Assessing the Respiratory Effects of Air Pollution from Biomass Cookstoves on Pregnant Women in Rural India
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting
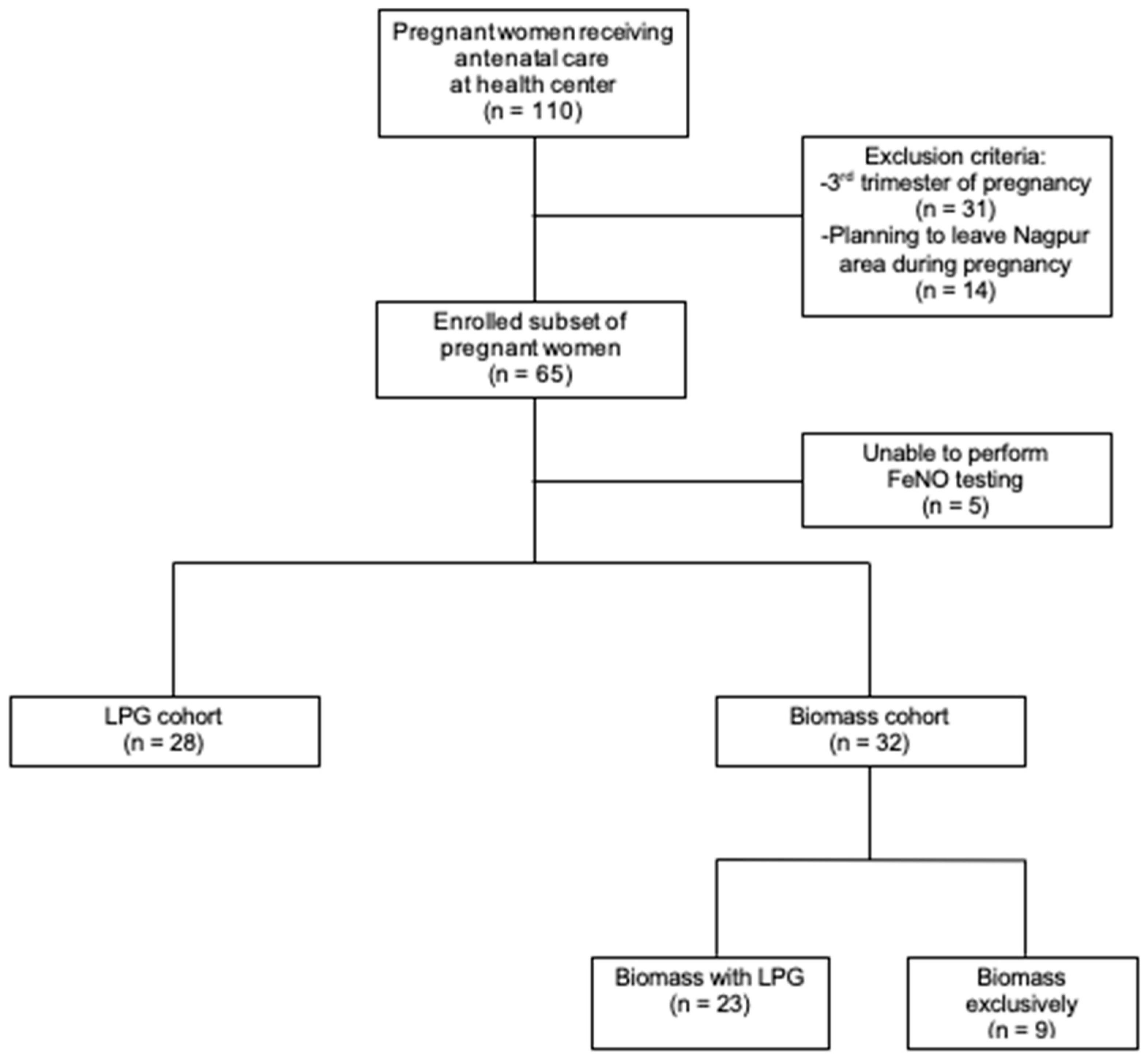
2.2. Participants
2.3. FeNO Testing
2.4. Subjective Testing
2.5. Covariates
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Respiratory Symptoms
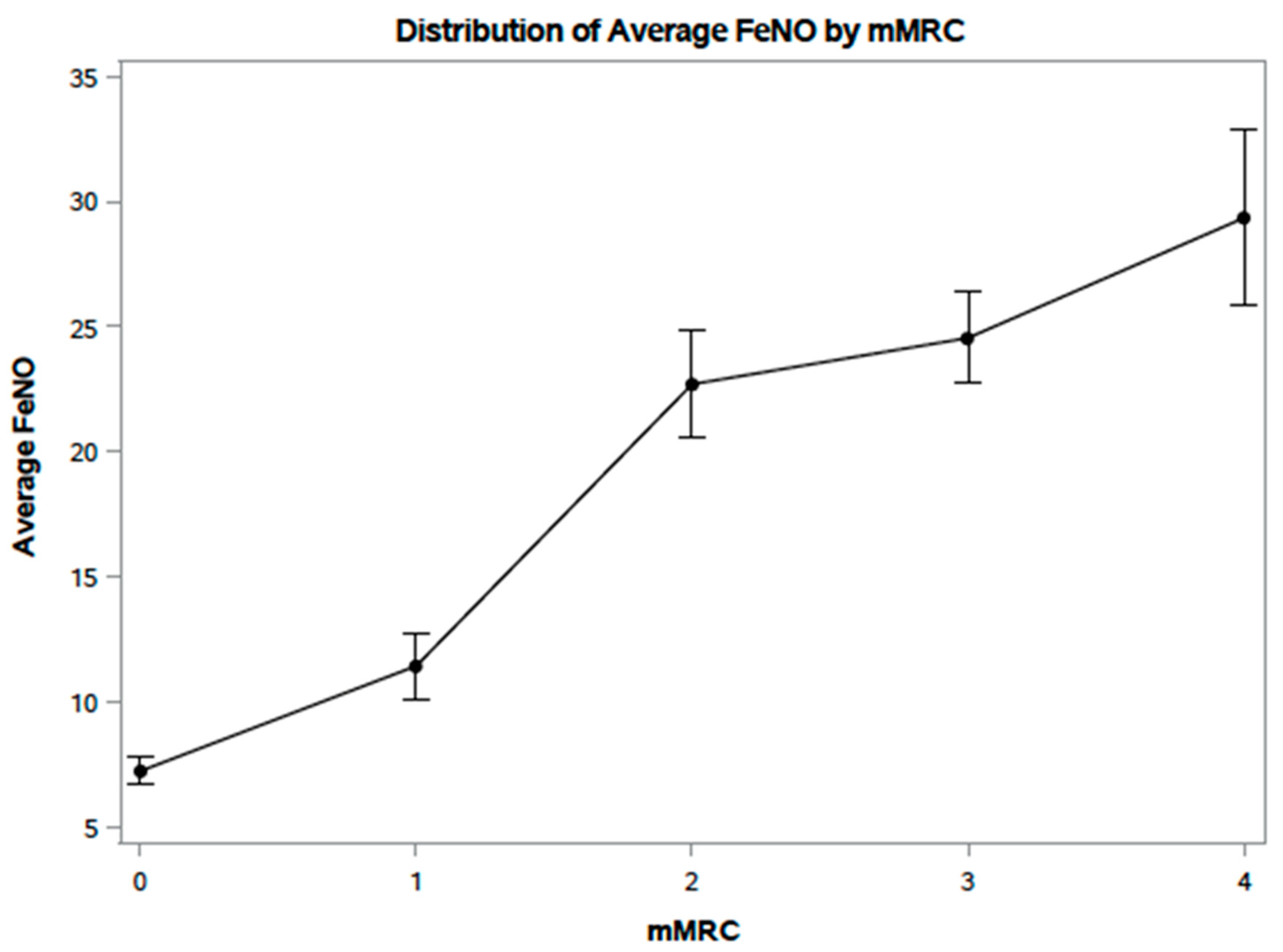
4.2. FeNO
4.3. Strengths
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mishra, V.K. Effect of indoor air pollution from biomass combustion on prevalence of asthma in the elderly. Environ. Health Perspect. 2003, 111, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Bonjour, S.; Adair-Rohani, H.; Wolf, J.; Bruce, N.G.; Mehta, S.; Prüss-Ustün, A.; Lahiff, M.; Rehfuess, E.A.; Mishra, V.; Smith, K.R. Solid Fuel Use for Household Cooking: Country and Regional Estimates for 1980–2010. Environ. Health Perspect. 2013, 121, 784–790. [Google Scholar] [CrossRef] [PubMed]
- International Energy Agency. WEO-2017 Special Report: Energy Access Outlook; International Energy Agency: Paris, France, 2017. [Google Scholar]
- Guilbert, J.J. The World Health Report 2002—Reducing Risks, Promoting Healthy Life; Educ Health: Abingdon, VA, USA, 2003; Volume 16, p. 230. [Google Scholar]
- Smith, K.R. Fuel combustion, air-pollution exposure, and health—The situation in developing-countries. Annu. Rev. Energy Environ. 1993, 18, 529–566. [Google Scholar] [CrossRef]
- Patel, A.B.; Meleth, S.; Pasha, O.; Goudar, S.S.; Esamai, F.; Garces, A.; Chomba, E.; McClure, E.M.; Wright, L.L.; Koso-Thomas, M.; et al. Impact of exposure to cooking fuels on stillbirths, perinatal, very early and late neonatal mortality—A multicenter prospective cohort study in rural communities in India, Pakistan, Kenya, Zambia and Guatemala. Matern. Health Neonatol. Perinatol. 2015, 1, 1–12. [Google Scholar] [CrossRef]
- Patel, A.; Prakash, A.A.; Pusdekar, Y.V.; Kulkarni, H.; Hibberd, P.L. Detection and risk stratification of women at high risk of pre-term birth in rural communities near Nagpur, India. BMC Pregnancy Childbirth 2017, 17, 311. [Google Scholar] [CrossRef]
- Page, C.M.; Patel, A.; Hibberd, P.L. Does Smoke from Biomass Fuel Contribute to Anemia in Pregnant Women in Nagpur, India? A Cross-Sectional Study. PLoS ONE 2015, 10, e0127890. [Google Scholar] [CrossRef]
- Kleimola, L.B.; Patel, A.B.; Borkar, J.A.; Hibberd, P.L. Consequences of household air pollution on child survival: Evidence from demographic and health surveys in 47 countries. Int. J. Occup. Environ. Health 2015, 21, 294–302. [Google Scholar] [CrossRef]
- Fullerton, D.G.; Bruce, N.; Gordon, S.B. Indoor air pollution from biomass fuel smoke is a major health concern in the develop-ing world. Trans. R Soc. Trop. Med. Hyg. 2008, 102, 843–851. [Google Scholar] [CrossRef]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Curtis, L.; Rea, W.; Smith-Willis, P.; Fenyves, E.; Pan, Y. Adverse health effects of outdoor air pollutants. Environ. Int. 2006, 32, 815–830. [Google Scholar] [CrossRef]
- Mishra, V.; Retherford, R.D. Does biofuel smoke contribute to anemia and stunting in early childhood? Int. J. Epidemiol. 2007, 36, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.; Northcross, A.; Wilson, N.; Dutta, A.; Pandya, R.; Ibigbami, T. Randomized controlled ethanol cookstove in-tervention and blood pressure in pregnant Nigerian women. Am. J. Respir. Crit. Care Med. 2017, 195, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Amegah, A.K.; Quansah, R.; Jaakkola, J.J.K. Household Air Pollution from Solid Fuel Use and Risk of Adverse Pregnancy Outcomes: A Systematic Review and Meta-Analysis of the Empirical Evidence. PLoS ONE 2014, 9, e113920. [Google Scholar] [CrossRef] [PubMed]
- Glinianaia, S.V.; Rankin, J.; Bell, R.; Pless-Mulloli, T.; Howel, D. Particulate air pollution and fetal health: A systematic review of the epidemiologic evidence. Epidemiology 2004, 15, 36–45. [Google Scholar] [CrossRef]
- Perera, F.P.; Whyatt, R.M.; Jedrychowski, W.; Rauh, V.; Manchester, D.; Santella, R.M. Recent developments in molecular epi-demiology: A study of the effects of environmental polycyclic aromatic hydrocarbons on birth outcomes in Poland. Am. J. Epidemiol. 1998, 147, 309–314. [Google Scholar] [CrossRef]
- Morello-Frosch, R.; Jesdale, B.M.; Sadd, J.L.; Pastor, M. Ambient air pollution exposure and full-term birth weight in California. Environ. Health 2010, 9, 44. [Google Scholar] [CrossRef]
- Engel, S.M.; Erichsen, H.C.; Savitz, D.A.; Thorp, J.; Chanock, S.J.; Olshan, A.F. Risk of Spontaneous Preterm Birth is Associated With Common Proinflammatory Cytokine Polymorphisms. Epidemiology 2005, 16, 469–477. [Google Scholar] [CrossRef]
- Ritz, B.; Wilhelm, M.; Hoggatt, K.J.; Ghosh, J.K.C. Ambient Air Pollution and Preterm Birth in the Environment and Pregnancy Outcomes Study at the University of California, Los Angeles. Am. J. Epidemiol. 2007, 166, 1045–1052. [Google Scholar] [CrossRef]
- Pope, D.P.; Mishra, V.; Thompson, L.; Siddiqui, A.R.; Rehfuess, E.A.; Weber, M. Risk of low birth weight and stillbirth associ-ated with indoor air pollution from solid fuel use in developing countries. Epidemiol. Rev. 2010, 32, 70–81. [Google Scholar] [CrossRef]
- Salam, M.T.; Millstein, J.; Li, Y.-F.; Lurmann, F.W.; Margolis, H.G.; Gilliland, F.D. Birth Outcomes and Prenatal Exposure to Ozone, Carbon Monoxide, and Particulate Matter: Results from the Children’s Health Study. Environ. Health Perspect. 2005, 113, 1638–1644. [Google Scholar] [CrossRef]
- Sangalli, M.; McLean, A.; Peek, M.J.; Rivory, L.P.; Le Couteur, D.G. Carbon Monoxide Disposition and Permeability-Surface Area Product in the Foetal Circulation of the Perfused Term Human Placenta. Placenta 2003, 24, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Di Cera, E.; Doyle, M.L.; Morgan, M.S.; De Cristofaro, R.; Landolfi, R.; Bizzi, B. Carbon monoxide and oxygen binding to hu-man hemoglobin F0. Biochemisty 1989, 28, 2631–2638. [Google Scholar] [CrossRef] [PubMed]
- Bosley, A.R.; Sibert, J.R.; Newcombe, R.G. Effects of maternal smoking on fetal growth and nutrition. Arch. Dis. Child 1981, 56, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.A.; Alexis, N.; Barnes, C.; Bernstein, I.L.; Nel, A.; Peden, D.; Diaz-Sanchez, D.; Tarlo, S.M.; Williams, P.B. Health effects of air pollution. J. Allergy Clin. Immunol. 2004, 114, 1116–1123. [Google Scholar] [CrossRef]
- Anderson, J.O.; Thundiyil, J.G.; Stolbach, A. Clearing the Air: A Review of the Effects of Particulate Matter Air Pollution on Human Health. J. Med. Toxicol. 2012, 8, 166–175. [Google Scholar] [CrossRef]
- Kelly, E.J.; Fussell, J.C. Air pollution and airway disease. Clin. Exp. Allergy 2011, 41, 1059–1071. [Google Scholar] [CrossRef]
- Holgate, S.T.; Sandstrom, T.; Frew, A.J.; Stenfors, N.; Nordenhall, C.; Salvi, S. Health effects of acute exposure to air pollution. Part 1: Health and asthmatic subjects exposed to diesal exhaust. Res. Rep. Health Eff. Inst. 2003, 112, 1–30. [Google Scholar]
- Van Eeden, S.F.; Tan, W.C.; Suwa, T.; Mukae, H.; Terashima, T.; Fuji, T. Cytokines involved in the systemic inflammatory re-sponse induced by exposure to particulate matter air pollutants (PM10). Am. J. Respir. Crit. Care Med. 2001, 164, 826–830. [Google Scholar] [CrossRef]
- Barregard, L.; Sallsten, G.; Andersson, L.; Almstrand, A.C.; Gustafson, P.; Andersson, M. Experimental exposure to wood smoke: Effects on airway inflammation and oxidative stress. Occup. Environ. Med. 2008, 65, 319–324. [Google Scholar] [CrossRef]
- Pope, D.; Diaz, E.; Smith-Sivertsen, T.; Lie, R.T.; Bakke, P.; Balmes, J.R. Exposure to household air pollution from wood com-bustion and association with respiratory symptoms and lung function in nonsmoking women: Results from the RESPIRE trial, Guatemala. Environ. Health Perspect. 2015, 123, 285–292. [Google Scholar] [CrossRef]
- Swiston, J.R.; Davidson, W.; Attridge, S.; Li, G.T.; Brauer, M.; Van Eeden, S.F. Wood smoke exposure induces a pulmonary and sys-temic inflammatory response in firefighters. Eur. Respir. J. 2008, 32, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, G.; Lu, S.-E.; Kipen, H.; Wang, Y.; Hu, M.; Lin, W.; Rich, D.; Ohman-Strickland, P.; Diehl, S.R.; et al. Inflammatory and Oxidative Stress Responses of Healthy Young Adults to Changes in Air Quality during the Beijing Olympics. Am. J. Respir. Crit. Care Med. 2012, 186, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Van Amsterdam, J.G.; Verlaan, B.P.; Van Loveren, H.; Elzakker, B.G.; Vos, S.G.; Opperhuizen, A. Air pollution is associated with increased level of exhaled nitric oxide in nonsmoking healthy subjects. Arch. Environ. Health 1999, 54, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Hou, J.; Cheng, J.; Zhang, R.; Yin, W.; Huang, C.; Zhu, X.; Chen, W.; Yuan, J. Estimated individual inhaled dose of fine particles and indicators of lung function: A pilot study among Chinese young adults. Environ. Pollut. 2018, 235, 505–513. [Google Scholar] [CrossRef]
- Shi, J.; Chen, R.; Yang, C.; Lin, Z.; Cai, J.; Xia, Y. Association between fine particulate matter chemical constituents and air-way inflammation: A panel among health adults in China. Enivron. Res. 2016, 150, 264–268. [Google Scholar] [CrossRef]
- Idavain, J.; Julge, K.; Rebane, T.; Lang, A.; Orru, H. Respiratory symptoms, asthma and levels of fractional exhaled nitric oxide in school children in the industrial areas of Estonia. Sci. Total. Environ. 2019, 650, 65–72. [Google Scholar] [CrossRef]
- Graveland, H.; Van Roosbroeck, S.A.; Rensen, W.M.; Brunekreef, B.; Gehring, U. Air pollution and exhaled nitric oxide in Dutch school children. Occup. Environ. Med. 2011, 68, 551–556. [Google Scholar] [CrossRef]
- La Grutta, S.; Ferrante, G.; Malizia, V.; Cibella, F.; Viegi, G. Environmental effects on fractional exhaled nitric oxide in allergic children. J. Allergy 2012, 2012, 916926. [Google Scholar] [CrossRef]
- Tamasi, L.; Bohacs, A.; Bikov, A.; Andorka, C.; Losonczyy, G.R., Jr. Exhaled nitric oxide in pregnant healthy and asth-matic women. J. Asthma 2009, 46, 786–791. [Google Scholar] [CrossRef]
- Fahy, J.V. Eosinophilic and Neutrophilic Inflammation in Asthma: Insights from Clinical Studies. Proc. Am. Thorac. Soc. 2009, 6, 256–259. [Google Scholar] [CrossRef]
- Barnes, P.J.; Dweik, R.A.; Gelb, A.F.; Gibson, P.G.; George, S.C.; Grasemann, H. Exhaled nitric oxide in pulmonary diseases: A comprehensive review. Chest 2010, 138, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Possa, S.S.; Leick, E.A.; Prado, C.M.; Martins, M.A.; Tibério, I.D.F.L.C. Eosinophilic Inflammation in Allergic Asthma. Front. Pharmacol. 2013, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Zamora, R.; Vodovotz, Y.; Billiar, T.R. Inducible Nitric Oxide Synthase and Inflammatory Diseases. Mol. Med. 2000, 6, 347–373. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.W.; Demchenko, I.T.; Piantadosi, C.A. Two faces of nitric oxide: Implications for cellular mechanisms of oxygen tox-icity. J. Appl. Physiol. 2009, 106, 662–667. [Google Scholar] [CrossRef]
- D’Sa, A.; Murthy, K.N. LPG as a cooking fuel option for India. Energy Sustain. Dev. 2004, 8, 91–106. [Google Scholar] [CrossRef]
- Mohsen, S.; Hanafy, F.Z.; Fathy, A.A.; El-Gilany, A.-H. Nonadherence to treatment and quality of life among patients with chronic obstructive pulmonary disease. Lung India 2019, 36, 193–198. [Google Scholar] [CrossRef]
- Pyasi, K.; Brashier, B.; Londhe, J.; Das, V.; Bulsara, N.; Moitra, S. Association between quality of life as assessed by St. George’s respiratory questionnaire (SGRQ), lung function indices and sputum inflammatory parameters in Indian COPD patients. Eur. Resp. J. 2014, 44, 2189. [Google Scholar]
- Pati, S.; Swain, S.; Patel, S.K.; Chauhan, A.S.; Panda, N.; Mahapatra, P.; Pati, S. An assessment of health-related quality of life among patients with chronic obstructive pulmonary diseases attending a tertiary care hospital in Bhubaneswar City, India. J. Fam. Med. Prim. Care 2018, 7, 1047–1053. [Google Scholar]
- Meguro, M.; Barley, E.A.; Spencer, S.; Jones, P.W. Development and Validation of an Improved, COPD-Specific Version of the St. George Respiratory Questionnaire. Chest 2007, 132, 456–463. [Google Scholar] [CrossRef]
- Jones, P.W.; Quirk, F.H.; Baveystock, C.M. The St George’s Respiratory Questionnaire. Respir. Med. 1991, 85, 25–31. [Google Scholar] [CrossRef]
- Bestall, J.C.; Paul, E.A.; Garrod, R.; Garnham, R.; Jones, P.W.; Wedzicha, J.A. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999, 54, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Suri, J.C.; Pathak, U.; Kumar, R.; Suri, T.M.; Gupta, N.C.; Pathak, S. Impact of biomass fuel exposure from traditional stoves on lung functions in adult women of a rural Indian village. Lung India 2019, 36, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.; Linnes, J.C.; Bolton, S.; Larson, T. Ventilated cookstoves associated with improvements in respiratory health-related quality of life in rural Bolivia. J. Public Health 2013, 36, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Kurmi, O.P.; Semple, S.; Devereux, G.S.; Gaihre, S.; Lam, K.B.; Sadhra, S. The effect of exposure to biomass smoke on respira-tory symptoms in adult rural and urban Nepalese populations. Environ. Health 2014, 13, 92. [Google Scholar] [CrossRef]
- KalagoudaMahishale, V.; Angadi, N.; Metgudmath, V.; Lolly, M.; Eti, A.; Khan, S. The prevalence of chronic obstructive pulmo-nary disease and the determinants of underdiagnosis in women exposed to biomass fuel in India—A cross section study. Chonnam. Med. J. 2016, 52, 117–122. [Google Scholar] [CrossRef]
- Van Vilet, E.D.S.; Kinney, P.L.; Owusu-Agyei, S.; Schluger, N.W.; Ae-Ngibise, K.A.; Whyatt, R.M. Current respiratory symptoms and risk factors in pregnant women cooking with biomass fuels in rural Ghana. Environ. Int. 2019, 124, 533–540. [Google Scholar] [CrossRef]
- Woodruff, P.G.; Barr, R.G.; Bleecker, E.; Christenson, S.A.; Couper, D.; Curtis, J.L.; Gouskova, N.A.; Hansel, N.N.; Hoffman, E.A.; Kanner, R.E.; et al. Clinical Significance of Symptoms in Smokers with Preserved Pulmonary Function. N. Engl. J. Med. 2016, 374, 1811–1821. [Google Scholar] [CrossRef]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O. An official ATS clinical practice guideline: In-terpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef]
- Benka-Coker, M.L.; Clark, M.L.; Rajkumar, S.; Young, B.N.; Bachand, A.M.; Balmes, J.R.; Brook, R.D.; Nelson, T.L.; Volckens, J.; Reynolds, S.J.; et al. Exposure to Household Air Pollution from Biomass Cookstoves and Levels of Fractional Exhaled Nitric Oxide (FeNO) among Honduran Women. Int. J. Environ. Res. Public Health 2018, 15, 2544. [Google Scholar] [CrossRef]
- Strak, M.; Boogaard, H.; Meliefste, K.; Oldenwening, M.; Zuurbier, M.; Brunekreef, B.; Hoek, G. Respiratory health effects of ultrafine and fine particle exposure in cyclists. Occup. Environ. Med. 2009, 67, 118–124. [Google Scholar] [CrossRef]
- Yoda, Y.; Otani, N.; Sakurai, S.; Shima, M. Acute effects of summer air pollution on pulmonary function and airway inflamma-tion in healthy young women. J. Epidemiol. 2014, 24, 312–320. [Google Scholar] [CrossRef]
- Berhane, K.; Zhang, Y.; Salam, M.T.; Eckel, S.P.; Linn, W.S.; Rappaport, E.B. Longitudinal effects of air pollution on exhaled ni-tric oxide; The Children’s Health Study. Occup. Environ. Med. 2014, 71, 507–513. [Google Scholar] [CrossRef]
- Liu, C.; Flexeder, C.; Fuertes, E.; Cyrys, J.; Bauer, C.-P.; Koletzko, S.; Hoffmann, B.; Von Berg, A.; Heinrich, J. Effects of air pollution on exhaled nitric oxide in children: Results from the GINIplus and LISAplus studies. Int. J. Hyg. Environ. Health 2014, 217, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Malerba, M.; Radaeli, A.; Olivini, A.; Damiani, G.; Ragnoli, B.; Montuschi, P.; Ricciardolo, F.L.M. Exhaled Nitric Oxide as a Biomarker in COPD and Related Comorbidities. BioMed Res. Int. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Olin, A.-C.; Rosengren, A.; Thelle, D.S.; Lissner, L.; Bake, B.; Torén, K. Height, Age, and Atopy Are Associated with Fraction of Exhaled Nitric Oxide in a Large Adult General Population Sample. Chest 2006, 130, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Erpenbeck, V.J.; Jörres, R.A.; Discher, M.; Krentel, H.; Tsikas, D.; Luettig, B. Local nitric oxide levels reflect the degree of aller-gic airway inflammation after segmental allergen challenge in asthmatics. Nitric Oxide 2005, 13, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Brightling, C.E.; Symon, F.A.; Birring, S.S.; Bradding, P.; Wardlaw, A.J.; Pavord, I.D. Comparison of airway immunopathology of eosinophilic bronchitis and asthma. Thorax 2003, 58, 528–532. [Google Scholar] [CrossRef] [PubMed]
| Participant Characteristics | Biomass (n = 32) | LPG (n = 28) | p-Value |
|---|---|---|---|
| Mean age (SD) | 23.3 (3.0) | 23.4 (3.1) | 0.987 |
| Mean BMI (SD) | 19.7 (2.1) | 18.9 (1.6) | 0.109 |
| Education | 1.0 | ||
| Primary | 9 (28.1%) | 8 (28.6%) | |
| Secondary | 12 (37.5%) | 10 (35.7%) | |
| College | 11 (34.4%) | 10 (35.7%) | |
| Parity | 0.802 | ||
| Nulliparous | 16 (50.0%) | 15 (53.6%) | |
| Multiparous | 16 (50.0%) | 13 (46.4%) | |
| Preterm birth history | 13 (40.6%) | 2 (7.1%) | 0.003 |
| Second hand smoke | 0.946 | ||
| Daily | 15 (46.9%) | 13 (46.4%) | |
| Weekly/Monthly | 8 (25.0%) | 6 (21.4%) | |
| Never | 9 (28.1%) | 9 (32.1%) | |
| Biomass fuel type | Not applicable | ||
| Wood | 24 (75.0%) | ||
| Crop/grass | 3 (9.4%) | ||
| Cow dung | 5 (15.6%) | ||
| Secondary fuel type | Not applicable | ||
| Crop/grass | 22 (68.8%) | ||
| Cow dung | 3 (9.4%) | ||
| Fuel source timeframe | <0.001 | ||
| <3 years | 4 (12.5%) | 18 (64.3%) | |
| ≥3 years | 28 (87.5%) | 10 (35.7%) | |
| Stove location within home | 0.201 | ||
| Within home | 27 (84.4%) | 27 (96.4%) | |
| Separate room | 5 (15.6%) | 1 (3.6%) |
| SGRQ-C, mMRC, FeNO Scores | Biomass (n = 32) | LPG (n = 28) | p-Value |
|---|---|---|---|
| SGRQ-C Symptoms | 47.0 (5.5) | 20.2 (11.1) | <0.001 |
| SGRQ-C Activity | 36.4 (8.5) | 16.5 (9.0) | <0.001 |
| SGRQ-C Impact | 15.9 (4.7) | 5.2 (3.5) | <0.001 |
| SGRQ-C Composite | 27.1 (3.8) | 10.8 (4.0) | <0.001 |
| Mean mMRC | 2.9 (0.9) | 0.5 (0.6) | <0.001 |
| FeNO (first test) ppb | 25.4 (7.9) | 8.6 (3.2) | <0.001 |
| FeNO (second test) ppb | 25.4 (8.1) | 8.6 (3.1) | <0.001 |
| SGRQ-C Symptoms | Biomass (n = 32) | LPG (n = 28) |
|---|---|---|
| Cough | ||
| Most days | 5 (15.6) | 0 (0.0) |
| Several days | 14 (43.8) | 3 (10.7) |
| Only with chest infections | 10 (31.3) | 16 (57.1) |
| Not at all | 3 (9.4) | 9 (32.1) |
| Phlegm | ||
| Most days | 4 (12.5) | 0 (0.0) |
| Several days | 16 (50.0) | 4 (14.3) |
| Only with chest infections | 12 (37.5) | 13 (46.4) |
| Not at all | 0 (0.0) | 11 (39.3) |
| Shortness of breath | ||
| Most days | 2 (6.3) | 0 (0.0) |
| Several days | 26 (81.3) | 10 (35.7) |
| Not at all | 4 (12.5) | 18 (64.3) |
| Wheezing | ||
| Most days | 0 (0.0) | 0 (0.0) |
| Several days | 0 (0.0) | 0 (0.0) |
| Few days | 0 (0.0) | 0 (0.0) |
| Only with chest infections | 5 (15.6) | 4 (14.3) |
| Not at all | 27 (84.4) | 24 (85.7) |
| Weighted Scores | Biomass (n = 32) | LPG (n = 28) | p-Value |
|---|---|---|---|
| Cough | 41.6 (21.9) | 21.0 (15.7) | <0.001 |
| Phlegm | 44.4 (14.7) | 20.7 (17.9) | <0.001 |
| Shortness of breath | 46.3 (19.9) | 18.0 (24.5) | <0.001 |
| Wheezing | 5.7 (13.4) | 5.2 (13.0) | 0.887 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parikh, R.; Rao, S.R.; Kukde, R.; O'Connor, G.T.; Patel, A.; Hibberd, P.L. Assessing the Respiratory Effects of Air Pollution from Biomass Cookstoves on Pregnant Women in Rural India. Int. J. Environ. Res. Public Health 2021, 18, 183. https://doi.org/10.3390/ijerph18010183
Parikh R, Rao SR, Kukde R, O'Connor GT, Patel A, Hibberd PL. Assessing the Respiratory Effects of Air Pollution from Biomass Cookstoves on Pregnant Women in Rural India. International Journal of Environmental Research and Public Health. 2021; 18(1):183. https://doi.org/10.3390/ijerph18010183
Chicago/Turabian StyleParikh, Raj, Sowmya R. Rao, Rakesh Kukde, George T. O'Connor, Archana Patel, and Patricia L. Hibberd. 2021. "Assessing the Respiratory Effects of Air Pollution from Biomass Cookstoves on Pregnant Women in Rural India" International Journal of Environmental Research and Public Health 18, no. 1: 183. https://doi.org/10.3390/ijerph18010183
APA StyleParikh, R., Rao, S. R., Kukde, R., O'Connor, G. T., Patel, A., & Hibberd, P. L. (2021). Assessing the Respiratory Effects of Air Pollution from Biomass Cookstoves on Pregnant Women in Rural India. International Journal of Environmental Research and Public Health, 18(1), 183. https://doi.org/10.3390/ijerph18010183




