Prospective Associations of Serum Adiponectin, Leptin, and Leptin-Adiponectin Ratio with Incidence of Metabolic Syndrome: The Korean Genome and Epidemiology Study
Abstract
1. Introduction
2. Methods
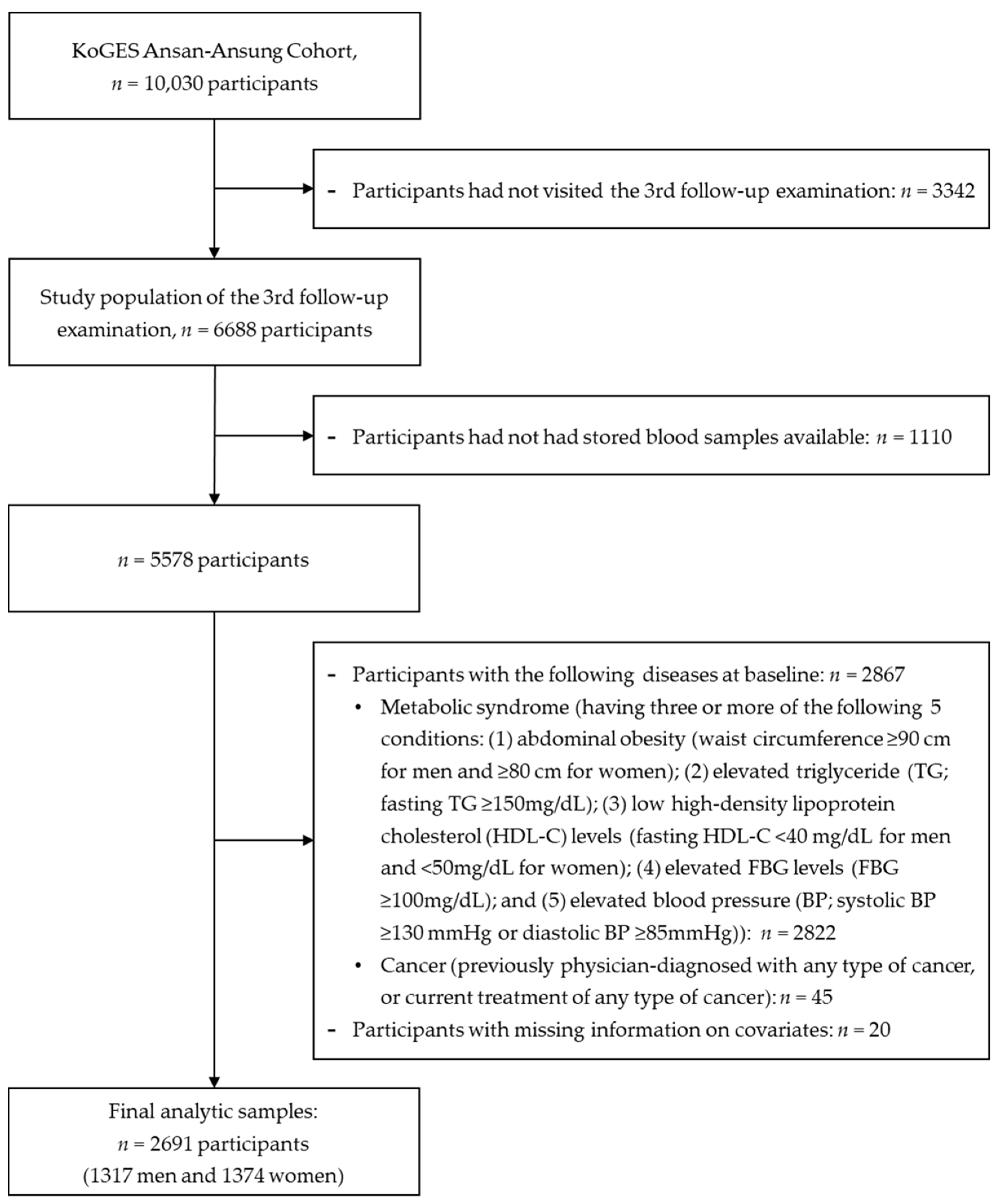
2.1. Data Source and Study Population
2.2. Measurements of Anthropometric and Biochemical Parameters
2.3. Ascertainment of Metabolic Syndrome
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Naghavi, M.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Adetokunboh, O.; Afshin, A.; Agrawal, A.; et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef]
- Korea Centers for Disease Control and Prevention. Fact book: Non-communicable Diseases 2019. Cheongju: Korea Centers for Disease Control and Prevention. 2019. Available online: http://nih.go.kr/gallery.es?mid=a20503020000&bid=0003&b_list=9&act=view&list_no=144581&nPage=1&vlist_no_npage=2&keyField=&keyWord=&orderby= (accessed on 10 January 2020).
- Grundy, S.M.; Brewer, H.B., Jr.; Cleeman, J.I.; Smith, S.C., Jr.; Lenfant, C. Definition of metabolic syndrome: Report of the national heart, lung, and blood institute/American heart association conference on scientific issues related to definition. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef]
- Korean Society of Cardiometabolic Syndrome. Metabolic syndrome fact sheet in Korea. 2018. Available online: http://kscms.org/en/uploads/Metabolic_Syndrome.pdf (accessed on 8 January 2020).
- Grundy, S.M. Metabolic syndrome: A multiplex cardiovascular risk factor. J. Clin. Endocrinol. Metab. 2007, 92, 399–404. [Google Scholar] [CrossRef]
- Haffner, S.M. The metabolic syndrome: Inflammation, diabetes mellitus, and cardiovascular disease. Am. J. Cardiol. 2006, 97, 3–11. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Catalán, V.; Rodríguez, A.; Andrada, P.; Ramírez, B.; Ibáñez, P.; Vila, N.; Romero, S.; Margall, M.A.; Gil, M.J.; et al. Increased cardiometabolic risk factors and inflammation in adipose tissue in obese subjects classified as metabolically healthy. Diabetes Care 2014, 37, 2813–2821. [Google Scholar] [CrossRef]
- Scherer, P.E.; Williams, S.; Fogliano, M.; Baldini, G.; Lodish, H.F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 1995, 270, 26746–26749. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef]
- Weyer, C.; Funahashi, T.; Tanaka, S.; Hotta, K.; Matsuzawa, Y.; Pratley, R.E.; Tataranni, P.A. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 2001, 86, 1930–1935. [Google Scholar] [CrossRef]
- Im, J.-A.; Kim, S.-H.; Lee, J.-W.; Shim, J.-Y.; Lee, H.-R.; Lee, D.-C. Association between hypoadiponectinemia and cardiovascular risk factors in nonobese healthy adults. Metabolism 2006, 55, 1546–1550. [Google Scholar] [CrossRef]
- Friedman, J. Leptin at 20: An overview. J. Endocrinol. 2014, 223, T1–T8. [Google Scholar] [CrossRef]
- Da Paz Filho, G.; Mastronardi, C.; Franco, C.; Wang, K.; Wong, M.-L.; Licinio, J. Leptin: Molecular mechanisms, systemic pro-inflammatory effects, and clinical implications [Leptina: Mecanismos moleculares, efeitos proinflamatorios sistmicos e implicoes clinicas]. Arquivos Bras. Endocrinol. Metabol. 2012, 56, 597–607. [Google Scholar] [CrossRef]
- Kumada, M.; Kihara, S.; Sumitsuji, S.; Kawamoto, T.; Matsumoto, S.; Ouchi, N.; Arita, Y.; Okamoto, Y.; Shimomura, I.; Hiraoka, H.; et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 85–89. [Google Scholar] [CrossRef]
- de Courten, M.; Zimmet, P.; Hodge, A.; Collins, V.; Nicolson, M.; Staten, M.; Dowse, G.; Alberti, K. Hyperleptinaemia: The missing link in the metabolic syndrome? Diabet. Med. 1997, 14, 200–208. [Google Scholar] [CrossRef]
- Lindberg, S.; Jensen, J.S.; Bjerre, M.; Frystyk, J.; Flyvbjerg, A.; Jeppesen, J.; Mogelvang, R. Low adiponectin levels at baseline and decreasing adiponectin levels over 10 years of follow-up predict risk of the metabolic syndrome. Diabetes Metab. 2017, 43, 134–139. [Google Scholar] [CrossRef]
- Zhuo, Q.; Wang, Z.; Fu, P.; Piao, J.; Tian, Y.; Xu, J.; Yang, X. Comparison of adiponectin, leptin and leptin to adiponectin ratio as diagnostic marker for metabolic syndrome in older adults of Chinese major cities. Diabetes Res. Clin. Pract. 2009, 84, 27–33. [Google Scholar] [CrossRef]
- Zamboni, M.; Zoico, E.; Fantin, F.; Panourgia, M.P.; Di Francesco, V.; Tosoni, P.; Solerte, B.; Vettor, R.; Bosello, O. Relation between leptin and the metabolic syndrome in elderly women. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2004, 59, M396–M400. [Google Scholar] [CrossRef]
- Koh, S.-B.; Yoon, J.; Kim, J.-Y.; Yoo, B.-S.; Lee, S.-H.; Park, J.-K.; Choe, K.-H. Relationships between serum adiponectin with metabolic syndrome and components of metabolic syndrome in non-diabetic Koreans: ARIRANG study. Yonsei Med. J. 2011, 52, 234–241. [Google Scholar] [CrossRef]
- Yun, J.E.; Kimm, H.; Jo, J.; Jee, S.H. Serum leptin is associated with metabolic syndrome in obese and nonobese Korean populations. Metabolism 2010, 59, 424–429. [Google Scholar] [CrossRef]
- Choi, K.M.; Lee, J.; Lee, K.; Seo, J.A.; Oh, J.; Kim, S.G.; Kim, N.H.; Choi, D.; Baik, S.-H. Serum adiponectin concentrations predict the developments of type 2 diabetes and the metabolic syndrome in elderly Koreans. Clin. Endocrinol. 2004, 61, 75–80. [Google Scholar] [CrossRef]
- Kang, D.R.; Yadav, D.; Koh, S.-B.; Kim, J.-Y.; Ahn, S.V. Impact of serum leptin to adiponectin ratio on regression of metabolic syndrome in high-risk individuals: The ARIRANG study. Yonsei Med. J. 2017, 58, 339–346. [Google Scholar] [CrossRef]
- Correia, M.; Haynes, W. Lessons from leptin’s molecular biology: Potential therapeutic actions of recombinant leptin and leptin-related compounds. Mini Rev. Med. Chem. 2007, 7, 31–38. [Google Scholar] [CrossRef]
- Kim, Y.; Han, B.-G.; Group, K. Cohort profile: The Korean genome and epidemiology study (KoGES) consortium. Int. J. Epidemiol. 2017, 46, e20. [Google Scholar] [CrossRef]
- Expert Panel on Detection, E. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001, 285, 2486. [Google Scholar]
- Alberti, K.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Matsuzawa, Y.; Funahashi, T.; Kihara, S.; Shimomura, I. Adiponectin and metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 29–33. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Franco, O.H.; Yu, Z.; Liu, Y.; Lin, X. Adiponectin and metabolic syndrome in middle-aged and elderly Chinese. Obesity 2008, 16, 172–178. [Google Scholar] [CrossRef]
- Ghadge, A.A.; Khaire, A.A. Leptin as a predictive marker for metabolic syndrome. Cytokine 2019, 121, 154735. [Google Scholar] [CrossRef]
- Chen, M.-C.; Hsu, B.-G.; Lee, C.-J.; Wang, J.-H. Hyperleptinemia positively correlates with cardiometabolic syndrome in hypertensive patients. Int. J. Clin. Exp. Pathol. 2016, 9, 12959–12967. [Google Scholar]
- Franks, P.W.; Brage, S.; Luan, J.A.; Ekelund, U.; Rahman, M.; Farooqi, I.S.; Halsall, I.; O’Rahilly, S.; Wareham, N.J. Leptin predicts a worsening of the features of the metabolic syndrome independently of obesity. Obes. Res. 2005, 13, 1476–1484. [Google Scholar] [CrossRef]
- Nappo, A.; Gonzalez-Gil, E.; Ahrens, W.; Bammann, K.; Michels, N.; Moreno, L.; Kourides, Y.; Iacoviello, L.; Mårild, S.; Fraterman, A.; et al. Analysis of the association of leptin and adiponectin concentrations with metabolic syndrome in children: Results from the IDEFICS study. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 543–551. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, C.E.; Everhart, J.E. Leptin concentrations in the United States: Relations with demographic and anthropometric measures. Am. J. Clin. Nutr. 2001, 74, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, M.; Maruoka, S.; Katayose, S. Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. Eur. J. Endocrinol. 2002, 147, 173–180. [Google Scholar] [CrossRef]
- Silha, J.V.; Krsek, M.; Skrha, J.V.; Sucharda, P.; Nyomba, B.; Murphy, L.J. Plasma resistin, adiponectin and leptin levels in lean and obese subjects: Correlations with insulin resistance. Eur. J. Endocrinol. 2003, 149, 331–336. [Google Scholar] [CrossRef]
- Gauthier, A.; Dubois, S.; Bertrais, S.; Gallois, Y.; Aube, C.; Gagnadoux, F. The leptin to adiponectin ratio is a marker of the number of metabolic syndrome criteria in French adults. J. Metab. Syndr. 2012, 1, 101. [Google Scholar] [CrossRef]
- Mirza, S.; Qu, H.-Q.; Li, Q.; Martinez, P.J.; Rentfro, A.R.; McCormick, J.B.; Fisher-Hoch, S.P. Adiponectin/leptin ratio and metabolic syndrome in a Mexican American population. Clin. Investig. Med. Med. Clin. Exp. 2011, 34, E290. [Google Scholar] [CrossRef]
- Falahi, E.; Rad, A.H.; Roosta, S. What is the best biomarker for metabolic syndrome diagnosis? Diabetes Metab. Syndr. 2015, 9, 366–372. [Google Scholar] [CrossRef]
- Mayeux, R. Biomarkers: Potential uses and limitations. NeuroRx 2004, 1, 182–188. [Google Scholar] [CrossRef]
- O’Neill, S.; Bohl, M.; Gregersen, S.; Hermansen, K.; O’Driscoll, L. Blood-based biomarkers for metabolic syndrome. Trends Endrocrinol. Metab. 2016, 27, 363–374. [Google Scholar] [CrossRef]
- Karastergiou, K.; Smith, S.R.; Greenberg, A.S.; Fried, S.K. Sex differences in human adipose tissues—The biology of pear shape. Biol. Sex Differ. 2012, 3, 13. [Google Scholar] [CrossRef]
- Lihn, A.S.; Bruun, J.M.; He, G.; Pedersen, S.B.; Jensen, P.F.; Richelsen, B. Lower expression of adiponectin mRNA in visceral adipose tissue in lean and obese subjects. Mol. Cell. Endocrinol. 2004, 219, 9–15. [Google Scholar] [CrossRef]
- Masuzaki, H.; Ogawa, Y.; Isse, N.; Satoh, N.; Okazaki, T.; Shigemoto, M.; Mori, K.; Tamura, N.; Hosoda, K.; Yoshimasa, Y.; et al. Human obese gene expression: Adipocyte-specific expression and regional differences in the adipose tissue. Diabetes 1995, 44, 855–858. [Google Scholar] [CrossRef]
- Van Harmelen, V.; Reynisdottir, S.; Eriksson, P.; Thörne, A.; Hoffstedt, J.; Lönnqvist, F.; Arner, P. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes 1998, 47, 913–917. [Google Scholar] [CrossRef]
- Christen, T.; Trompet, S.; Noordam, R.; van Klinken, J.B.; van Dijk, K.W.; Lamb, H.J.; Cobbaert, C.M.; den Heijer, M.; Jazet, I.M.; Jukema, J.W.; et al. Sex differences in body fat distribution are related to sex differences in serum leptin and adiponectin. Peptides 2018, 107, 25–31. [Google Scholar] [CrossRef]
- Storlien, L.; Oakes, N.D.; Kelley, D.E. Metabolic flexibility. Proc. Nutr. Soc. 2004, 63, 363–368. [Google Scholar] [CrossRef]
- Bouchard, C.; Despres, J.-P.; Mauriège, P. Genetic and nongenetic determinants of regional fat distribution. Endocr. Rev. 1993, 14, 72–93. [Google Scholar] [CrossRef]
- Sparks, L.M.; Pasarica, M.; Sereda, O.; dejonge, L.; Thomas, S.; Loggins, H.; Xie, H.; Miles, J.M.; Smith, S.R. Effect of adipose tissue on the sexual dimorphism in metabolic flexibility. Metabolism 2009, 58, 1564–1571. [Google Scholar] [CrossRef]
- Ong, K.K.; Frystyk, J.; Flyvbjerg, A.; Petry, C.J.; Ness, A.; Dunger, D.B. Sex-discordant associations with adiponectin levels and lipid profiles in children. Diabetes 2006, 55, 1337–1341. [Google Scholar] [CrossRef][Green Version]
- Asterholm, I.W.; Scherer, P.E. Enhanced metabolic flexibility associated with elevated adiponectin levels. Am. J. Pathol. 2010, 176, 1364–1376. [Google Scholar] [CrossRef]
- Berg, A.H.; Combs, T.P.; Scherer, P.E. ACRP30/adiponectin: An adipokine regulating glucose and lipid metabolism. Trends Endocrinol. Metab. 2002, 13, 84–89. [Google Scholar] [CrossRef]
- Rasouli, N.; Kern, P.A. Adipocytokines and the metabolic complications of obesity. J. Clin. Endocrinol. Metab. 2008, 93, s64–s73. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M.; Brennan, A.M.; Kelesidis, T.; Kratzsch, J.; Fasshauer, M.; Kralisch, S.; Williams, C.J.; Mantzoros, C.S. Total and high-molecular weight adiponectin in relation to metabolic variables at baseline and in response to an exercise treatment program: Comparative evaluation of three assays. Diabetes Care 2007, 30, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, R.; Yamane, K.; Kamei, N.; Nakanishi, S.; Kohno, N. Low serum levels of total and high-molecular-weight adiponectin predict the development of metabolic syndrome in Japanese-Americans. J. Endocrinol. Investig. 2011, 34, 615–619. [Google Scholar]
- Sierksma, A.; Patel, H.; Ouchi, N.; Kihara, S.; Funahashi, T.; Heine, R.J.; Grobbee, D.E.; Kluft, C.; Hendriks, H.F. Effect of moderate alcohol consumption on adiponectin, tumor necrosis factor-α, and insulin sensitivity. Diabetes Care 2004, 27, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Pravdova, E.; Macho, L.; Fickova, M. Alcohol intake modifies leptin, adiponectin and resistin serum levels and their mRNA expressions in adipose tissue of rats. Endocr. Regul. 2009, 43, 117–125. [Google Scholar] [PubMed]
- Pischon, T.; Girman, C.J.; Rifai, N.; Hotamisligil, G.S.; Rimm, E.B. Association between dietary factors and plasma adiponectin concentrations in men. Am. J. Clin. Nutr. 2005, 81, 780–786. [Google Scholar] [CrossRef]

| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Tertile of LA Ratio1 | Tertile of LA Ratio | |||||||
| T1 (Lowest) | T2 | T3 (Highest) | P3 | T1 (Lowest) | T2 | T3 (Highest) | P | |
| (n = 439) | (n = 439) | (n = 439) | (n = 458) | (n = 458) | (n = 458) | |||
| Age (years) | 59.07 ± 8.532 | 56.63 ± 8.51 | 54.53 ± 7.68 | <0.0001 | 56.84 ± 8.62 | 53.86 ± 6.95 | 53.06 ± 6.70 | <0.0001 |
| Area of residence | <0.0001 | <0.0001 | ||||||
| Ansan | 245 (55.81) | 180 (41.00) | 121 (27.56) | 230 (50.22) | 162 (35.37) | 124 (27.07) | ||
| Ansung | 194 (44.19) | 259 (59.00) | 318 (72.44) | 228 (49.78) | 296 (64.63) | 334 (72.93) | ||
| Education level | <0.0001 | <0.0001 | ||||||
| ≤elementary school | 102 (23.23) | 82 (18.68) | 38 (8.66) | 157 (34.28) | 122 (26.64) | 98 (21.40) | ||
| middle/high school | 260 (59.23) | 254 (57.86) | 265 (60.36) | 266 (58.08) | 292 (63.76) | 311 (67.90) | ||
| ≥college | 77 (17.54) | 103 (23.46) | 136 (30.98) | 35 (7.64) | 44 (9.61) | 49 (10.70) | ||
| Smoking status | 0.0688 | 0.4408 | ||||||
| Never | 114 (25.97) | 134 (30.52) | 114 (25.97) | 452 (98.69) | 451 (98.47) | 449 (98.03) | ||
| Past | 151 (34.40) | 170 (38.72) | 193 (43.96) | 2 (0.44) | 3 (0.66) | 3 (0.66) | ||
| Current | 174 (39.64) | 135 (30.75) | 132 (30.07) | 4 (0.87) | 4 (0.87) | 6 (1.31) | ||
| Alcohol consumption (grams/day)4 | 4.70 (0.00–18.81) | 4.70 (0.00–20.26) | 10.13 (0.60–25.25) | 0.0020 | 0.00 (0.00–0.26) | 0.00 (0.00–0.87) | 0.00 (0.00–0.31) | 0.1584 |
| Regular physical activity | 0.006 | 0.1405 | ||||||
| Yes | 161 (36.67) | 174 (39.64) | 201 (45.79) | 171 (37.34) | 208 (45.41) | 193 (42.14) | ||
| No | 278 (63.33) | 265 (60.36) | 238 (54.21) | 287 (62.66) | 250 (54.59) | 265 (57.86) | ||
| Family history of diabetes | 0.0535 | 0.6564 | ||||||
| Yes | 3 (0.68) | 7 (1.59) | 10 (2.28) | 11 (2.40) | 7 (1.53) | 13 (2.84) | ||
| No | 436 (99.32) | 432 (98.41) | 429 (97.72) | 447 (97.60) | 451 (98.47) | 445 (97.16) | ||
| Body mass index (kg/m2) | 21.16 ± 2.04 | 23.20 ± 1.87 | 24.83 ± 2.07 | <0.0001 | 21.70 ± 2.11 | 23.53 ± 2.04 | 25.19 ± 2.41 | <0.0001 |
| hs-CRP (mg/dL)4 | 0.49 (0.27–1.19) | 0.55 (0.30–1.12) | 0.86 (0.44–1.68) | <0.0001 | 0.35 (0.22–0.64) | 0.43 (0.28–0.77) | 0.65 (0.37–1.22) | <0.0001 |
| Adiponectin (μg/mL)4 | 6.52 (5.37–7.91) | 5.03 (4.06–6.15) | 4.00 (3.17–4.84) | <0.0001 | 8.74 (7.36–10.93) | 6.87 (5.66–8.22) | 5.21 (4.10–6.51) | <0.0001 |
| Leptin (ng/mL)4 | 1.01 (1.01–1.11) | 2.15 (1.64–2.70) | 4.15 (3.21–5.76) | <0.0001 | 5.18 (3.49–6.67) | 9.93 (8.16–12.06) | 16.35 (13.15–21.10) | <0.0001 |
| LA ratio (ng/μg)4 | 0.17 (0.14–0.21) | 0.43 (0.34–0.52) | 1.01 (0.81–1.41) | <0.0001 | 0.63 (0.40–0.80) | 1.46 (1.20–1.74) | 3.00 (2.46–3.99) | <0.0001 |
| MetS components, n (%) | ||||||||
| Abdominal obesity | 8 (1.82) | 39 (8.88) | 84 (19.13) | <0.0001 | 31 (6.77) | 55 (12.01) | 94 (20.52) | <0.0001 |
| Elevated BP | 97 (22.10) | 126 (28.70) | 108 (24.60) | 0.3923 | 108 (23.58) | 71 (15.50) | 77 (16.81) | 0.0085 |
| Elevated FBG | 58 (13.21) | 77 (17.54) | 100 (22.78) | 0.0002 | 19 (4.15) | 20 (4.37) | 31 (6.77) | 0.0715 |
| Elevated TG | 35 (7.97) | 77 (17.54) | 125 (28.47) | <0.0001 | 17 (3.71) | 47 (10.26) | 73 (15.94) | <0.0001 |
| Low HDL-C | 92 (20.96) | 125 (28.47) | 134 (30.52) | 0.0014 | 207 (45.20) | 252 (55.02) | 272 (59.39) | <0.0001 |
| No. of MetS components at baseline | <0.0001 | <0.0001 | ||||||
| 0 | 210 (47.84) | 118 (26.88) | 73 (16.63) | 165 (36.03) | 127 (27.73) | 85 (18.56) | ||
| 1 | 168 (38.27) | 198 (45.10) | 181 (41.23) | 204 (44.54) | 217 (47.38) | 199 (43.45) | ||
| 2 | 61 (13.90) | 123 (28.02) | 185 (42.14) | 89 (19.43) | 114 (24.89) | 174 (37.99) | ||
| Tertile | Ptrend2 | Per 1 SD Increment | P | |||
|---|---|---|---|---|---|---|
| T1 (Lowest) | T2 | T3 (Highest) | ||||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| Adiponectin | ||||||
| Person-years | 2748 | 2947 | 3075 | 8770 | ||
| Incident cases (n) | 167 | 113 | 79 | 359 | ||
| Rate per 1000person-years | 60.8 | 38.3 | 25.7 | 40.9 | ||
| Median | 3.5 | 5.1 | 7.3 | 5.1 | ||
| Ranges | 0.48–4.34 | 4.36–5.93 | 5.94–25.04 | 0.48–25.04 | ||
| Model 1 1 | 1.00 | 0.62 (0.49–0.79) | 0.39 (0.30–0.52) | <0.0001 | 0.85 (0.80–0.90) | <0.0001 |
| Model 2 | 1.00 | 0.71 (0.56–0.91) | 0.53 (0.40–0.70) | <0.0001 | 0.90 (0.85–0.95) | 0.0003 |
| Leptin | ||||||
| Person-years | 3145 | 2951 | 2674 | 8770 | ||
| Incident cases (n) | 55 | 119 | 185 | 359 | ||
| Rate per 1000 person-years | 17.5 | 40.3 | 69.2 | 40.9 | ||
| Median | 1.0 | 2.1 | 4.3 | 2.1 | ||
| Ranges | 1.01–1.37 | 1.38–2.91 | 2.91–24.08 | 1.01–24.08 | ||
| Model 1 | 1.00 | 2.39 (1.73–3.29) | 4.11 (3.04–5.57) | <0.0001 | 1.14 (1.11–1.17) | <0.0001 |
| Model 2 | 1.00 | 1.95 (1.39–2.74) | 2.88 (2.01–4.13) | <0.0001 | 1.05 (1.01–1.08) | 0.0176 |
| LA ratio | ||||||
| Person-years | 3150 | 2953 | 2667 | 8770 | ||
| Incident cases (n) | 51 | 124 | 184 | 359 | ||
| Rate per 1000 person-years | 16.2 | 42.0 | 69.0 | 40.9 | ||
| Median | 0.2 | 0.4 | 1.0 | 0.4 | ||
| Ranges | 0.1–0.3 | 0.3–0.6 | 0.6–5.3 | 0.1–5.3 | ||
| Model 1 | 1.00 | 2.72 (1.96–3.77) | 4.66 (3.39–6.39) | <0.0001 | 1.74 (1.56–1.94) | <0.0001 |
| Model 2 | 1.00 | 2.16 (1.53–3.03) | 3.07 (2.13–4.44) | <0.0001 | 1.40 (1.22–1.62) | <0.0001 |
| Tertile | Ptrend2 | Per 1 SD Increment | P | |||
|---|---|---|---|---|---|---|
| T1 (Lowest) | T2 | T3 (Highest) | ||||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| Adiponectin | ||||||
| Person-years | 3069 | 3132 | 3191 | 9392 | ||
| Incident cases (n) | 154 | 131 | 100 | 385 | ||
| Rate per 1000 person-years | 50.2 | 41.8 | 31.3 | 41.0 | ||
| Median | 4.7 | 6.9 | 9.6 | 6.9 | ||
| Ranges | 0.87–5.88 | 5.88–8.09 | 8.10–27.78 | 0.9–27.8 | ||
| Model 1 1 | 1.00 | 0.81 (0.64–1.02) | 0.51 (0.39–0.66) | <0.0001 | 0.90 (0.86–0.93) | <0.0001 |
| Model 2 | 1.00 | 0.82 (0.65–1.03) | 0.54 (0.42–0.71) | <0.0001 | 0.91 (0.88–0.95) | <0.0001 |
| Leptin | ||||||
| Person-years | 3296 | 3145 | 2951 | 9392 | ||
| Incident cases (n) | 86 | 128 | 171 | 385 | ||
| Rate per 1000 person-years | 26.1 | 40.7 | 57.9 | 41.0 | ||
| Median | 5.1 | 9.7 | 17.1 | 9.7 | ||
| Ranges | 1.01–7.33 | 7.37–12.62 | 12.65–52.36 | 1.0–52.4 | ||
| Model 1 | 1.00 | 1.79 (1.36–2.36) | 2.47 (1.90–3.21) | <0.0001 | 1.04 (1.03–1.05) | <0.0001 |
| Model 2 | 1.00 | 1.45 (1.09–1.94) | 1.55 (1.13–2.13) | <0.0001 | 1.01 (0.99–1.02) | 0.4084 |
| LA ratio | ||||||
| Person-years | 3273 | 3169 | 2950 | 9392 | ||
| Incident cases (n) | 88 | 120 | 177 | 385 | ||
| Rate per 1000 person-years | 26.9 | 37.9 | 60.0 | 41.0 | ||
| Median | 5.1 | 9.7 | 17.1 | 1.5 | ||
| Ranges | 1.0–7.3 | 7.4–12.6 | 12.6–52.4 | 0.1–24.6 | ||
| Model 1 | 1.00 | 1.67 (1.26–2.20) | 2.78 (2.14–3.62) | <0.0001 | 1.11 (1.08–1.15) | <0.0001 |
| Model 2 | 1.00 | 1.37 (1.02–1.84) | 1.94 (1.41–2.66) | <0.0001 | 1.04 (0.99–1.09) | 0.1096 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.W.; Shin, D. Prospective Associations of Serum Adiponectin, Leptin, and Leptin-Adiponectin Ratio with Incidence of Metabolic Syndrome: The Korean Genome and Epidemiology Study. Int. J. Environ. Res. Public Health 2020, 17, 3287. https://doi.org/10.3390/ijerph17093287
Lee KW, Shin D. Prospective Associations of Serum Adiponectin, Leptin, and Leptin-Adiponectin Ratio with Incidence of Metabolic Syndrome: The Korean Genome and Epidemiology Study. International Journal of Environmental Research and Public Health. 2020; 17(9):3287. https://doi.org/10.3390/ijerph17093287
Chicago/Turabian StyleLee, Kyung Won, and Dayeon Shin. 2020. "Prospective Associations of Serum Adiponectin, Leptin, and Leptin-Adiponectin Ratio with Incidence of Metabolic Syndrome: The Korean Genome and Epidemiology Study" International Journal of Environmental Research and Public Health 17, no. 9: 3287. https://doi.org/10.3390/ijerph17093287
APA StyleLee, K. W., & Shin, D. (2020). Prospective Associations of Serum Adiponectin, Leptin, and Leptin-Adiponectin Ratio with Incidence of Metabolic Syndrome: The Korean Genome and Epidemiology Study. International Journal of Environmental Research and Public Health, 17(9), 3287. https://doi.org/10.3390/ijerph17093287





