Assessing the Health Loss from Kashin-Beck Disease and Its Relationship with Environmental Selenium in Qamdo District of Tibet, China
Abstract
:1. Introduction
2. Materials and Methods
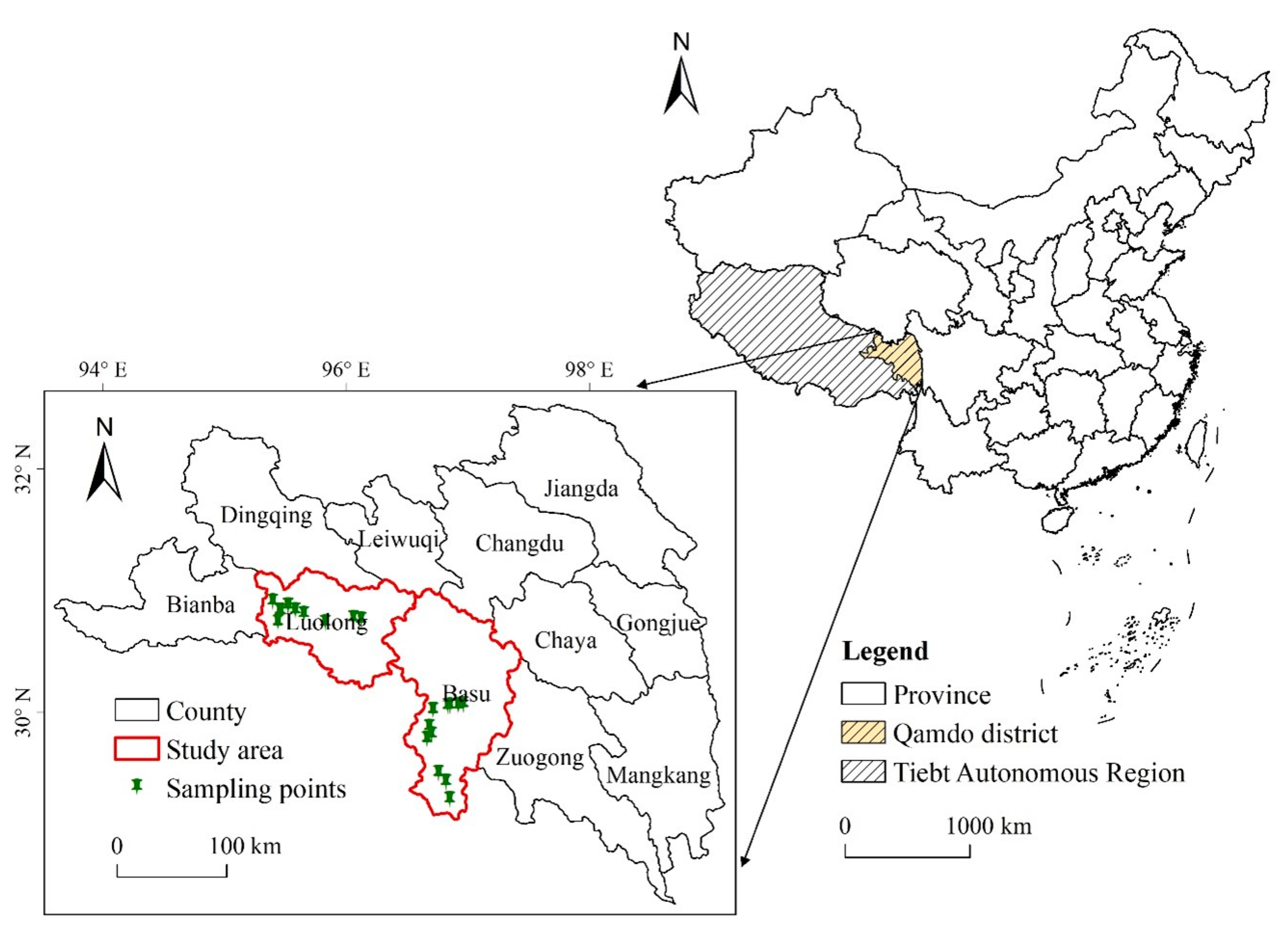
2.1. Study Area
2.2. Study Populations
2.3. Sampling and Analyses
2.4. Calculation of YLDs and YLD Rate
2.5. Multiple Linear Regression Model
3. Results
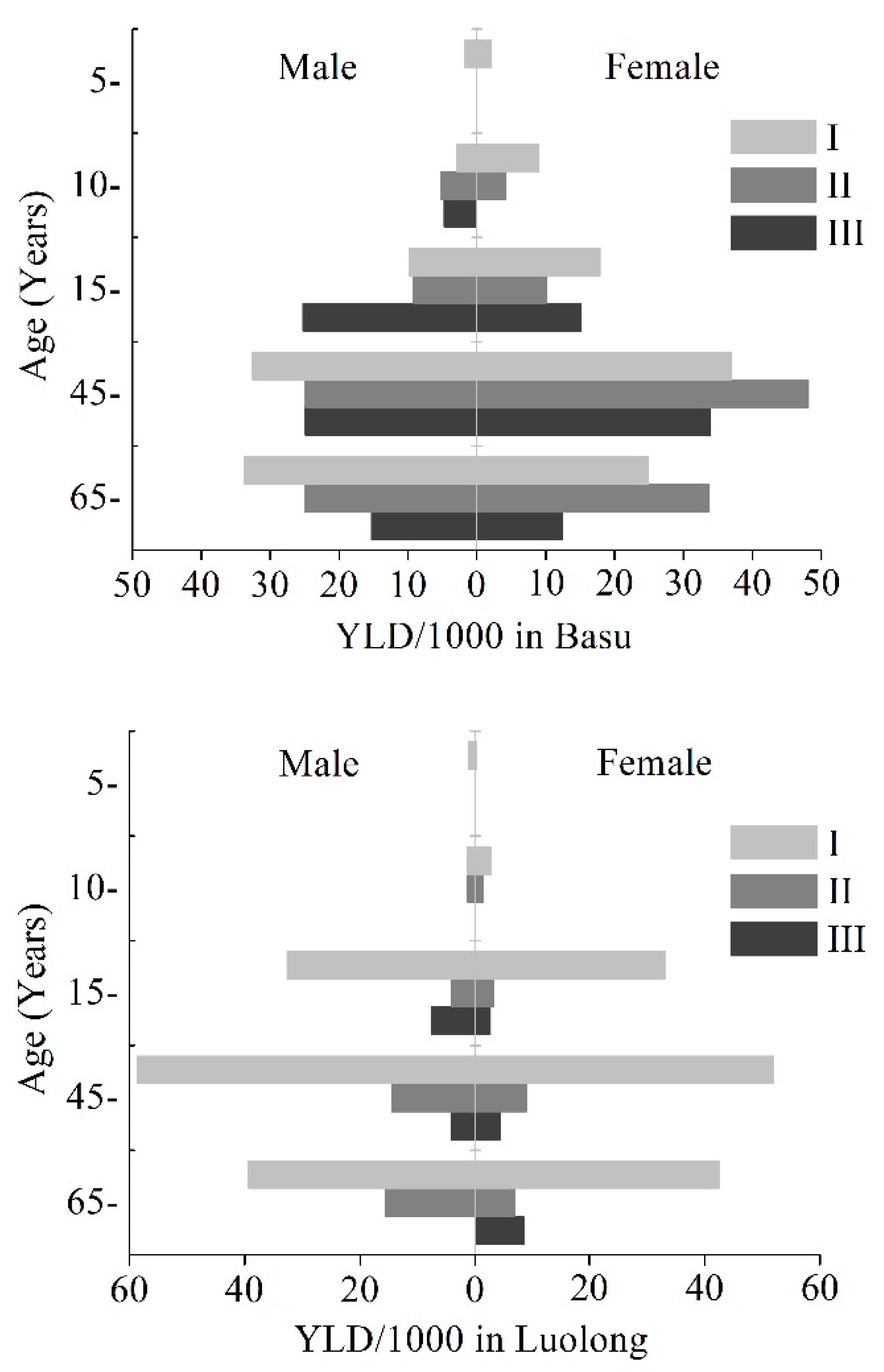
3.1. YLDs and YLD Rate of KBD
3.2. Patterns by Age Groups
3.3. Se Content in the Environment
3.4. Correlation Analysis
3.5. MLR Modeling
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kraus, V.B. 185-Rare osteoarthritis: Ochronosis and Kashin-Beck disease. Rheumatology 2015, 2, 1536–1547. [Google Scholar]
- Cao, J.; Li, S.; Shi, Z.; Yue, Y.; Sun, J.; Chen, J.; Fu, Q.; Hughes, C.E.; Caterson, B. Articular cartilage metabolism in patients with Kashin-Beck Disease: An endemic osteoarthropathy in China. Osteoarthr. Cartil. 2008, 16, 680–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Ma, W.J.; Zhang, F.; Ren, F.L.; Qu, C.J.; Lammi, M.J. Recent advances in the research of an endemic osteochondropathy in China: Kashin-Beck disease. Osteoarthr. Cartil. 2014, 22, 1774–1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooq, U.; Guo, X.; Irshad, R.; Yaqoob, S. Pattern of joints involvement in Kashin-Beck disease: A local osteochondropathy in China. J. Ayub. Med. Coll. Abbottabad. 2010, 22, 97–100. [Google Scholar] [PubMed]
- Zhao, S.C.; Deji, Y.Z.; Tu, D.; Li, R.J.; Gong, H.Q.; Guo, M. Surveillance report of Kashin Beck disease in Tibet in 2017. Chin. J. Ctrl. Endem. Dis. 2019, 34, 155–157. [Google Scholar]
- National Health Commission. China Health Statistics Yearbook 2018; Beijing Union Medical University Press: Beijing, China, 2018. [Google Scholar]
- Murray, C.J.; Lopez, A.D. Evidence-based health policy-lessons from the Global Burden of Disease Study. Science 1996, 274, 740–743. [Google Scholar] [CrossRef] [Green Version]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Abraham, J. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abdollahpour, I.; GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [Green Version]
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Fleming, T.; Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar]
- Spronk, I.; Edgar, D.W.; Baar, M.E.; Wood, F.M.; Van Loey, N.E.E.; Middelkoop, E.; Nieuwenhuis, M. Improved and standardized method for assessing years lived with disability after burns and its application to estimate the non-fatal burden of disease of burn injuries in Australia, New Zealand and the Netherlands. BMC Public Health 2020, 20, 121. [Google Scholar] [CrossRef] [Green Version]
- Zha, X.J.; Gao, X. Ecological analysis of Kashin-Beck osteoarthropathy risk factors in Tibet’s Qamdo City, China. Sci. Rep. 2019, 9, 2471. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ning, Y.; Wang, X. Selenium and Kashin-Beck Disease. In Selenium: Chemistry, Analysis, Function and Effects; Preedy, V.R., Ed.; Royal Society of Chemistry: London, UK, 2015; pp. 552–571. [Google Scholar]
- Tan, J.; Zhu, W.Y.; Wang, W.Y.; Li, R.B.; Hou, S.F.; Wang, D.C.; Yang, L.S. Selenium in soil and endemic diseases in China. Sci. Total Environ. 2020, 284, 227–235. [Google Scholar] [CrossRef]
- Li, S.; Li, W.; Hu, X.; Yang, L.; Xirao, R. Soil selenium concentration and Kashin-Beck disease prevalence in Tibet, China. Front. Environ. Sci. Eng. 2009, 3, 62–68. [Google Scholar] [CrossRef]
- Zhang, B.J.; Yang, L.S.; Wang, W.Y.; Li, Y.H. Environmental selenium in the Kaschin–Beck disease area, Tibetan Plateau, China. Environ. Geochem. Health 2011, 33, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, H.R.; Li, Y.H.; Yu, J.P.; Yang, L.S.; Feng, F.J.; Chen, Z. Speciation, distribution and bioavailability of soil selenium in the Tibetan Plateau Kashin–Beck disease area—A case study in Songpan County, Sichuan Province, China. Biol. Trace Elem. Res. 2013, 156, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.H.Q.; Guo, L.L. Observations on effect of Kashin-Beck disease prevention by supplying selenium for 20 years in JingChuan County, Gansu Province. Endem. Dis. Bull. 2002, 17, 40–42. [Google Scholar]
- Bai, M.C.W.; Za, X.S.D.; Xi, R.R.D.; Gong, H.Q.; Zhao, S.C. The investigation on effect of supplement selenium in KBD-affected area of Linzhou and Sangri County, Tibet. Chin. J. Endemiol. 2007, 26, 710. [Google Scholar]
- Li, Q.L.; Xiao, W.; Ding, Y.F.; Zhang, T. Analyzing the effect of Se-I salt on Kashin-Beck disease by mathematical statistics method. Med. Anim. Prev. 2007, 23, 659–660. [Google Scholar]
- Zhao, Z.J.; Li, Q.; Yang, P.Z.; Wang, H.; Kong, L.C.; Wang, L.H.; Sun, L.Y. Selenium: A protective factor for Kaschin-Beck disease in Qing-Tibet plateau. Biol. Trace Elem. Res. 2013, 153, 1–4. [Google Scholar] [CrossRef]
- Thomson, C.D.; Robinson, M.F. Selenium in human health and disease with emphasis on those aspects peculiar to New Zealand. Am. J. Clin. Nutr. 1980, 33, 303–323. [Google Scholar] [CrossRef] [Green Version]
- Sundström, H. Annual variation of serum selenium in patients with gynaecological cancer during 1978–1983 in Finland, a low selenium area. Int. J. Vitam. Nutr. Res. 1985, 55, 433–438. [Google Scholar] [PubMed]
- Wang, J.; Li, H.R.; Yang, L.S. Selenium levels in the environment, food, and human hair in Kashin-Beck Disease endemic areas of the Qinghai-Tibet Plateau. Prog. Geogr. 2020, 39, 1677–1686. [Google Scholar] [CrossRef]
- Gong, H.Q.; Zhao, S.C.; Nima, C.J.; Guo, M.; Li, Q.W. Monitoring report of Kashin-Beck disease in Changdu region of Tibet in 2014. Foreign Med. Sci. Sect. Medgeogr. 2015, 36, 270–273. [Google Scholar]
- Statistics Bureau of Qamdo City. Statistical bulletin of national economic and social development of Qamdo City. In Changdu Yearbook; Wang, X.L., Ed.; Chinese Cultural and Historical Press: Beijing, China, 2015; pp. 41–42. [Google Scholar]
- Xu, Y.F.; Li, Y.H.; Li, H.R.; Wang, L.; Liao, X.Y.; Wang, J.; Kong, C. Effects of topography and soil properties on soil selenium distribution and bioavailability (phosphate extraction): A case study in Yongjia County, China. Sci. Total Environ. 2018, 633, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Maredza, M.; Bertram, M.Y.; Tollman, S.M. Disease burden of stroke in rural South Africa: An estimate of incidence, mortality and disability adjusted life years. BMC Neurol. 2015, 15, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, T.W.; Sun, L.P.; Hong, Q.B.; Deng, Y.; Zhang, G.H.; Wang, H.; Yi, P.; Guo, J.G.; Zhou, X.N. Burden of disease in schistosomiasis japonica I Calculation and evaluation of years lived with disability of chronic schistosomiasis. Chin. J. Schisto. Control 2011, 23, 243–248. [Google Scholar]
- Mathers, C.D.; Vos, T.; Lopez, A.D.; Salomon, J.; Ezzati, M. National Burden of Disease Studies: A Practical Guide, 2nd ed.; World Health Organization: Geneva, Switzerland, 2001; p. 96. Available online: https://www.who.int/healthinfo/nationalburdenofdiseasemanual.pdf?ua=1 (accessed on 22 November 2020).
- Duoji, Z.D. (Ed.) Statistics Bureau of Tibet Autonomous Region. Tibet Statistical Yearbook; China Statistical Press: Beijing, China, 2015; pp. 50–52. [Google Scholar]
- Jia, T.W.; Zhou, X.N.; Wang, X.H.; Utzinger, J.; Sternmann, P.; Wu, X.H. Assessment of the age-specific disability weight of chronic schistosomiasis japonica. Bull. World Health Organ. 2007, 85, 458–465. [Google Scholar]
- Yang, L.P.; Liang, S.Y.; Wang, X.J.; Li, X.J.; Wu, Y.L.; Ma, W. Burden of disease measured by disability-adjusted life years and a disease forecasting time series model of scrub typhus in Laiwu, China. PLoS Negl. Trop. Dis. 2015, 9, e3420. [Google Scholar] [CrossRef]
- Cai, L.; Bi, W.H.; Wan, C.H.; Huang, W.X.; Li, J.M.; Yang, R. Study on burden of stroke in Shilin county of Kunming. Chin. J. Public Health 2007, 23, 45–46. [Google Scholar]
- Liu, D.; Wang, Z.L. Differential diagnosis of Kashin-Beck disease and rheumatoid arthritis. Chin. J. Ctrl. Endem. Dis. 2009, 24, 195–197. [Google Scholar]
- Wu, W.H.; He, A.W.; Wen, Y.; Xiao, X.; Hao, J.C.; Zhang, F.; Guo, X. Comparison of micro RNA expression profiles of Kashin-Beck disease, osteoarthritis and rheumatoid arthritis. Sci. Rep. 2017, 7, 540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2017 (GBD 2017) Disability Weights; Institute for Health Metrics and Evaluation (IHME): Seattle, DC, USA, 2018; Available online: http://ghdx.healthdata.org/record/ihme-data/gbd-2017-disability-weights (accessed on 22 November 2020).
- World Health Organization (WHO). YLD Template. 1989. Available online: https://www.who.int/healthinfo/global_burden_disease/tools_national/en/ (accessed on 22 November 2020).
- Abdul-Wahab, S.A.; Bakheit, C.S.; Al-Alawi, S.M. Principal component and multiple regression analysis in modelling of ground-level ozone and factors affecting its concentrations. Environ. Modell. Softw. 2005, 20, 1263–1271. [Google Scholar] [CrossRef]
- Tariq, M.M.; Eyduran, E.; Bajwa, M.A.; Waheed, A.; Iqbal, F.; Javed, Y. Prediction of body weight from testicular and morphological characteristics in indigenous Mengali sheep of Pakistan: Using factor analysis scores in multiple linear regression analysis. Int. J. Agric. Biol. 2012, 14, 590–594. [Google Scholar]
- Gènova-Maleras, R.; Álvarez-Martín, E.; Morant-Ginestar, C.; Larrea-Baz, N.F.; Catalá-López, F. Measuring the burden of disease and injury in Spain using disability-adjusted life years: An updated and policy-oriented overview. Public Health 2012, 126, 1024–1031. [Google Scholar] [CrossRef]
- Jiang, Y.W.; Hesser, J.E. Using disability-adjusted life years to assess the burden of disease and injury in Rhode Island. Public Health Rep. 2012, 127, 293–303. [Google Scholar] [CrossRef] [Green Version]
- Dodhia, H.; Phillips, K. Measuring burden of disease in two inner London boroughs using disability adjusted life years. J. Public Health 2008, 30, 313–321. [Google Scholar] [CrossRef]
- Kominski, G.F.; Simon, P.A.; Ho, A.; Luck, J.; Fielding, J.E. Assessing the burden of disease and injury in Los Angeles County using disability-adjusted life years. Public Health Rep. 2002, 117, 185–191. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Murray, C.J.L.; Lopez, A.D.; World Health Organization; World Bank; Harvard School of Public Health. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries and Risk Factors in 1990 and Projected to 2020: Summary; Christopher, J., Murray, L., Lopez, A.D., Eds.; Harvard University Press: Boston, MA, USA, 1996; pp. 6–13. Available online: https://extranet.who.int/iris/restricted/handle/10665/41864 (accessed on 22 November 2020).
- Anand, S.; Hanson, K. Disability-adjusted life years: A critical review. J. Health Econ. 1997, 16, 685–702. [Google Scholar] [CrossRef]
| County | KBD Grades | Male | Female | Total | Prevalence (%) | |||
|---|---|---|---|---|---|---|---|---|
| YLDs | YLD/1000 | YLDs | YLD/1000 | YLDs | YLD/1000 | |||
| Basu | I | 249.92 | 12.15 | 349.77 | 17.49 | 599.69 | 14.78 | 11.71 |
| II | 212.49 | 10.33 | 302.42 | 15.13 | 514.91 | 12.69 | 3.83 | |
| III | 379.65 | 18.45 | 275.04 | 13.76 | 654.69 | 16.14 | 2.31 | |
| Total | 842.06 | 40.93 | 927.24 | 46.37 | 1769.30 | 43.61 | 17.86 | |
| Luolong | I | 651.57 | 26.13 | 631.12 | 25.83 | 1282.69 | 25.98 | 24.92 |
| II | 121.76 | 4.88 | 84.32 | 3.45 | 206.08 | 4.17 | 1.53 | |
| III | 108.14 | 4.34 | 60.07 | 2.46 | 168.21 | 3.41 | 0.62 | |
| Total | 881.48 | 35.34 | 775.51 | 31.74 | 1656.98 | 33.56 | 27.06 | |
| County | NS (μg/kg) | CS (μg/kg) | SAF (μg/kg) | HB (μg/kg) | TB (μg/kg) | Rice (μg/kg) | Flour (μg/kg) | DW (μg/L) |
|---|---|---|---|---|---|---|---|---|
| Basu | 143.0 ± 54.4 (16) | 245.3 ± 91.0 (36) | 15.0 ± 4.3 (16) | 9.0 ± 4.3 (26) | 13.1 ± 7.4 (26) | 49.8 ± 16.9 (26) | 27.5 ± 10.8 (22) | 0.79 ± 0.32 (39) |
| Luolong | 207.6 ± 37.1 (10) | 260.3 ± 71.0 (21) | 13.7 ± 3.1 (14) | 6.9 ± 2.9 (14) | 6.4 ± 4.8 (14) | 50.7 ± 16.3 (14) | 16.9 ± 10.3 (11) | 0.49 ± 0.35 (46) |
| Total | 167.8 ± 57.4 (26) | 250.9 ± 83.8 (57) | 14.5 ± 3.7 (30) | 8.4 ± 4.0 (40) | 11.2 ± 7.1 (40) | 50.0 ± 16.5 (40) | 22.6 ± 9.1 (33) | 0.63 ± 0.37 (85) |
| YLD Rate | Prev | NS | CS | SAF | HB | TB | Rice | Flour | DW | |
|---|---|---|---|---|---|---|---|---|---|---|
| YLD rate (%) | 1.000 | |||||||||
| Prev (%) | 0.718 ** | 1.000 | ||||||||
| NS (µg/kg) | −0.628 * | −0.171 | 1.000 | |||||||
| CS (µg/kg) | −0.853 ** | −0.518 | 0.552 * | 1.000 | ||||||
| SAF (µg/kg) | −0.429 | −0.241 | 0.390 | 0.447 | 1.000 | |||||
| HB (µg/kg) | −0.727 ** | −0.837 ** | 0.155 | 0.236 | 0.195 | 1.000 | ||||
| TB (µg/kg) | −0.454 | −0.541 * | −0.193 | −0.008 | −0.255 | 0.768 ** | 1.000 | |||
| Rice (µg/kg) | −0.168 | −0.327 | 0.181 | 0.023 | −0.159 | 0.431 | 0.332 | 1.000 | ||
| Flour (µg/kg) | −0.142 | −0.433 | −0.315 | −0.273 | −0.116 | 0.547 | 0.542 | 0.558 | 1.000 | |
| DW (µg/L) | −0.371 | −0.534 * | −0.114 | 0.125 | −0.171 | 0.707 ** | 0.598 * | 0.057 | 0.436 | 1.000 |
| Independent Variables | Coefficients | Standard Error | Sig. | VIF | ||
|---|---|---|---|---|---|---|
| Intercept | 97.848 | 8.882 | 0.000 | |||
| CS | −0.178 ** | 0.042 | 0.005 | 1.315 | ||
| HB | −2.603 * | 0.781 | 0.016 | 1.315 | ||
| R2 | 0.905 | Adjusted R2 | 0.873 | |||
| F | 28.442 | Sig. | 0.001 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhao, S.; Yang, L.; Gong, H.; Li, H.; Nima, C. Assessing the Health Loss from Kashin-Beck Disease and Its Relationship with Environmental Selenium in Qamdo District of Tibet, China. Int. J. Environ. Res. Public Health 2021, 18, 11. https://doi.org/10.3390/ijerph18010011
Wang J, Zhao S, Yang L, Gong H, Li H, Nima C. Assessing the Health Loss from Kashin-Beck Disease and Its Relationship with Environmental Selenium in Qamdo District of Tibet, China. International Journal of Environmental Research and Public Health. 2021; 18(1):11. https://doi.org/10.3390/ijerph18010011
Chicago/Turabian StyleWang, Jing, Shengcheng Zhao, Linsheng Yang, Hongqiang Gong, Hairong Li, and Cangjue Nima. 2021. "Assessing the Health Loss from Kashin-Beck Disease and Its Relationship with Environmental Selenium in Qamdo District of Tibet, China" International Journal of Environmental Research and Public Health 18, no. 1: 11. https://doi.org/10.3390/ijerph18010011
APA StyleWang, J., Zhao, S., Yang, L., Gong, H., Li, H., & Nima, C. (2021). Assessing the Health Loss from Kashin-Beck Disease and Its Relationship with Environmental Selenium in Qamdo District of Tibet, China. International Journal of Environmental Research and Public Health, 18(1), 11. https://doi.org/10.3390/ijerph18010011





