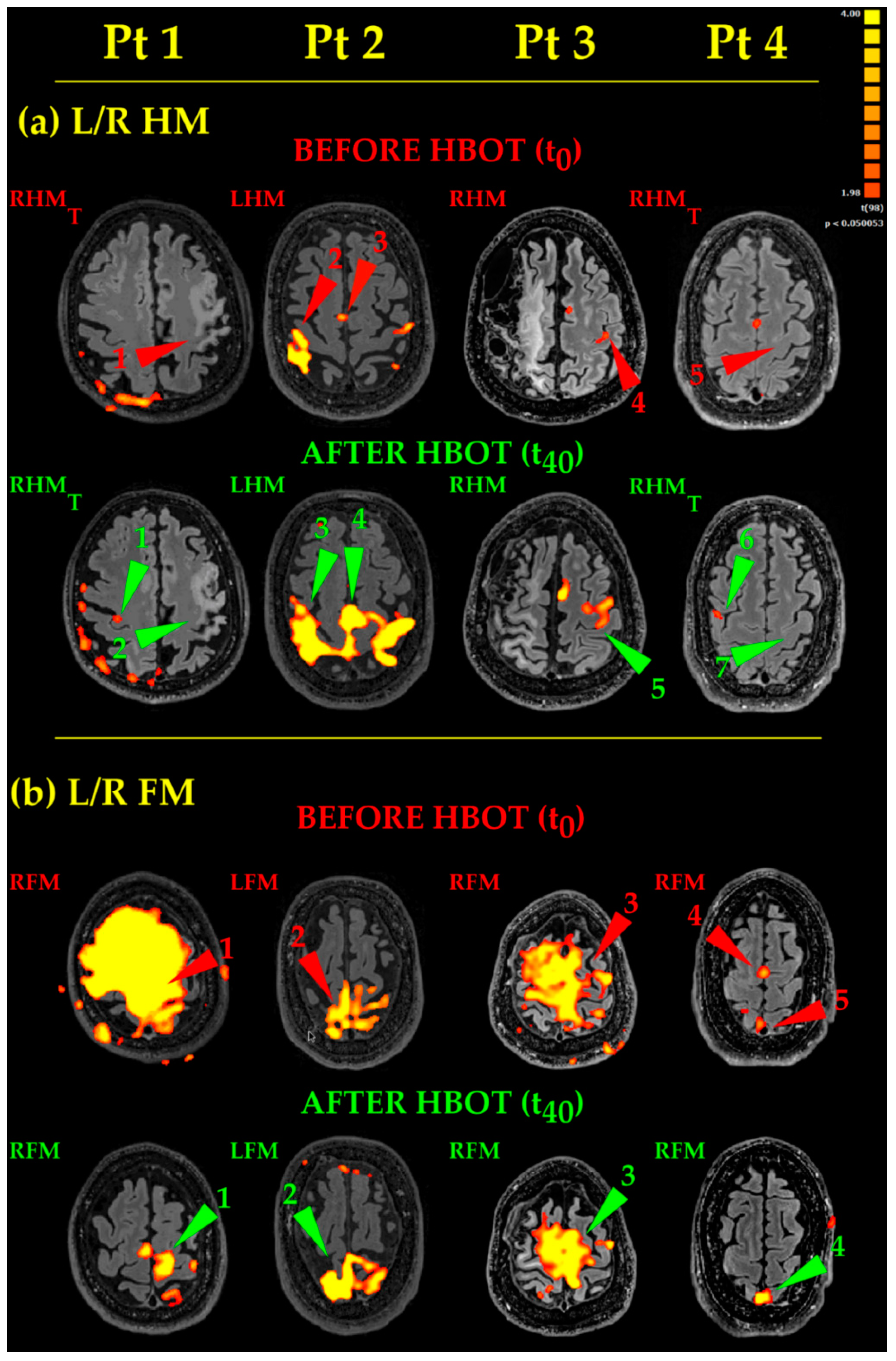
- Hand Movements (Figure 4, L/R HM). Pt 1 and Pt 4 thought to the movement of their right hand, but no activation occurred in the classical hand motor area (central omega/epsilon) (Figure 4, L/R HM, Before HBOT, Pt 1 and Pt 4, RHMT, red arrowheads 1 and 5, respectively). After HBOT, the same thinking to RHM did not evoke again any activations in the expected left hand motor areas (Figure 4, L/R HM, After HBOT, Pt1 and 4, RHMT, green arrowheads 2 and 7). It evoked instead a small, dot-like activation in the “wrong” right motor cortex only (Figure 4, L/R HM, After HBOT, Pt 1 and Pt 4, RHMT, green arrowheads 1 and 6). Likewise, Pt 3 moved his right uncoordinated upper limb, generating a very small activation before HBOT (Figure 4, L/R HM, Before HBOT, Pt3, RHM, red arrowhead 3). After HBOT, Pt 3 accomplished a near normal RHM (Figure 4, L/R HM, After HBOT, Pt3, RHM, green arrowhead 5). Pt 2 was the least compromised in his motor functions and performed his RHM almost normally before HBOT (Figure 4, L/R HM, Before HBOT, Pt2, RHM, red arrowhead 2). After HBOT, eloquent areas become much more extended, bilateral and more significant (Figure 4, L/R HM, After HBOT, Pt 2, RHM, green arrowhead 3), together with the enlargement of the supplementary motor area (compare red arrowhead 3 with green arrowhead 4, respectively, in Figure 4, L/R HM, Pt 2, Before and After HBOT). Activated areas were very extended: this because Pt 2 used excessive energy in performing his task.
- Foot Movements (Figure 4, L/R FM). As to Pt 1 and Pt 3, the huge yellow activated areas (of lesser extent in Pt 3) are coherent with very large, uncoordinated movements, occurring during the unsuccessful attempts to execute properly the RFM task before HBOT (Figure 4, L/R FM, Before HBOT, Pt 1 and Pt 3, RFM, red arrowheads 1 and 3, respectively). After HBOT, Pt 1 accomplished RFM task more easily and with more coordination, giving rise to an almost normal activation (Figure 4, L/R FM, After HBOT, Pt 1, RFM, green arrowhead 1). On the other hand, Pt 3, while showing some improvement, did not succeeded in performing his RFM task properly; RFM eloquent areas decreased a bit, while remaining more extended than normal (Figure 4, L/R FM, After HBOT, Pt 3, RFM, green arrowhead 3). In Pt2, the LFM before HBOT showed an irregular fragmented map (Figure 4, L/R FM, Before HBOT, Pt 2, LFM, red arrowhead 2); after HBOT, Pt 2 greatly improved the execution of his task, showing more significant and better defined eloquent areas (Figure 4, L/R FM, After HBOT, Pt 2, LFM, green arrowhead 2). Finally, in Pt 4, RFM resulted in a very small activation before HBOT (Figure 4, L/R FM, Before HBOT, Pt 4, RFM, red arrowhead 5) and in an activated supplementary area, due to his coordination effort to complete the task (Figure 4, L/R FM, Before HBOT, Pt 4, RFM, red arrowhead 4). After HBOT, actually, he succeeded in performing his RFM more smoothly and with less effort, showing, as a result, a bit more extended, more significant eloquent area (Figure 4, L/R FM, After HBOT, Pt 4, RFM, green arrowhead 4).
4. Discussion
4.1. HBOT Recommendation in Chronic Stroke Pts
4.2. HBOT Brain Effects And Mechanisms
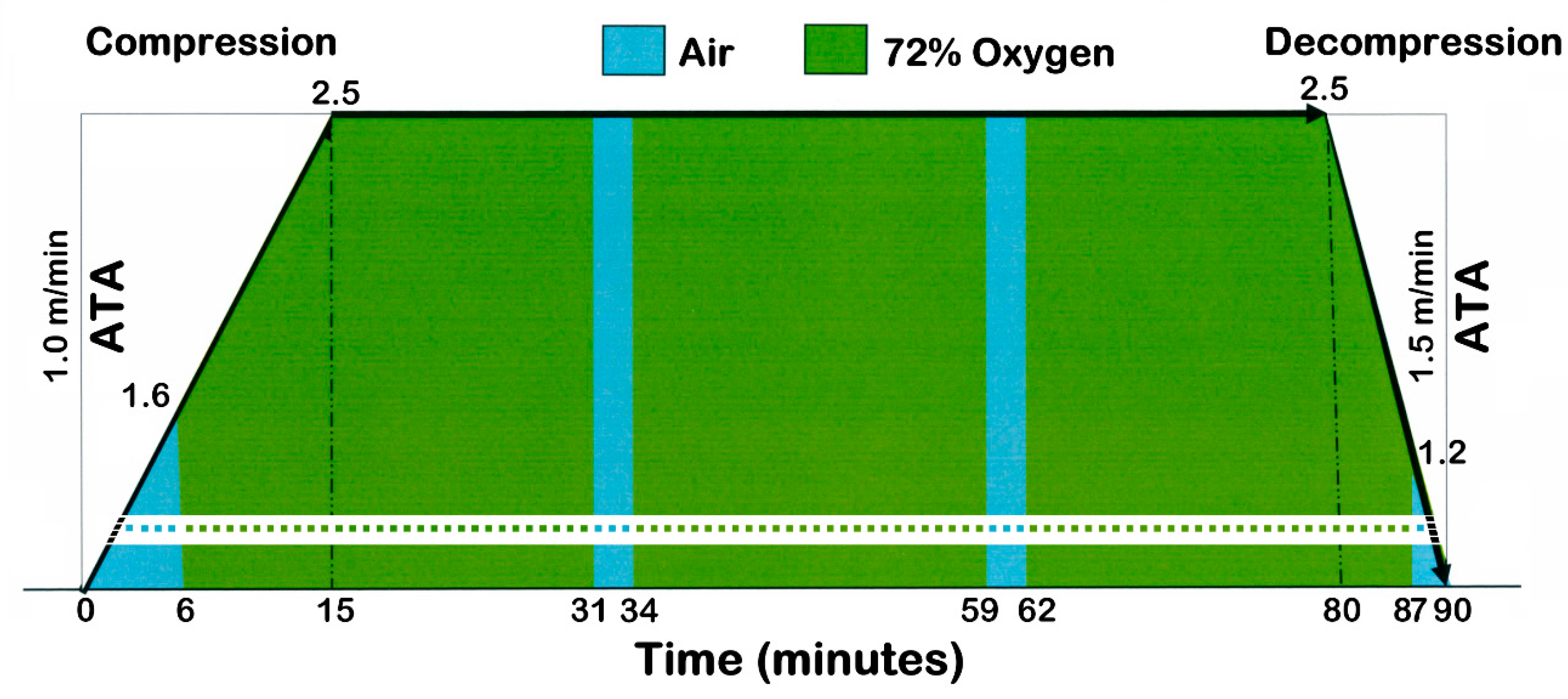
4.3. Pressure Values in the Hyperbaric Chamber
4.4. fMRI
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mathieu, D.; Marroni, A.; Kot, J. Tenth European Consensus Conference on Hyperbaric Medicine: Recommendations for and non-accepted clinical indications and practice of hyperbaric oxygen treatment. Diving Hyperb. Med. 2017, 47, 24–32. [Google Scholar] [CrossRef]
- Marroni, A. Hyperbaric Oxygen and In-Water Rehabilitation in Complete Stroke. J. Hyperb. Med. 1988, 3, 15–23. [Google Scholar]
- Di Donato, F. L’orecchio in Immersione; Editrice la Mandragora: Imola (Bologna), Italy, 2017; p. 202. [Google Scholar]
- Cevolani, D.; Agati, R.; Leonardi, M. Use of fMRI activation paradigms: A presurgical tool for mapping brain function. In High Field Brain MRI. Use in Clinical Practice, 2nd ed.; Scarabino, T., Pollice, S., Propolizio, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; Chapter 21; pp. 333–355. [Google Scholar]
- FitzGerald, D.B.; Cosgrove, G.R.; Ronner, S.; Jiang, H.; Buchbinder, B.R.; Belliveau, J.W.; Rosen, B.R.; Benson, R.R. Location of language in the cortex: A comparison between functional MR imaging and electrocortical stimulation. AJNR Am. J. Neuroradiol. 1997, 18, 1529–1539. [Google Scholar]
- Gaillard, W.D.; Balsamo, L.; Xu, B.; McKinney, C.; Papero, P.H.; Weinstein, S.; Conry, J.; Pearl, P.L.; Sachs, B.; Sato, S.; et al. fMRI language task panel improves determination of language dominance. Neurology 2004, 63, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Holland, S.K.; Plante, E.; Weber Byars, A.; Strawsburg, R.H.; Schmithorst, V.J.; Ball, W.S., Jr. Normal fMRI brain activation patterns in children performing a verb generation task. NeuroImage 2001, 14, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Sunaert, S. Presurgical planning for tumor resectioning. J. Magn. Reson. Imaging 2006, 23, 887–905. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Ashe, J.; Georgopoulos, A.P.; Merkle, H.; Ellermann, J.M.; Menon, R.S.; Ogawa, S.; Ugurbil, K. Functional imaging of human motor cortex at high magnetic field. J. Neurophysiol. 1993, 69, 297–302. [Google Scholar] [CrossRef]
- Naito, E.; Kochiyama, T.; Kitada, R.; Nakamura, S.; Matsumura, M.; Yonekura, Y.; Sadato, N. Internally simulated movement sensations during motor imagery activate cortical motor areas and the cerebellum. J. Neurosci. 2002, 22, 3683–3691. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, T.; Szarkowski, R.; Rios, C.; Ashe, J.; He, B. Negative covariation between task-related responses in alpha/beta-band activity and BOLD in human sensorimotor cortex: An EEG and fMRI study of motor imagery and movements. NeuroImage 2010, 49, 2596–2606. [Google Scholar] [CrossRef]
- Hermes, D.; Vansteensel, M.J.; Albers, A.M.; Bleichner, M.G.; Benedictus, M.R.; Mendez Orellana, C.; Aarnoutse, E.J.; Ramsey, N.F. Functional MRI-based identification of brain areas involved in motor imagery for implantable brain–computer interfaces. J. Neural Eng. 2011, 8, 025007. [Google Scholar] [CrossRef]
- Zhai, W.V.; Sun, L.; Yu, Z.Q.; Chen, G. Hyperbaric oxygen therapy in experimental and clinical stroke. Med. Gas Res. 2016, 6, 111–118. [Google Scholar] [PubMed]
- Hu, Q.; Manaenko, A.; Bian, H.; Guo, Z.; Huang, J.L.; Guo, Z.N.; Yang, P.; Tang, J.; Zhang, J.H. Hyperbaric oxygen reduces infarction volume and hemorrhagic transformation through ATP/NAD+/Sirt1 pathway in hyperglycemic middle cerebral artery occlusion rats. Stroke 2017, 48, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, R.P.; Stępień, K.; Pucko, E.; Matyja, E. The efficacy of hyperbaric oxygen in hemorrhagic stroke: Experimental and clinical implications. Arch. Med. Sci. 2017, 13, 1217–1223. [Google Scholar] [CrossRef]
- Tal, S.; Hadanny, A.; Sasson, E.; Suzin, G.; Efrati, S. Hyperbaric Oxygen Therapy can induce angiogenesis and regeneration of nerve fibers in traumatic brain injury patients. Front. Hum. Neurosci. 2017, 11, 508. [Google Scholar] [CrossRef] [PubMed]
- Liska, G.M.; Lippert, T.; Russo, E.; Nieves, N.; Borlongan, C.V. A dual role for hyperbaric oxygen in stroke neuroprotection: Preconditioning of the brain and stem cells. Cond. Med. 2018, 1, 151–166. [Google Scholar] [PubMed]
- Gonzales-Portillo, B.; Lippert, T.; Nguyen, H.; Lee, J.Y.; Borlongan, C.V. Hyperbaric oxygen therapy: A new look on treating stroke and traumatic brain injury. Brain Circ. 2019, 5, 101–105. [Google Scholar]
- Golan, H.; Makogon, B.; Volkov, O.; Smolyakov, Y.; Hadanny, A.; Efrati, S. Imaging-based predictors for hyperbaric oxygen therapy outcome in post-stroke patients. Report 1. Med. Hypotheses 2020, 136, 109510. [Google Scholar] [CrossRef]
- Schiavo, S.; Richardson, D.; Santa Mina, D.; Buryk-Iggers, S.; Uehling, J.; Carroll, J.; Clarke, H.; Djaiani, C.; Gershinsky, M.; Katznelson, R. Hyperbaric oxygen and focused rehabilitation program: A feasibility study in improving upper limb motor function after stroke. Appl. Physiol. Nutr. Metab. 2020, 45, 1345–1352. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Lu, M.; Liu, Y. Long-term functional prognosis of patients with aneurysmal subarachnoid hemorrhage treated with rehabilitation combined with hyperbaric oxygen. Case-series study. Medicine 2020, 99, 3. [Google Scholar] [CrossRef]
- Zhong, X.; Shan, A.; Xu, J.; Liang, J.; Long, Y.; Du, B. Hyperbaric oxygen for severe traumatic brain injury: A randomized trial. J. Int. Med. Res. 2020, 48, 7. [Google Scholar] [CrossRef]
- Hadanny, A.; Rittblat, M.; Bitterman, M.; May-Raz, I.; Suzin, G.; Boussi-Gross, R.; Zemel, Y.; Bechor, Y.; Catalogna, M.; Efrati, S. Hyperbaric oxygen therapy improves neurocognitive functions of post-stroke patients—A retrospective analysis. Restor. Neurol. Neurosci. 2020, 38, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Mozayeni, B.R.; Duncan, W.; Zant, E.; Love, T.L.; Beckman, R.L.; Stoller, K.P. The National Brain Injury, Rescue and Rehabilitation Study—A multicenter observational study of hyperbaric oxygen for mild traumatic brain injury with post-concussive symptoms. Med. Gas Res. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Sun, L.; Yang, J.; Liu, X.H.; Zhang, J.; Zhu, W.Q.; Yang, L.; Nan, D. The effect of different atmosphere absolute hyperbaric oxygen on the expression of extracellular histones after traumatic brain injury in rats. Cell Stress Chaperones 2020, 25, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Boussi-Gross, R.; Golan, H.; Volkov, O.; Bechor, Y.; Hoofien, D.; Beeri, M.S.; Ben-Jacob, H.; Efrati, S. Improvement of memory impairments in poststroke patients by hyperbaric oxygen therapy. Neuropsychology 2015, 29, 610–621. [Google Scholar] [CrossRef]
- Efrati, S.; Fishlev, G.; Bechor, Y.; Volkov, O.; Bergan, J.; Kliakhandler, K.; Kamiager, I.; Gal, N.; Friedman, M.; Ben-Jacob, E.; et al. Hyperbaric oxygen induces late neuroplasticity in post stroke patients—Randomized, prospective trial. PLoS ONE 2013, 8, e53716. [Google Scholar] [CrossRef]
- Rosario, E.R.; Kaplan, S.E.; Khonsari, S.; Vazquez, G.; Solanki, N.; Lane, M.; Brownell, H.; Rosenberg, S.S. The effect of hyperbaric oxygen therapy on functional impairments caused by ischemic stroke. Neurol. Res. Int. 2018, 2018, 3172679. [Google Scholar] [CrossRef]
- Thom, S.R. Hyperbaric oxygen: Its mechanisms and efficacy. Plast. Reconstr. Surg. 2011, 127, 131S–141S. [Google Scholar] [CrossRef]
- Alleva, R.; Di Donato, F.; Strafella, E.; Staffolani, S.; Nocchi, L.; Borghi, B.; Pignotti, E.; Santarelli, L.; Tomasetti, M. Effect of ascorbic acid-rich diet on in vivo-induced oxidative stress. Br. J. Nutr. 2011, 107, 1645–1654. [Google Scholar] [CrossRef]
- Sheikh, A.Y.; Gibson, J.J.; Rollins, M.D.; Hopf, H.W.; Hussain, Z.; Hunt, T.K. Effect of Hyperoxia on Vascular Endothelial Growth Factor Levels in a Wound Model. Arch. Surg. 2000, 135, 1293–1297. [Google Scholar] [CrossRef]
- Goldstein, L.J.; Gallagher, K.A.; Bauer, S.M.; Bauer, R.J.; Baireddy, V.; Liu, Z.J.; Buerk, D.G.; Thom, S.R.; Velazquez, O.C. Stem Cell mobilization by hyperbaric oxygen. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H1378–H1386. [Google Scholar]
- Hamilton, R.W. Tolerating oxygen exposure. SPUMS J. 1997, 27, 14. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cevolani, D.; Di Donato, F.; Santarella, L.; Bertossi, S.; Cellerini, M. Functional MRI (fMRI) Evaluation of Hyperbaric Oxygen Therapy (HBOT) Efficacy in Chronic Cerebral Stroke: A Small Retrospective Consecutive Case Series. Int. J. Environ. Res. Public Health 2021, 18, 190. https://doi.org/10.3390/ijerph18010190
Cevolani D, Di Donato F, Santarella L, Bertossi S, Cellerini M. Functional MRI (fMRI) Evaluation of Hyperbaric Oxygen Therapy (HBOT) Efficacy in Chronic Cerebral Stroke: A Small Retrospective Consecutive Case Series. International Journal of Environmental Research and Public Health. 2021; 18(1):190. https://doi.org/10.3390/ijerph18010190
Chicago/Turabian StyleCevolani, Daniela, Ferruccio Di Donato, Luigi Santarella, Simone Bertossi, and Martino Cellerini. 2021. "Functional MRI (fMRI) Evaluation of Hyperbaric Oxygen Therapy (HBOT) Efficacy in Chronic Cerebral Stroke: A Small Retrospective Consecutive Case Series" International Journal of Environmental Research and Public Health 18, no. 1: 190. https://doi.org/10.3390/ijerph18010190
APA StyleCevolani, D., Di Donato, F., Santarella, L., Bertossi, S., & Cellerini, M. (2021). Functional MRI (fMRI) Evaluation of Hyperbaric Oxygen Therapy (HBOT) Efficacy in Chronic Cerebral Stroke: A Small Retrospective Consecutive Case Series. International Journal of Environmental Research and Public Health, 18(1), 190. https://doi.org/10.3390/ijerph18010190





