Crepis vesicaria L. subsp. taraxacifolia Leaves: Nutritional Profile, Phenolic Composition and Biological Properties
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Material and Extract Preparation
2.2. Chemical Characterization
2.2.1. Nutrient Composition Analysis
2.2.2. Phenolic Profile HPLC-PDA-ESI/MSn
2.2.3. Quantification by HPLC-PDA
2.3. Antioxidant Activity
2.3.1. 2,2-Diphenyl-1-Picrylhydrazyl Radical Assay (DPPH)
2.3.2. Ferric Reducing Antioxidant Power Assay (FRAP)
2.3.3. 2,2′-Azinobis-(3-ethylbenzothiazoline-6-sulfonate) Assay (pH = 7) (ABTS)
2.4. Anti-Inflammatory Activity Evaluation
2.4.1. Nitrite Production by Griess Assay
2.4.2. Assessment of Cell Viability by Resazurin Assay
2.5. Statistical Analysis
3. Results and Discussion
3.1. Nutrient Composition of C. vesicaria
3.1.1. Nutritional Analysis of Crepis vesicaria subsp. taraxacifolia Leaves
3.1.2. Lipid and Fatty Acids Composition of Crepis vesicaria subsp. taraxacifolia Leaves
3.1.3. Minerals and Heavy Metal Composition of Cv Leaves
3.2. Screening for Antioxidant/Scavenging Activity
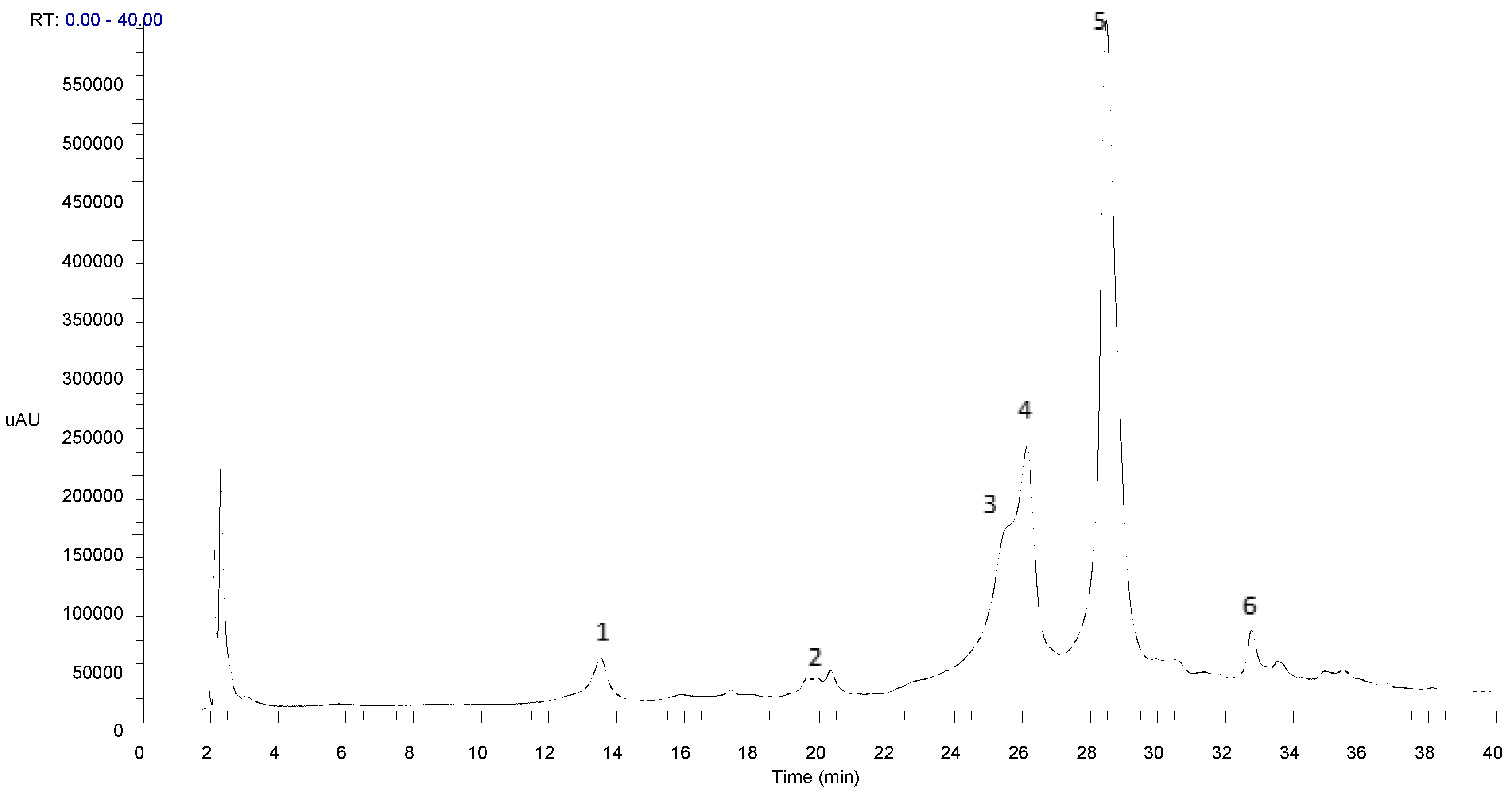
3.3. Phenolic Profile of 70% Ethanolic Extract from Crepis vesicaria subsp. taraxacifolia
3.4. Quantification of Chicoric Acid
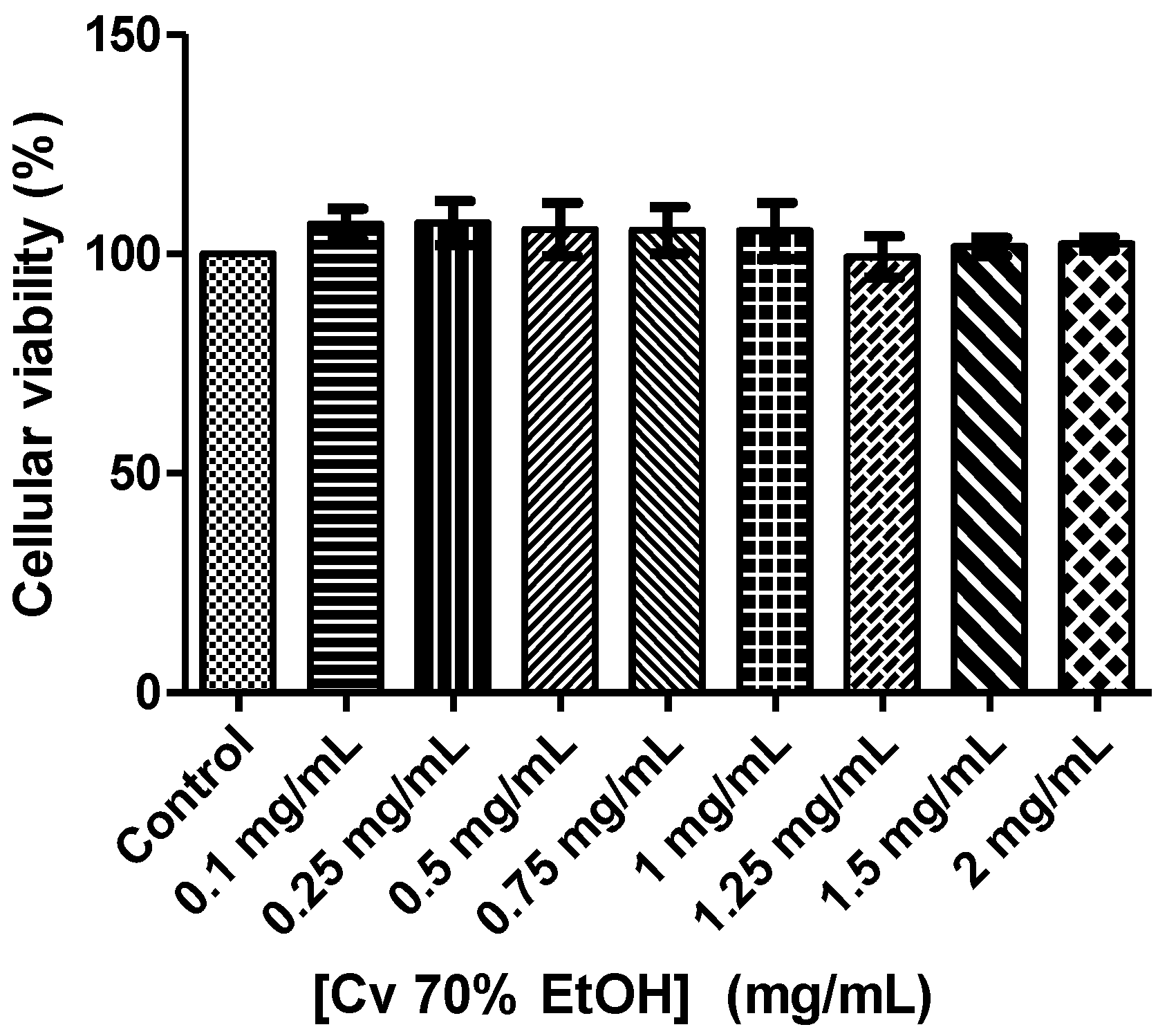
3.5. Assessment of Cell Viability of the Cv 70% EtOH Extract
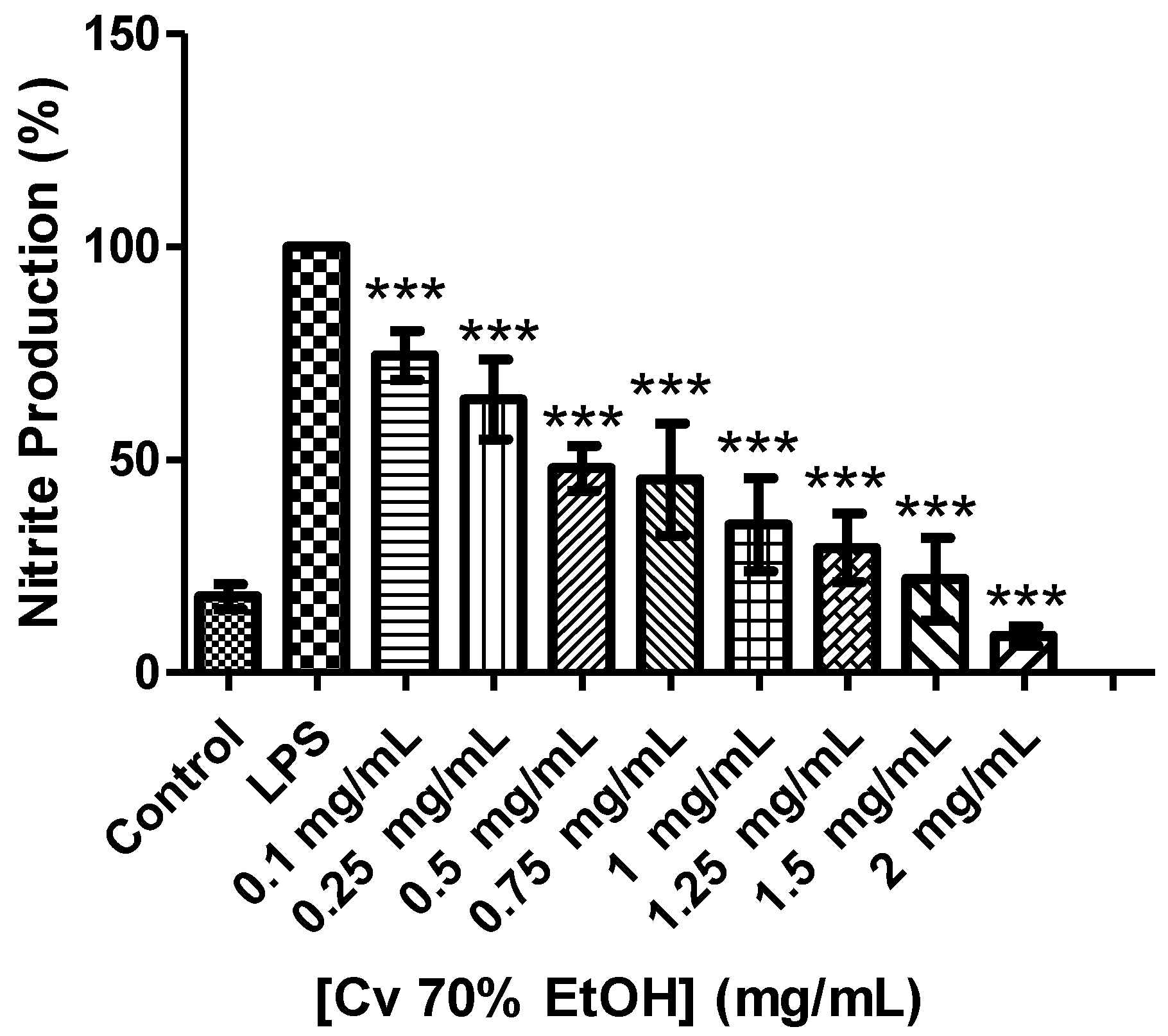
3.6. Antioxidant and Anti-Inflammatory Activity of the Cv 70% Ethanol Extract
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Enke, N.; Gemeinholzer, B. Babcock revisited: New insights into generic delimitation and character evolution in Crepis L. (Compositae: Cichorieae) from ITS and matK sequence data. Taxon 2008, 57, 756–768. [Google Scholar] [CrossRef]
- Sansanelli, S.; Tassoni, A. Wild food plants traditionally consumed in the area of Bologna (Emilia Romagna region, Italy). J. Ethnobiol. Ethnomed. 2014, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Orhan, D.D.; Özçelik, B.; Hoşbaş, S.; Vural, M. Assessment of antioxidant, antibacterial, antimycobacterial, and antifungal activities of some plants used as folk remedies in Turkey against dermatophytes and yeast-like fungi. Turk. J. Biol. 2016, 36, 672–686. [Google Scholar]
- Dalar, A. Plant taxa used in the treatment of diabetes in Van Province, Turkey. Int. J. Second. Metab. 2018, 5, 170–184. [Google Scholar] [CrossRef]
- Singh, K.; Lal, B. Ethnomedicines used against four common ailments by the tribal communities of Lahaul-Spiti in western Himalaya. J. Ethnopharmacol. 2008, 115, 147–159. [Google Scholar] [CrossRef]
- Ebada, S.S.; Al-Jawabri, N.A.; Youssef, F.S.; Albohy, A.; Aldalaien, S.M.; Disi, A.M.; Proksch, P. In vivo antiulcer activity, phytochemical exploration, and molecular modelling of the polyphenolic-rich fraction of Crepis sancta extract. Inflammopharmacology 2019, 28, 321–331. [Google Scholar] [CrossRef]
- Bakar, F.; Acikara, Ö.B.; Ergene, B.; Nebioğlu, S.; Çitoğlu, G.S. Antioxidant activity and phytochemical screening of some Asteraceae plants. Turk. J. Pharm. Sci. 2015, 12, 36–45. [Google Scholar] [CrossRef]
- Rahman, M. An ethnobotanical investigation on Asteraceae family at Rajshahi, Bangladesh. Acad. J. Med. Plants 2013, 1, 92–100. [Google Scholar]
- Zengin, G. Crepis foetida L. subsp. rhoeadifolia (Bieb.) Celak. as a source of multifunctional agents: Cytotoxic and phytochemical evaluation. J. Funct. Foods 2015, 17, 698–708. [Google Scholar] [CrossRef]
- Ooi, L.S.; Wang, H.; Luk, C.-W.; Ooi, V.E. Anticancer and antiviral activities of Youngia japonica (L.) DC (Asteraceae, Compositae). J. Ethnopharmacol. 2004, 94, 117–122. [Google Scholar] [CrossRef]
- Ooi, L.S.; Wang, H.; He, Z.; Ooi, V.E. Antiviral activities of purified compounds from Youngia japonica (L.) DC (Asteraceae, Compositae). J. Ethnopharmacol. 2006, 106, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Munira, S.; Kabir, H.; Bulbul, I.J.; Nesa, L.; Muhit, A.; Haque, I. Pharmacological activities of Youngia japonica extracts. Annu. Res. Rev. Biol. 2018, 25, 1–14. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zheng, Y.-W.; Liu, Q.; Liu, L.-P.; Luo, F.-L.; Zhou, H.-C.; Isoda, H.; Ohkohchi, N.; Li, Y.-M. Reactive oxygen species in skin repair, regeneration, aging, and inflammation. In Reactive Oxygen Species (ROS) in Living Cells; Intech: London, UK, 2018. [Google Scholar]
- Rapa, S.F.; Di Iorio, B.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and oxidative stress in chronic kidney disease—potential therapeutic role of minerals, vitamins and plant-derived metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Jiménez, F.J.; Lozano-Sánchez, J.; Cádiz-Gurrea, M.D.L.L.; Arráez-Román, D.; Segura-Carretero, A. Functional ingredients based on nutritional phenolics. A Case Study against inflammation: Lippia Genus. Nutrients 2019, 11, 1646. [Google Scholar] [CrossRef] [PubMed]
- Francisco, V.; Costa, G.; Figueirinha, A.; Marques, C.; Pereira, P.; Neves, B.M.; Lopes, M.C.; García-Rodríguez, C.; Cruz, M.T.; Batista, M.T. Anti-inflammatory activity of Cymbopogon citratus leaves infusion via proteasome and nuclear factor-κB pathway inhibition: Contribution of chlorogenic acid. J. Ethnopharmacol. 2013, 148, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Francisco, V.L.G.; Figueirinha, A.; Neves, B.M.; García-Rodríguez, C.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Cymbopogon citratus as source of new and safe anti-inflammatory drugs: Bio-guided assay using lipopolysaccharide-stimulated macrophages. J. Ethnopharmacol. 2011, 133, 818–827. [Google Scholar] [CrossRef]
- Yen, G.-C.; Duh, P.-D.; Huang, D.-W.; Hsu, C.-L.; Fu, T.Y.-C. Protective effect of pine (Pinus morrisonicola Hay.) needle on LDL oxidation and its anti-inflammatory action by modulation of iNOS and COX-2 expression in LPS-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2008, 46, 175–185. [Google Scholar] [CrossRef]
- Zhang, T.-T.; Hu, T.; Jiang, J.-G.; Zhao, J.-W.; Zhu, W. Antioxidant and anti-inflammatory effects of polyphenols extracted from Ilex latifolia Thunb. RSC Adv. 2018, 8, 7134–7141. [Google Scholar] [CrossRef]
- Saji, N.; Francis, N.; Blanchard, C.L.; Schwarz, L.J.; Santhakumar, A.B. Rice bran phenolic compounds regulate genes associated with antioxidant and anti-inflammatory activity in human umbilical vein endothelial cells with induced oxidative stress. Int. J. Mol. Sci. 2019, 20, 4715. [Google Scholar] [CrossRef] [PubMed]
- Cunniff, P. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 1997. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Boly, R.; Boly, R.; Lamkami, T.; Lompo, M.; Dubois, J.; Guissou, I. DPPH free radical scavenging activity of two extracts from Agelanthus dodoneifolius (Loranthaceae) Leaves. Int. J. Toxicol. Pharmacol. Res. 2016, 8, 29–34. Available online: www.ijtpr.com (accessed on 27 January 2020).
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Green, L.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Rampersad, S.N. Multiple Applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef]
- Chou, T.-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- García-Herrera, P.; Sanchezmata, M.C.; Cámara, M.; Fernandezruiz, V.; Díez-Marqués, C.; Molina, M.G.A.; Tardio, J. Nutrient composition of six wild edible Mediterranean Asteraceae plants of dietary interest. J. Food Compos. Anal. 2014, 34, 163–170. [Google Scholar] [CrossRef]
- Opitz, B.; Smith, P.M.; Kienzle, E.; Earle, K.E.; Maskell, I.E. The measurement of dietary fibre in pet food: A comparison of methods. J. Anim. Physiol. Anim. Nutr. 1998, 79, 146–152. [Google Scholar] [CrossRef]
- Albishi, T.; Banoub, J.H.; De Camargo, A.C.; Shahidi, F. Wood extracts as unique sources of soluble and insoluble-bound phenolics: Reducing power, metal chelation and inhibition of oxidation of human LDL-cholesterol and DNA strand scission. J. Food Bioact. 2019, 8. [Google Scholar] [CrossRef]
- Barnett, J.K.; Crawford, J.A. Pre-Laying Nutrition of Sage Grouse Hens in Oregon. J. Range Manag. 1994, 47, 114. [Google Scholar] [CrossRef]
- Guil, J.L.; E Torija, M.; Giménez, J.J.; Rodríguez, I. Identification of fatty acids in edible wild plants by gas chromatography. J. Chromatogr. A 1996, 719, 229–235. [Google Scholar] [CrossRef]
- Sales-Campos, H.; De Souza, P.R.; Peghini, B.C.; Da Silva, J.S.; Cardoso, C.R. An overview of the modulatory effects of oleic acid in health and disease. Mini Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar] [PubMed]
- Zeghichi, S.; Kallithraka, S.; Simopoulos, A.P.; Kypriotakis, Z. Nutritional composition of selected wild plants in the diet of Crete. World Rev. Nutr. Diet. 2003, 91, 22–40. [Google Scholar] [PubMed]
- Mir-Marqués, A.; Cervera, M.; De La Guardia, M. Mineral analysis of human diets by spectrometry methods. TrAC Trends Anal. Chem. 2016, 82, 457–467. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. Mol. Ecol. Evol. Approaches Appl. 2012, 101, 133–164. [Google Scholar] [CrossRef]
- Commission of the European Communities. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364. Available online: https://www.ecolex.org/details/legislation/commission-regulation-ec-no-18812006-setting-maximum-levels-for-certain-contaminants-in-foodstuffs-lex-faoc068134/ (accessed on 23 December 2020).
- Falcão, H.G. Optimizing the potential bioactivity of isoflavones from soybeans via ultrasound pretreatment: Antioxidant potential and NF-κB activation. J. Food Biochem. 2019, 43, e13018. [Google Scholar] [CrossRef]
- El Jemli, M.; Kamal, R.; Marmouzi, I.; Zerrouki, A.; Cherrah, Y.; Alaoui, K. Radical-Scavenging activity and ferric reducing ability of Juniperus thurifera (L.), J. oxycedrus (L.), J. phoenicea (L.) and Tetraclinis articulata (L.). Adv. Pharmacol. Sci. 2016, 2016. [Google Scholar] [CrossRef]
- Costa, G.; Ferreira, J.P.; Vitorino, C.; Pina, M.E.T.; Sousa, J.; Figueiredo, I.; Batista, M.T. Polyphenols from Cymbopogon citratus leaves as topical anti-inflammatory agents. J. Ethnopharmacol. 2016, 178, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Liu, R.; Liu, S.; Zhang, X.; Wang, Z.; Lu, J. HPLC-LTQ-Orbitrap MSn profiling method to comprehensively characterize multiple chemical constituents in Xiao-er-qing-jie granules. Anal. Methods 2015, 7, 7511–7526. [Google Scholar] [CrossRef]
- Vukovic, N.; Vukic, M.D.; Đelić, G.T.; Kačániová, M.; Cvijovic, M. The investigation of bioactive secondary metabolites of the methanol extract of eryngium amethystinum. Kragujev. J. Sci. 2018, 113–129. [Google Scholar] [CrossRef]
- Ferreres, F.; Bernardo, J.; Andrade, P.B.; E Sousa, C.A.; Gilizquierdo, A.; Valentão, P. Pennyroyal and gastrointestinal cells: Multi-target protection of phenolic compounds against t-BHP-induced toxicity. RSC Adv. 2015, 5, 41576–41584. [Google Scholar] [CrossRef]
- Lee, J.M.; Scagel, C. Chicoric acid: Chemistry, distribution, and production. Front. Chem. 2013, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-P.; Liu, L.; Wang, L.; Hu, Y.; Zhang, W.-D.; Liu, R. Structural characterization and identification of major constituents in Jitai tablets by High-Performance Liquid Chromatography/Diode-Array Detection Coupled with Electrospray Ionization Tandem Mass Spectrometry. Molecules 2012, 17, 10470–10493. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, F.; Xiang, X.; Fan, M.; Chen, T. Evaluation of the potential of chicoric acid as a natural food antioxidant. Exp. Ther. Med. 2018, 16, 3651–3657. [Google Scholar] [CrossRef]
- Biskup, I.; Golonka, I.; Gamian, A.; Sroka, Z. Antioxidant activity of selected phenols estimated by ABTS and FRAP methods. Adv. Hyg. Exp. Postępy Hig. Med. Doświadczalnej 2013, 67, 958–963. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Krętowski, R.; Kalinowska, M.; Świderski, G.; Cechowska-Pasko, M.; Lewandowski, W. Possible mechanisms of the prevention of doxorubicin toxicity by cichoric acid—antioxidant nutrient. Nutrients 2018, 10, 44. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Barros, L.; Ferreira, I.C. A comparison of the phenolic profile and antioxidant activity of different Cichorium spinosum L. ecotypes. J. Sci. Food Agric. 2017, 98, 183–189. [Google Scholar] [CrossRef]
- Peng, Y.; Sun, Q.; Park, Y. The bioactive effects of chicoric acid as a functional food ingredient. J. Med. Food 2019, 22, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, Y.; Shen, C.; Xiao, Y.; Wang, Y.; Liu, Z.; Liu, X. Chicoric acid supplementation prevents systemic inflammation-induced memory impairment and amyloidogenesis via inhibition of NF-κB. FASEB J. 2017, 31, 1494–1507. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Ci, X.; Cheng, H.; Yu, Q.; Li, D. Chicoric acid alleviates lipopolysaccharide-induced acute lung injury in mice through anti-inflammatory and anti-oxidant activities. Int. Immunopharmacol. 2019, 66, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhang, B.; Li, S.; Zhao, Q. A formal model for analyzing drug combination effects and its application in TNF-α-induced NFκB pathway. BMC Syst. Biol. 2010, 4, 50. [Google Scholar] [CrossRef] [PubMed]
| Composition | Raw Matter | Dry Matter | |
|---|---|---|---|
| Energy (KJ/100 g) | 175.190 ± 0.259 | 1211.80 ± 2.11 | |
| Energy (Kcal/100 g) | 41.840 ± 0.062 | 289.43 ± 0.50 | |
| Moisture (g/100 g) | 85.540 ± 0.006 | - | |
| Protein (g/100 g) | 1.040 ± 0.003 | 7.18 ± 0.02 | |
| Dietary fiber (g/100 g) | 4.240 ± 0.015 | 29.35 ± 0.11 | |
| Insoluble Dietary fiber (g/100 g) | 3.490 ± 0.026 | 24.14 ± 0.18 | |
| Acid detergent fiber (ADF) (g/100 g) | 3.120 ± 0.011 | 21.59 ± 0.08 | |
| Cellulose (g/100 g) | 2.550 ± 0.002 | 17.61 ± 0.02 | |
| Crude fiber (g/100 g) | 2.460 ± 0.009 | 17.00 ± 0.06 | |
| Hemicellulose (g/100 g) | 0.620 ± 0.012 | 4.27 ± 0.09 | |
| Lignin (g/100 g) | 0.440 ± 0.013 | 3.03 ± 0.09 | |
| Acid detergent lignin (ADL) (g/100 g) | 0.430 ± 0.006 | 2.99 ± 0.04 | |
| Nitrogen-free extractives (g/100 g) | 7.530 ± 0.010 | 52.11 ± 0.07 | |
| Carbohydrates | Maltose (g/100 g) | 2.470 ± 0.015 | 17.11 ± 0.11 |
| Fructose (g/100 g) | 0.940 ± 0.012 | 6.53 ± 0.08 | |
| Glucose (g/100 g) | 0.340 ± 0.012 | 2.37 ± 0.08 | |
| Composition | Raw Matter | Dry Matter |
|---|---|---|
| Fatty acids, total polyunsaturated (mg/100 g) | 402.840 ± 0.146 | 2786.53 ± 1.01 |
| Fatty acids, total saturated (mg/100 g) | 159.820 ± 0.207 | 1105.48 ± 1.44 |
| Fatty acids, total monounsaturated (mg/100 g) | 123.710 ± 0.063 | 855.75 ± 0.44 |
| α-Linolenic acid (C18:3n-3) (mg/100 g) | 343.240 ± 0.065 | 2374.30 ± 0.57 |
| Linoleic acid (C18:2n-6) (mg/100 g) | 59.600 ± 0.084 | 412.23 ± 0.45 |
| Oleic acid (C18:1n-9) (mg/100 g) | 60.490 ± 0.087 | 418.43 ± 0.58 |
| Palmitic acid (C16:0) (mg/100 g) | 108.750 ± 0.004 | 752.26 ± 0.56 |
| Gondoic acid (C20:1) (mg/100 g) | 63.220 ± 0.086 | 437.32 ± 0.59 |
| Arachidic acid (C20:0) (mg/100 g) | 17.490 ± 0.091 | 121.00 ± 0.63 |
| Stearic acid (C18:0) (mg/100 g) | 21.520 ± 0.047 | 148.84 ± 0.60 |
| Margaric acid (C17:0) (mg/100 g) | 12.050 ± 0.081 | 83.38 ± 0.33 |
| Lipids (g/100 g) | 0.690 ± 0.004 | 4.78 ± 0.03 |
| Composition | Raw Matter | Dry Matter | |
|---|---|---|---|
| Ash (g/100 g) | 2.740 ± 0.007 | 18.94 ± 0.05 | |
| Minerals | Potassium (mg/100 g) | 591.290 ± 0.058 | 4090.07 ± 0.31 |
| Calcium (mg/100 g) | 309.930 ± 0.090 | 2143.84 ± 0.62 | |
| Sodium (mg/100 g) | 76.780 ± 0.084 | 531.12 ± 0.42 | |
| Phosphorus (mg/100 g) | 59.910 ± 0.074 | 412.33 ± 0.51 | |
| Magnesium (mg/100 g) | 45.460 ± 0.066 | 314.46 ± 0.34 | |
| Zinc (mg/100 g) | 0.860 ± 0.012 | 5.97 ± 0.09 | |
| Manganese (mg/100 g) | 0.830 ± 0.006 | 5.71 ± 0.04 | |
| Iron (mg/100 g) | 0.590 ± 0.003 | 4.05 ± 0.02 | |
| Copper (mg/100 g) | 0.420 ± 0.012 | 2.89 ± 0.09 | |
| Nickel (mg/100 g) | 0.110 ± 0.011 | 0.79 ± 0.08 | |
| Chromium (mg/100 g) | 0.020 ± 0.003 | 0.11 ± 0.02 | |
| Heavy metals | Cadmium (µg/100 g) | 19.300 ± 0.076 | 133.52 ± 0.53 |
| Lead (µg/100 g) | 2.300 ± 0.094 | 15.92 ± 0.65 | |
| Mercury (µg/100 g) | 0.050 ± 0.002 | 0.36 ± 0.01 | |
| IC50 (µg/mL) | TE * | |
|---|---|---|
| DPPH● | 26.20 ± 1.86 | 111.980 ± 0.041 |
| ABTS● (pH = 7) | 18.92 ± 2.24 | 21.670 ± 0.012 |
| FRAP | - | 0.678 ± 0.168 |
| Compound | Partial Identification | Rt (min.) | λmax. by HPLC/PDA (nm) | [M-H]− | MS 2 | MS 3 |
|---|---|---|---|---|---|---|
| 1 | Caffeic acid [44] | 13.54 | 238, 251, 291 sh, 299 sh, 328 | 179 | [179]: 135 (100) | [179 135]: 151 (13), 135 (61), 125 (11), 107 (24), 91 (100) |
| 2 | Quinic acid [44] | 20.33 | 238, 253, 291 sh, 300 sh, 326 | 191 | [191]: 173 (82), 171 (24), 147 (12), 127 (100), 111 (45), 109 (27), 93 (46), 87 (14), 85 (66) | --- |
| 3 | Chicoric acid isomer [45] | 25.59 | 238, 251, 291 sh, 299 sh, 329 | 473 | [473]: 311 (100), 293 (80) | [473 311]: 179 (62), 149 (100) |
| 4 | Chicoric acid isomer [45] | 26.12 | 238, 251, 292 sh, 300 sh, 329 | 473 | [473]: 311 (100), 293 (80) | [473 311]: 179 (58), 149 (100) |
| 5 | Chicoric acid isomer [45] | 28.47 | 238, 251, 292 sh, 299 sh, 330 | 473 | [473]: 311 (100), 293 (80) | [473 311]: 179 (61), 149 (100) |
| 6 | Feruloyl hexosylpentoside [46] | 32.77 | 238, 253, 292 sh, 299 sh, 329 | 487 | [487]: 325 (100), 307 (46), 293 (77) | [487 325]: 193 (100) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedreiro, S.; da Ressurreição, S.; Lopes, M.; Cruz, M.T.; Batista, T.; Figueirinha, A.; Ramos, F. Crepis vesicaria L. subsp. taraxacifolia Leaves: Nutritional Profile, Phenolic Composition and Biological Properties. Int. J. Environ. Res. Public Health 2021, 18, 151. https://doi.org/10.3390/ijerph18010151
Pedreiro S, da Ressurreição S, Lopes M, Cruz MT, Batista T, Figueirinha A, Ramos F. Crepis vesicaria L. subsp. taraxacifolia Leaves: Nutritional Profile, Phenolic Composition and Biological Properties. International Journal of Environmental Research and Public Health. 2021; 18(1):151. https://doi.org/10.3390/ijerph18010151
Chicago/Turabian StylePedreiro, Sónia, Sandrine da Ressurreição, Maria Lopes, Maria Teresa Cruz, Teresa Batista, Artur Figueirinha, and Fernando Ramos. 2021. "Crepis vesicaria L. subsp. taraxacifolia Leaves: Nutritional Profile, Phenolic Composition and Biological Properties" International Journal of Environmental Research and Public Health 18, no. 1: 151. https://doi.org/10.3390/ijerph18010151
APA StylePedreiro, S., da Ressurreição, S., Lopes, M., Cruz, M. T., Batista, T., Figueirinha, A., & Ramos, F. (2021). Crepis vesicaria L. subsp. taraxacifolia Leaves: Nutritional Profile, Phenolic Composition and Biological Properties. International Journal of Environmental Research and Public Health, 18(1), 151. https://doi.org/10.3390/ijerph18010151







