Surface-Modified Sewage Sludge-Derived Carbonaceous Catalyst as a Persulfate Activator for Phenol Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation and Characterization of the Sludge-Derived Carbonaceous Catalysts (SCs)
2.3. Batch Experiments and Analytical Methods
3. Results and Discussion
3.1. Characterization of SCs
3.1.1. Composition Analysis
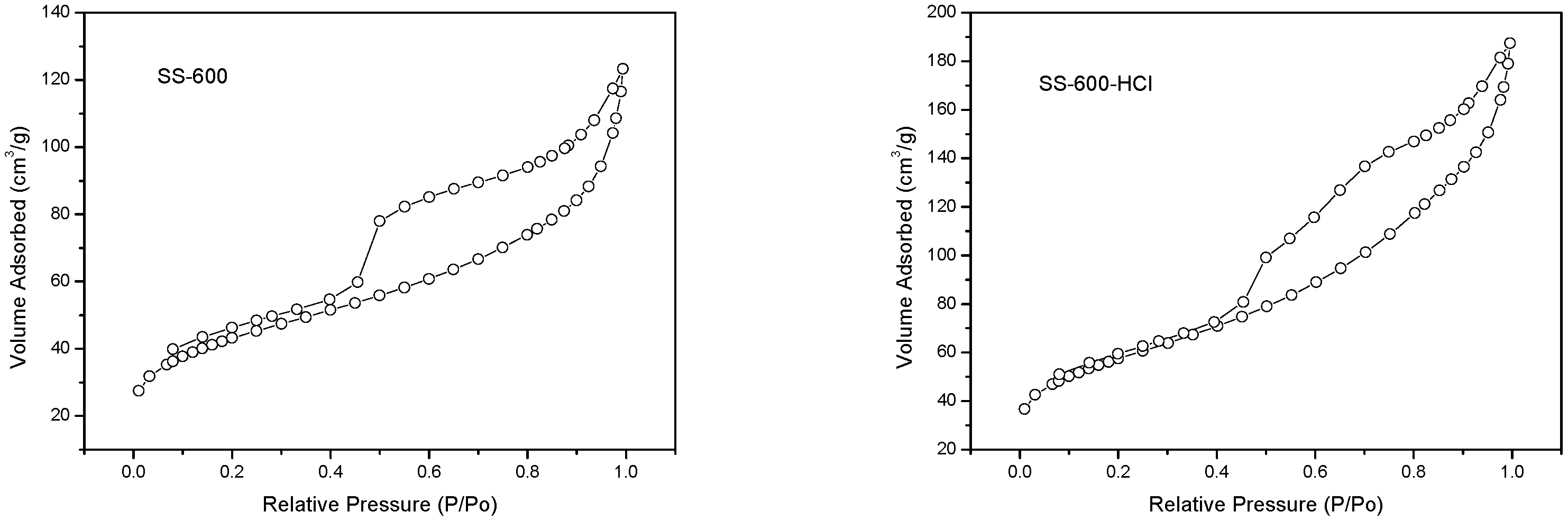
3.1.2. Nitrogen Adsorption-Desorption Isotherms and Pore Size Distributions
3.1.3. Scanning Electron Microscopy (SEM) Analysis
3.1.4. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
3.2. Performance of SCs on Phenol Degradation
3.3. Effect of Reaction Parameters on Phenol Degradation
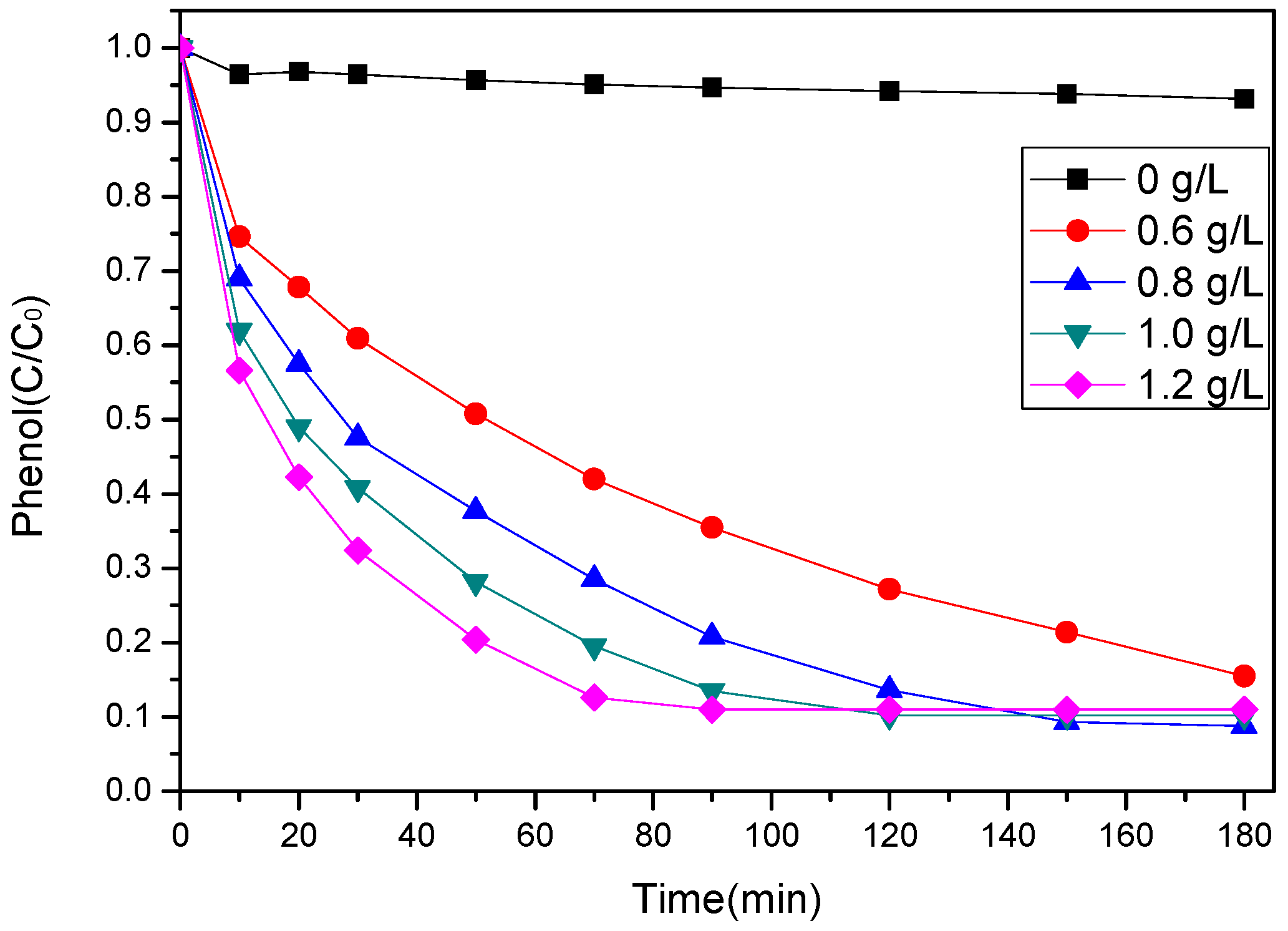
3.3.1. Effect of SS-600-HCl Dosage
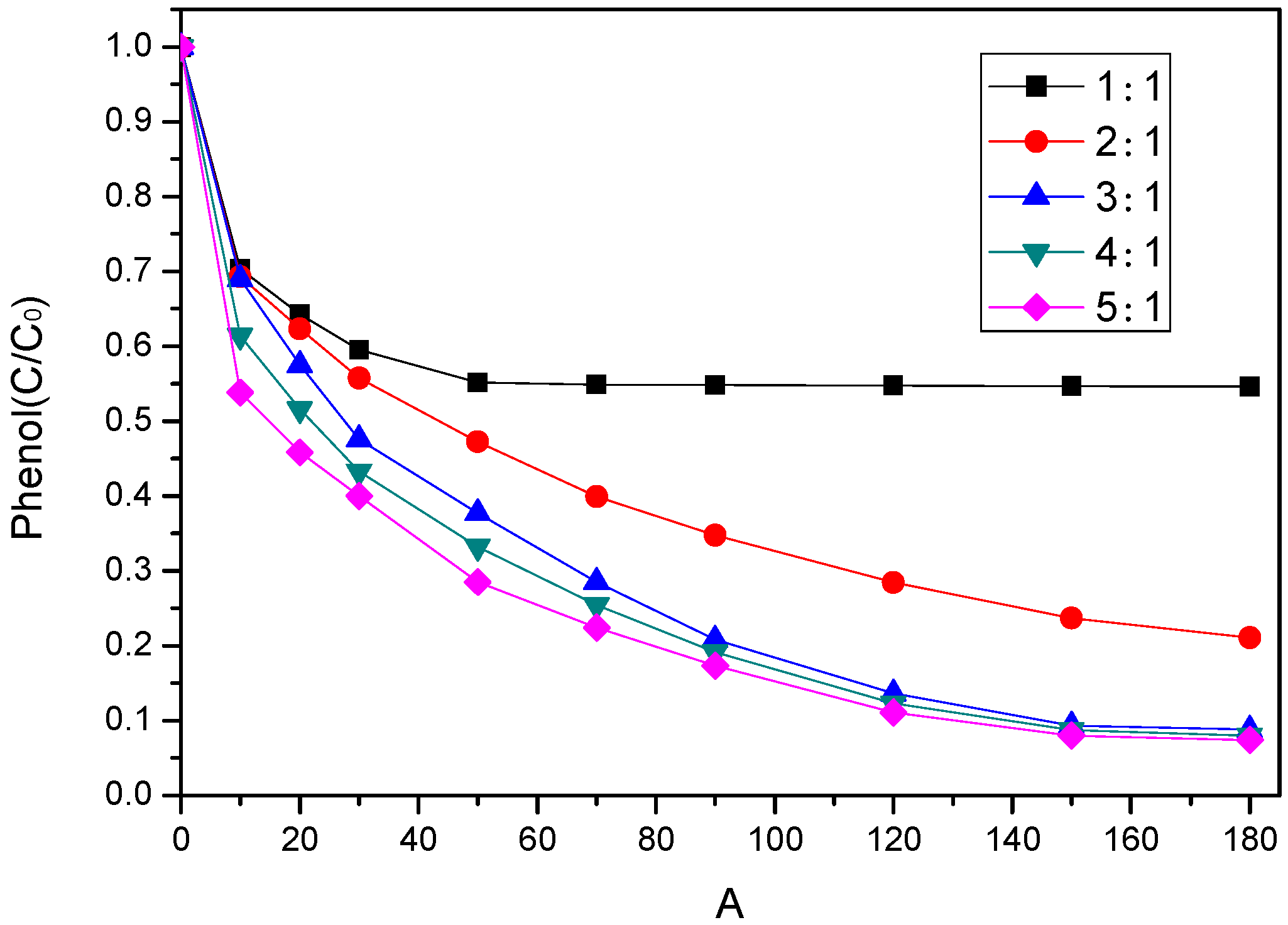
3.3.2. Effect of Sodium Persulfate (PS) Dosage
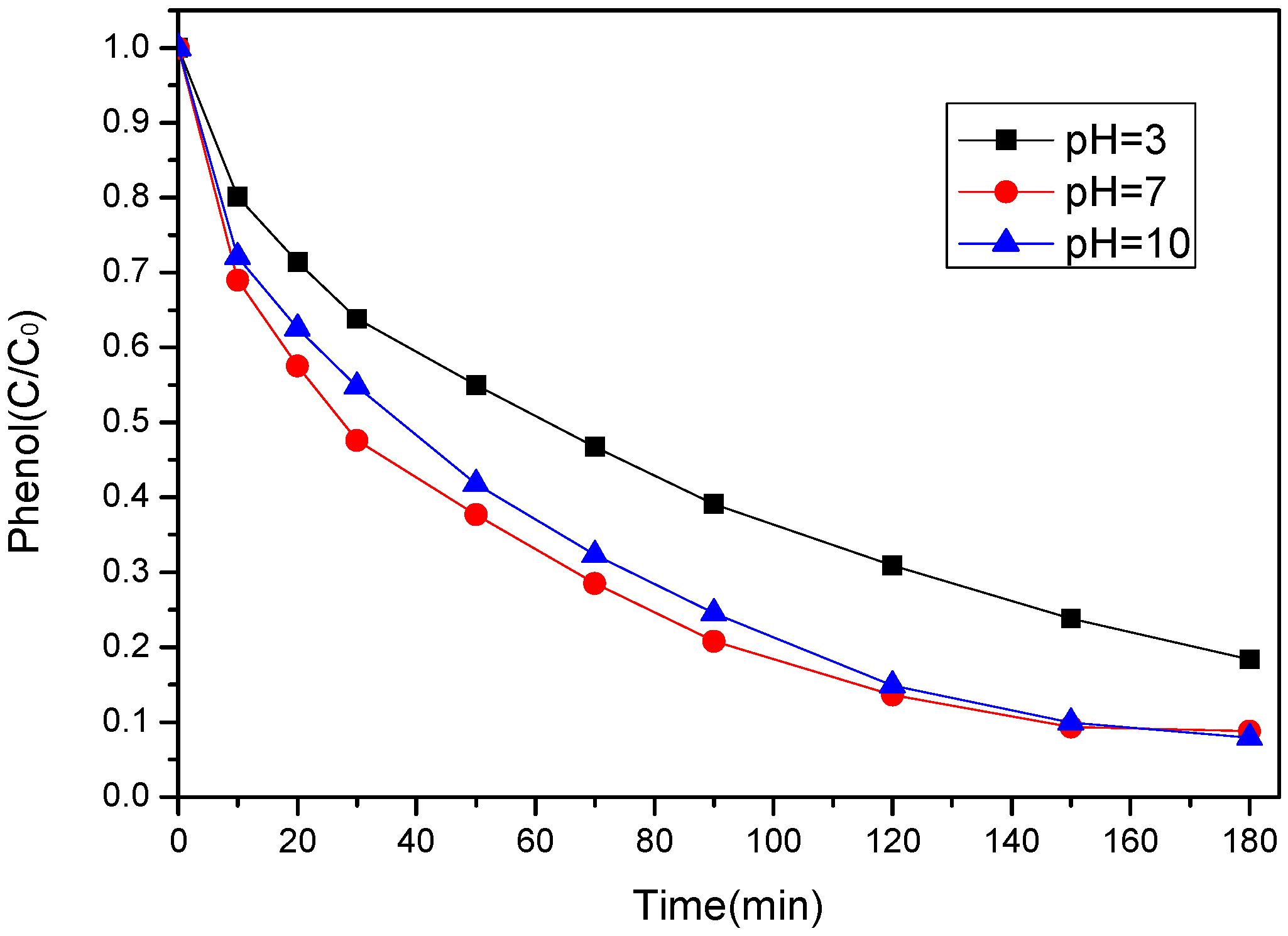
3.3.3. Effect of Initial pH
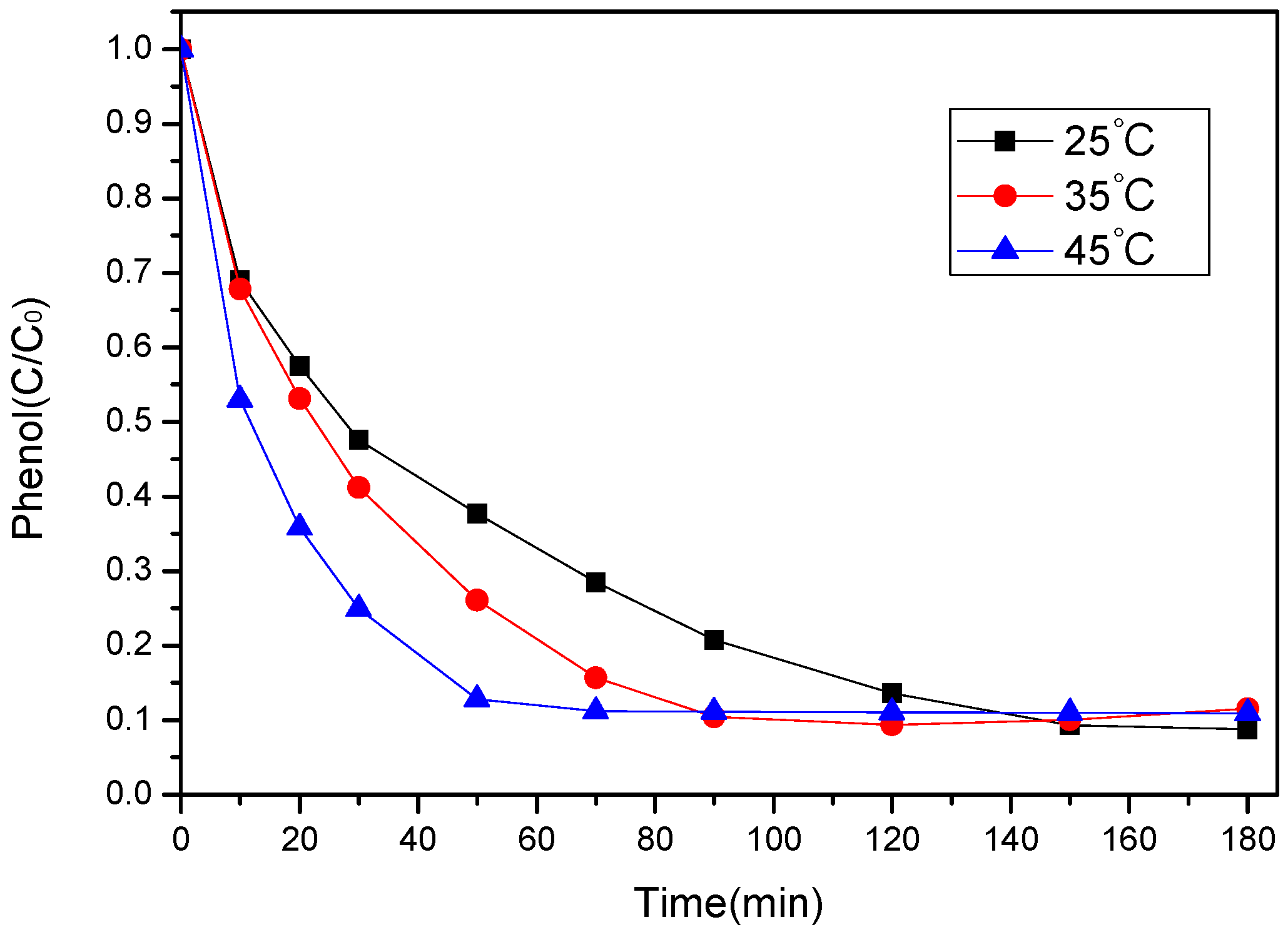
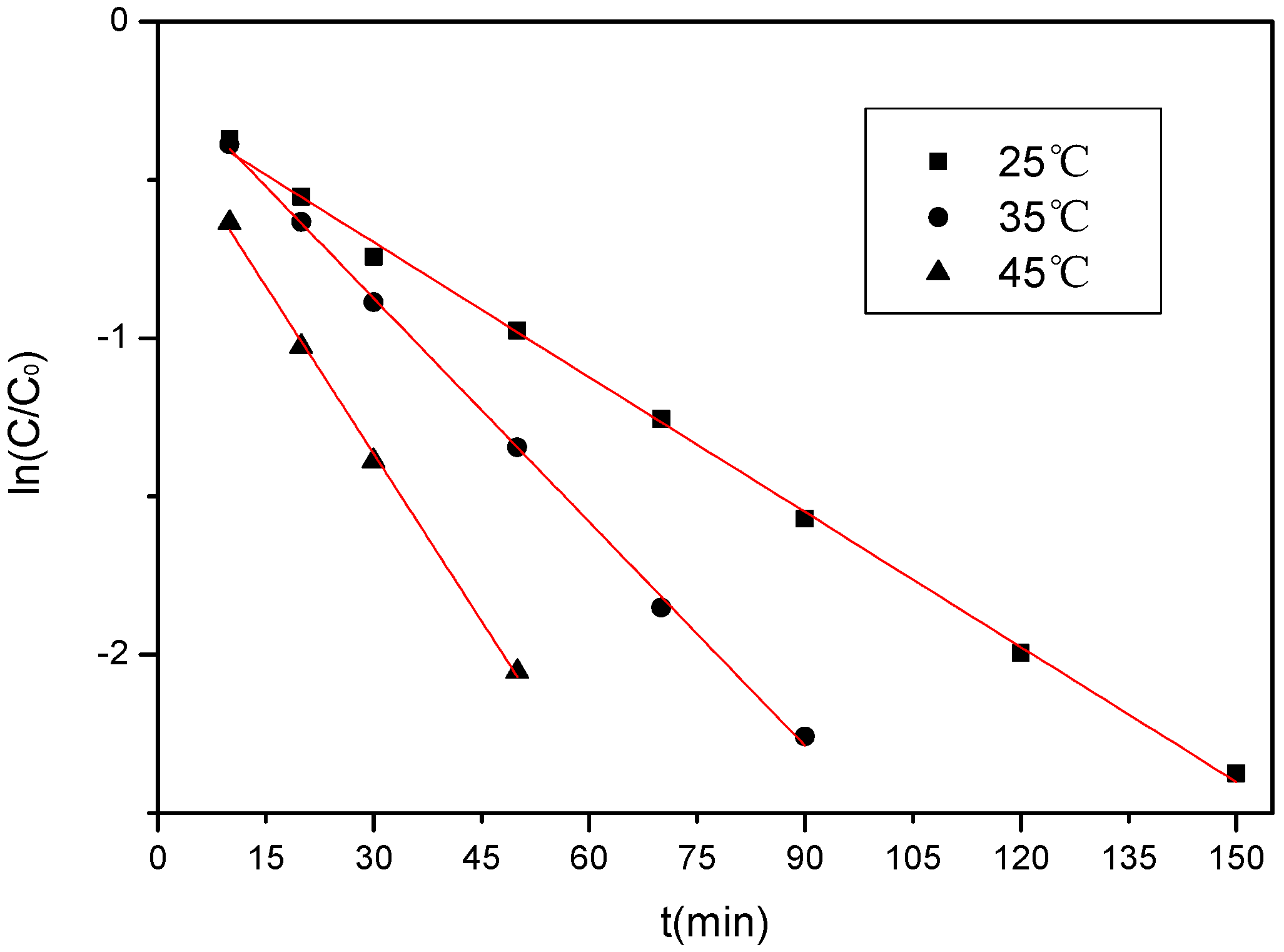
3.3.4. Effect of Temperature
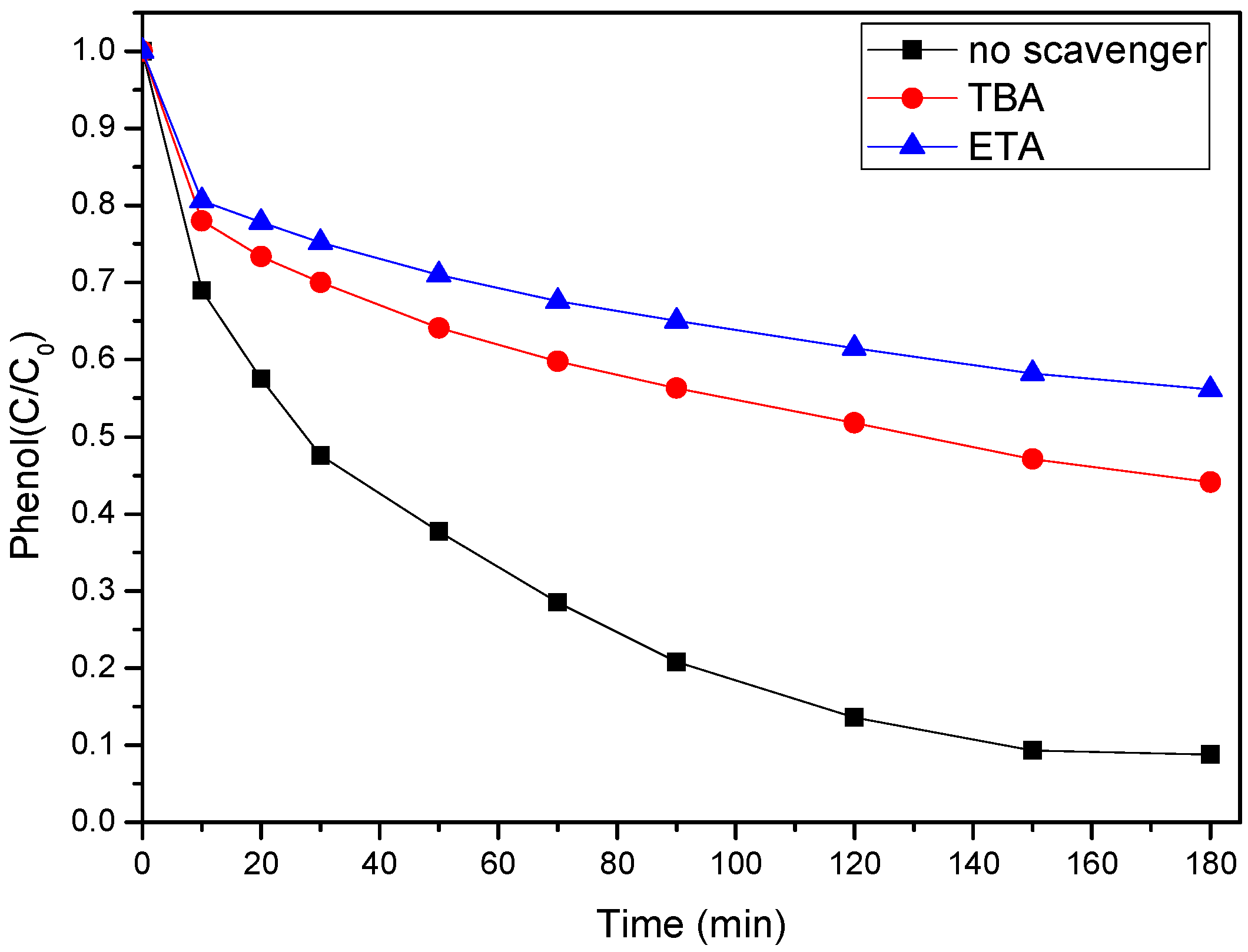
3.4. Mechanism Analysis of Phenol Degradation by SS-600-HCl/PS
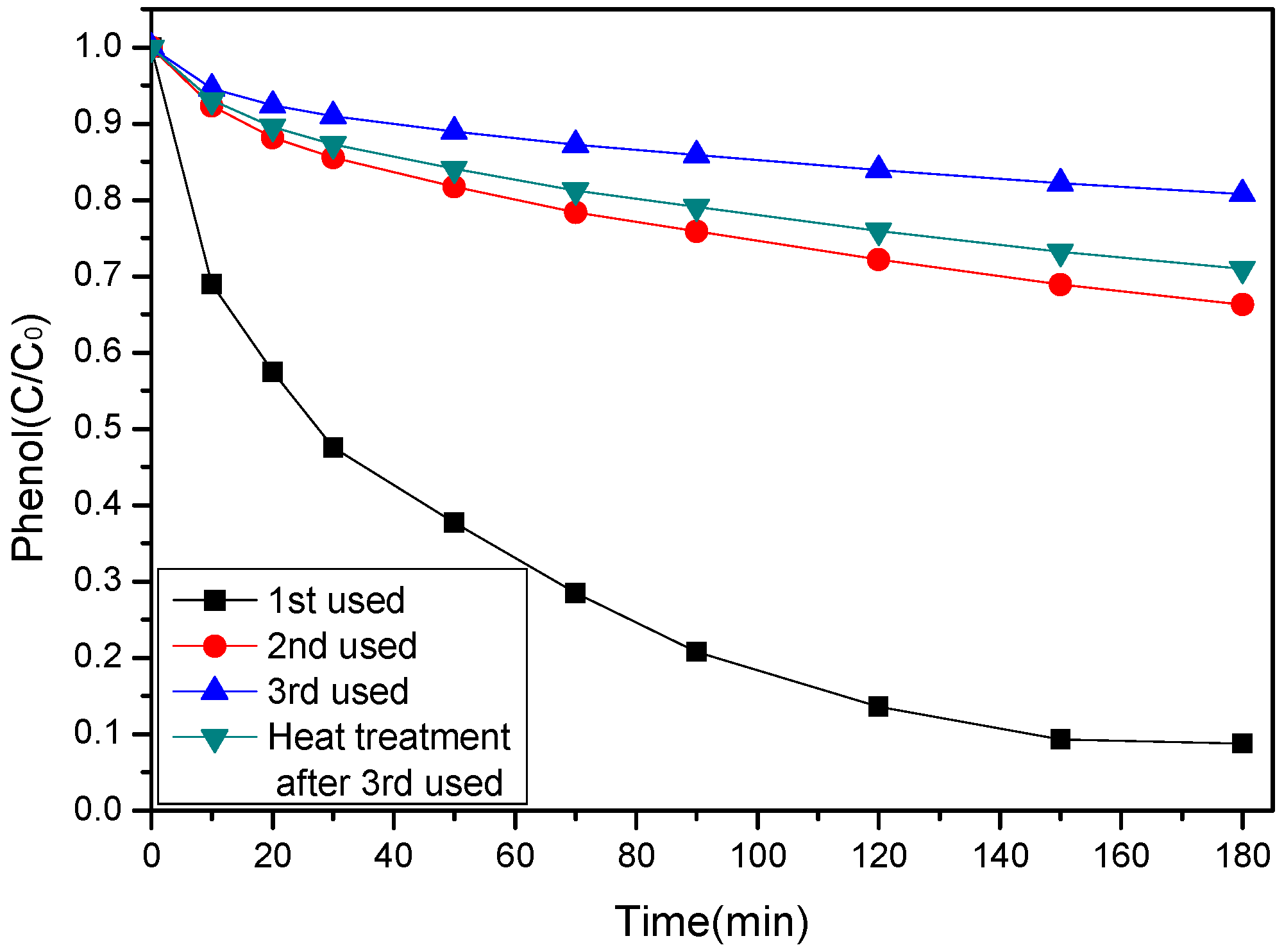
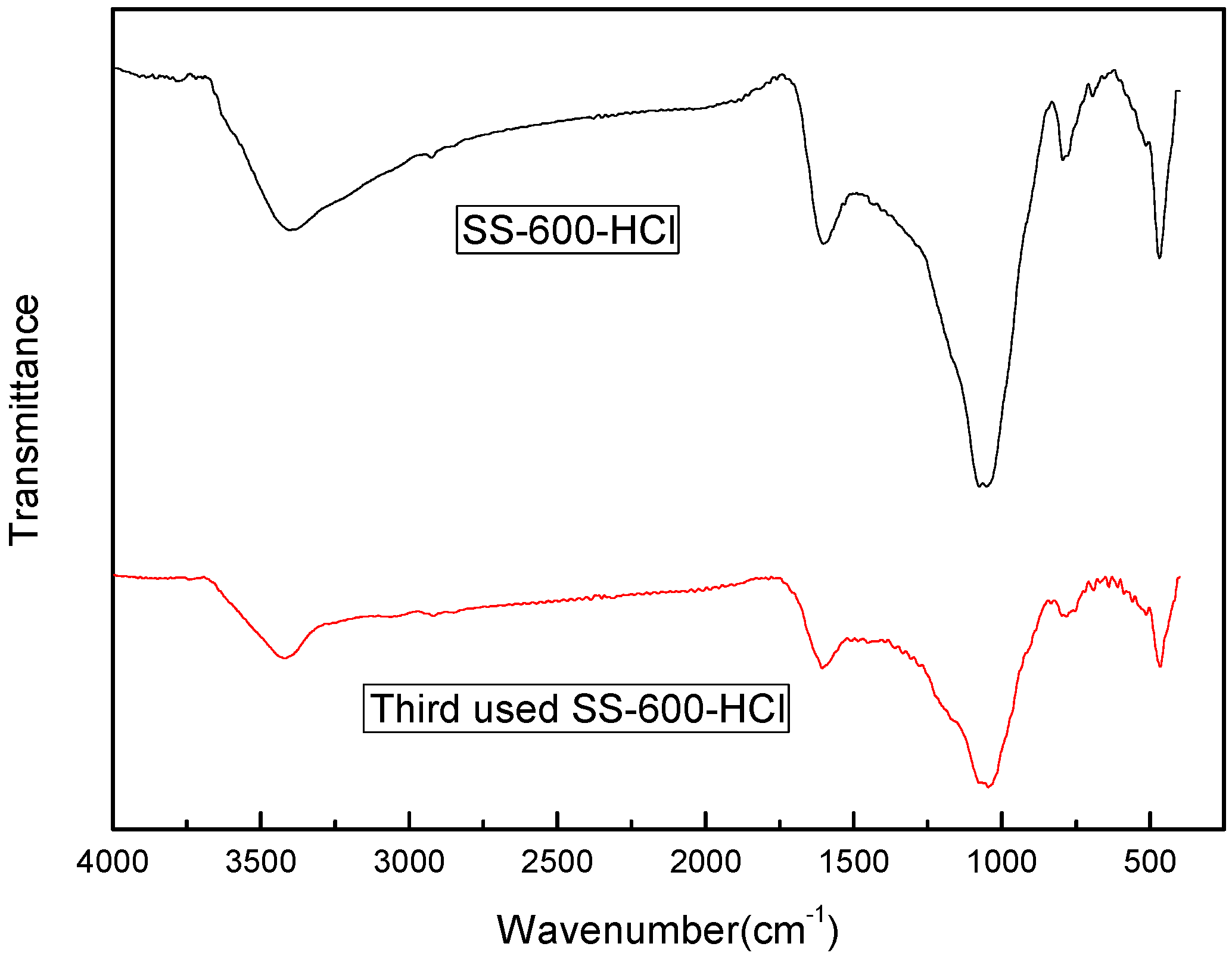
3.5. Stability and Reuse of SS-600-HCl Catalyst
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olaniran, A.O.; Igbinosa, E.O. Chlorophenols and other related derivatives of environmental concern: Properties, distribution and microbial degradation processes. Chemosphere 2011, 83, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Krastanov, A.; Alexieva, Z.; Yemendzhiev, H. Microbial degradation of phenol and phenolic derivatives. Eng. Life Sci. 2013, 13, 76–87. [Google Scholar] [CrossRef]
- Biglari, H.; Afsharnia, M.; Alipour, V.; Khosravi, R.; Sharafi, K.; Mahvi, A.H. A review and investigation of the effect of nanophotocatalytic ozonation process for phenolic compound removal from real effluent of pulp and paper industry. Environ. Sci. Pollut. Res. 2017, 24, 4105–4116. [Google Scholar] [CrossRef] [PubMed]
- Villegas, L.G.C.; Mashhadi, N.; Chen, M.; Mukherjee, D.; Taylor, K.E.; Biswas, N. A short review of techniques for phenol removal from wastewater. Curr. Pollut. Rep. 2016, 2, 157–167. [Google Scholar] [CrossRef]
- Issabayeva, G.; Hang, S.Y.; Wong, M.C.; Aroua, M.K. A review on the adsorption of phenols from wastewater onto diverse groups of adsorbents. Rev. Chem. Eng. 2018, 34, 855–873. [Google Scholar] [CrossRef]
- Alshabib, M.; Onaizi, S.A. A review on phenolic wastewater remediation using homogeneous and heterogeneous enzymatic processes: Current status and potential challenges. Sep. Purif. Technol. 2019, 219, 186–207. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.; Wang, F.F.; Xiong, X.G.Y.; Tian, K.; Sun, Y.B.; Yu, T.T. Application of heterogeneous catalytic ozonation for refractory organics in wastewater. Catalysts 2019, 9, 241. [Google Scholar] [CrossRef]
- Zhou, L.B.; Cao, H.B.; Descorme, C.; Xie, Y.B. Phenolic compounds removal by wet air oxidation based processes. Front. Environ. Sci. Eng. 2018, 12, 1. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Zhou, M.H.; Martinez-Huitle, C.A. Heterogeneous electro-Fenton and photoelectro- Fenton processes: A critical review of fundamental principles and application for water/wastewater treatment. Appl. Catal. B Environ. 2018, 235, 103–129. [Google Scholar] [CrossRef]
- Marquez, J.J.R.; Levchuk, I.; Sillanpaa, M. Application of catalytic wet peroxide oxidation for industrial and urban wastewater treatment: A review. Catalysts 2018, 8, 673. [Google Scholar] [CrossRef]
- Ike, I.A.; Linden, K.G.; Orbell, J.D.; Duke, M. Critical review of the science and sustainability of persulphate advanced oxidation processes. Chem. Eng. J. 2018, 338, 651–669. [Google Scholar] [CrossRef]
- Matzek, L.W.; Carter, K.E. Activated persulfate for organic chemical degradation: A review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef]
- Oh, W.D.; Dong, Z.L.; Lim, T.T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Shao, Y.S.; Gao, N.Y.; Lu, X.; An, N. Degradation kinetic of phthalate esters and the formation of brominated byproducts in heat-activated persulfate system. Chem. Eng. J. 2019, 359, 1086–1096. [Google Scholar] [CrossRef]
- Tian, S.C.; Zhang, Z.H. Photo-electrochemical oxidation of hypophosphite and phosphorous recovery by UV/Fe2+/peroxydisulfate with electrochemical process. Chem. Eng. J. 2019, 359, 1075–1085. [Google Scholar] [CrossRef]
- Gu, N.; Wu, Y.X.; Gao, J.L.; Meng, X.Y.; Zhao, P.; Qin, H.H.; Wang, K.T. Microcystis aeruginosa removal by in situ chemical oxidation using persulfate activated by Fe2+ ions. Ecol. Eng. 2017, 99, 290–297. [Google Scholar] [CrossRef]
- Shukla, P.R.; Wang, S.B.; Sun, H.Q.; Ang, H.M.; Tade, M. Activated carbon supported cobalt catalysts for advanced oxidation of organic contaminants in aqueous solution. Appl. Catal. B Environ. 2010, 100, 529–534. [Google Scholar] [CrossRef]
- Forouzesh, M.; Ebadi, A.; Aghaeinejad-Meybodi, A. Degradation of metronidazole antibiotic in aqueous medium using activated carbon as a persulfate activator. Sep. Purif. Technol. 2019, 210, 145–151. [Google Scholar] [CrossRef]
- Guan, C.T.; Jiang, J.; Luo, C.W.; Pang, S.Y.; Yang, Y.; Wang, Z.; Ma, J.; Yu, J.; Zhao, X. Oxidation of bromophenols by carbon nanotube activated peroxymonosulfate (PMS) and formation of brominated products: Comparison to peroxydisulfate (PDS). Chem. Eng. J. 2018, 337, 40–50. [Google Scholar] [CrossRef]
- Tang, L.; Liu, Y.I.; Wang, J.J.; Zeng, G.M.; Deng, Y.C.; Dong, H.R.; Feng, H.P.; Wang, J.J.; Peng, B. Enhanced activation process of persulfate by mesoporous carbon for degradation of aqueous organic pollutants: Electron transfer mechanism. Appl. Catal. B Environ. 2018, 231, 1–10. [Google Scholar] [CrossRef]
- Duan, X.G.; Sun, H.Q.; Tade, M.; Wang, S.B. Metal-free activation of persulfate by cubic mesoporous carbons for catalytic oxidation via radical and nonradical processes. Catal. Today 2018, 307, 140–146. [Google Scholar] [CrossRef]
- Olmez-Hanci, T.; Arslan-Alaton, I.; Gurmen, S.; Gafarli, I.; Khoei, S.; Safaltin, S.; Ozcelik, D.Y. Oxidative degradation of Bisphenol A by carbocatalytic activation of persulfate and peroxymonosulfate with reduced graphene oxide. J. Hazard. Mater. 2018, 360, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Magioglou, E.; Frontistis, Z.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Activation of persulfate by biochars from valorized olive stones for the degradation of sulfamethoxazole. Catalysts 2019, 9, 419. [Google Scholar] [CrossRef]
- Zhu, K.M.; Wang, X.S.; Chen, D.; Ren, W.; Lin, H.; Zhang, H. Wood-based biochar as an excellent activator of peroxydisulfate for Acid Orange 7 decolorization. Chemosphere 2019, 231, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liao, Z.W.; Ifthikar, J.; Shi, L.R.; Du, Y.N.; Zhu, J.Y.; Xi, S.; Chen, Z.Q.; Chen, Z.L. Treatment of refractory contaminants by sludge-derived biochar/persulfate system via both adsorption and advanced oxidation process. Chemosphere 2017, 185, 754–763. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpaa, M. An overview of the modification methods of activated carbon for its water treatment applications. Chem. Eng. J. 2013, 219, 499–511. [Google Scholar]
- Zhu, K.M.; Wang, X.S.; Geng, M.Z.; Chen, D.; Lin, H.; Zhang, H. Catalytic oxidation of clofibric acid by peroxydisulfate activated with wood-based biochar: Effect of biochar pyrolysis temperature, performance and mechanism. Chem. Eng. J. 2019, 374, 1253–1263. [Google Scholar] [CrossRef]
- Ding, Z.H.; Hu, X.; Wan, Y.S.; Wang, S.S.; Gao, B. Removal of lead, copper, cadmium, zinc, and nickel from aqueous solutions by alkali-modified biochar: Batch and column tests. J. Ind. Eng. Chem. 2016, 33, 239–245. [Google Scholar] [CrossRef]
- Yu, Y.; Wei, H.Z.; Yu, L.; Gu, B.; Li, X.R.; Rong, X.; Zhao, Y.; Chen, L.L.; Sun, C.L. Catalytic wet air oxidation of m-cresol over a surface-modified sewage sludge-derived carbonaceous catalyst. Catal. Sci. Technol. 2016, 6, 1085–1093. [Google Scholar] [CrossRef]
- Yang, S.Y.; Li, L.; Xiao, T.; Zhang, Y.T.; Zheng, D. Promoting effect of ammonia modification on activated carbon catalyzed peroxymonosulfate oxidation. Sep. Purif. Technol. 2016, 160, 81–88. [Google Scholar] [CrossRef]
- Sun, H.W.; Peng, X.X.; Zhang, S.P.; Liu, S.W.; Xiong, Y.; Tian, S.H.; Fang, J.Y. Activation of peroxymonosulfate by nitrogen-functionalized sludge carbon for efficient degradation of organic pollutants in water. Bioresour. Technol. 2017, 241, 244–251. [Google Scholar] [CrossRef]
- Wang, J.; Liao, Z.W.; Ifthikar, J.; Shi, L.R.; Chen, Z.Q.; Chen, Z.L. One-step preparation and application of magnetic sludge-derived biochar on acid orange 7 removal via both adsorption and persulfate based oxidation. RSC Adv. 2017, 30, 18696–18706. [Google Scholar] [CrossRef]
- Yu, Y.; Wei, H.Z.; Yu, L.; Wang, W.; Zhao, Y.; Gu, B.; Sun, C.L. Sewage-sludge-derived carbonaceous materials for catalytic wet hydrogen peroxide oxidation of m-cresol in batch and continuous reactors. Environ. Technol. 2016, 37, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.Y.; Tian, Y.; Weng, M.Q. Preparation of expandable graphite with silicate assistant intercalation and its effect on flame retardancy of ethylene vinyl acetate composite. Polym. Compos. 2015, 36, 1407–1416. [Google Scholar] [CrossRef]
- Ouyang, D.; Chen, Y.; Yan, J.C.; Qian, L.B.; Han, L.; Chen, M.F. Activation mechanism of peroxymonosulfate by biochar for catalytic degradation of 1,4-dioxane: Important role of biochar defect structures. Chem. Eng. J. 2019, 370, 614–624. [Google Scholar] [CrossRef]
- Marques, R.R.N.; Stuber, F.; Smith, K.M.; Fabregat, A.; Bengoa, C.; Font, J.; Fortuny, A.; Pullket, S.; Fowler, G.D.; Graham, N.J.D. Sewage sludge based catalysts for catalytic wet air oxidation of phenol: Preparation, characterisation and catalytic performance. Appl. Catal. B Environ. 2011, 101, 306–316. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, F.; He, Y.D.; Liu, X.Y.; Xu, Y.H.; Zhang, Y.J. Surface modification of sludge-derived carbon by phosphoric acid as new electrocatalyst for degradation of acetophenone. Environ. Sci. Pollut. Res. 2018, 25, 25496–25503. [Google Scholar] [CrossRef] [PubMed]
- Bedia, J.; Monsalvo, V.M.; Rodriguez, J.J.; Mohedano, A.F. Iron catalysts by chemical activation of sewage sludge with FeCl3 for CWPO. Chem. Eng. J. 2017, 318, 224–230. [Google Scholar] [CrossRef]
- Saputra, E.; Muhammad, S.; Sun, H.Q.; Wang, S.B. Activated carbons as green and effective catalysts for generation of reactive radicals in degradation of aqueous phenol. RSC Adv. 2013, 3, 21905–21910. [Google Scholar] [CrossRef]
- Yin, R.L.; Guo, W.Q.; Wang, H.Z.; Du, J.S.; Wu, Q.L.; Chang, J.S.; Ren, N.Q. Singlet oxygen-dominated peroxydisulfate activation by sludge-derived biochar for sulfamethoxazole degradation through a nonradical oxidation pathway: Performance and mechanism. Chem. Eng. J. 2019, 357, 589–599. [Google Scholar] [CrossRef]
- Sun, C.; Zhou, R.; Sun, J.; Ren, H. Magnetic CuO@Fe3O4 nanocomposite as a highly active heterogeneous catalyst of persulfate for 2,4-dichlorophenol degradation in aqueous solution. RSC Adv. 2015, 5, 57058–57066. [Google Scholar] [CrossRef]
- Yu, Y.; Wei, H.Z.; Yu, L.; Zhang, T.; Wang, S.; Li, X.N.; Wang, J.H.; Sun, C.L. Surface modification of sewage sludge derived carbonaceous catalyst form-cresol catalytic wet peroxide oxidation and degradation mechanism. RSC Adv. 2015, 5, 41867–41876. [Google Scholar] [CrossRef]
- Xu, X.C.; Li, Y.F.; Zhang, G.Q.; Yang, F.L.; He, P. NiO-NiFe2O4-rGO Magnetic Nanomaterials for Activated Peroxymonosulfate Degradation of Rhodamine B. Water 2019, 11, 384. [Google Scholar] [CrossRef]
- Chan, K.H.; Chu, W. Degradation of atrazine by cobalt-mediated activation of peroxy- monosulfate: Different cobalt counteranions in homogenous process and cobalt oxide catalysts in photolytic heterogeneous process. Water Res. 2009, 43, 2513–2521. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Shi, D.J.; Wu, Z.T.; Zhang, L.F.; Zhai, Z.C.; Fang, Y.S.; Sun, P.; Han, R.R.; Wu, J.Q.; Liu, H. CoMn2O4 Catalyst Prepared Using the Sol-Gel Method for the Activation of Peroxymonosulfate and Degradation of UV Filter 2-Phenylbenzimidazole-5-sulfonic Acid (PBSA)2. Nanomaterials 2019, 9, 774. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.L.; Sun, C.; He, H.; Dai, Y.X.; Yang, S.G.; Lin, Y.S.; Zhan, X.H.; Li, Q.; Zhou, Y. Catalytic Degradation of Diatrizoate by Persulfate Activation with Peanut Shell Biochar-Supported Nano Zero-Valent Iron in Aqueous Solution. Int. J. Environ. Res. Public Health 2018, 15, 1937. [Google Scholar] [CrossRef]
- Liao, Z.W.; Zhu, J.Y.; Jawad, A.; Muzi, J.J.; Chen, Z.Q.; Chen, Z.L. Degradation of Phenol Using Peroxymonosulfate Activated by a High Efficiency and Stable CoMgAl-LDH Catalyst. Materials 2019, 12, 968. [Google Scholar] [CrossRef]




| Sample | C (%) | H (%) | N (%) | S (%) | Ash (%) |
|---|---|---|---|---|---|
| SS | 24.71 | 4.01 | 4.15 | 0.354 | 53.00 |
| SS-600 | 16.97 | 0.522 | 1.54 | 0.679 | 13.60 |
| SS-600-HCl | 30.07 | 0.904 | 2.97 | 1.23 | 9.40 |
| Sample | SBET (m2/g) | Total Pore Volume (Vp) (cm3/g) | Pore Size (L0) (nm) |
|---|---|---|---|
| SS-600 | 147 | 0.19 | 5.21 |
| SS-600-HCl | 197 | 0.29 | 5.89 |
| t (°C) | kobs (min−1) | R2 |
|---|---|---|
| 25 | 0.01421 | 0.99819 |
| 35 | 0.02358 | 0.99884 |
| 45 | 0.03527 | 0.99776 |
| Sample | SBET (m2/g) | Vt (cm3/g) | L0 (nm) |
|---|---|---|---|
| Fresh SS-600-HCl | 197 | 0.29 | 5.89 |
| Three times used SS-600-HCl | 3 | 0.005 | 5.71 |
| Regenerated SS-600-HCl | 9 | 0.043 | 19.09 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, M.; Zhang, J.; Chu, W.; Zhou, G.; Chen, J. Surface-Modified Sewage Sludge-Derived Carbonaceous Catalyst as a Persulfate Activator for Phenol Degradation. Int. J. Environ. Res. Public Health 2020, 17, 3286. https://doi.org/10.3390/ijerph17093286
Han M, Zhang J, Chu W, Zhou G, Chen J. Surface-Modified Sewage Sludge-Derived Carbonaceous Catalyst as a Persulfate Activator for Phenol Degradation. International Journal of Environmental Research and Public Health. 2020; 17(9):3286. https://doi.org/10.3390/ijerph17093286
Chicago/Turabian StyleHan, Meiling, Jin Zhang, Wen Chu, Gongfu Zhou, and Jiahao Chen. 2020. "Surface-Modified Sewage Sludge-Derived Carbonaceous Catalyst as a Persulfate Activator for Phenol Degradation" International Journal of Environmental Research and Public Health 17, no. 9: 3286. https://doi.org/10.3390/ijerph17093286
APA StyleHan, M., Zhang, J., Chu, W., Zhou, G., & Chen, J. (2020). Surface-Modified Sewage Sludge-Derived Carbonaceous Catalyst as a Persulfate Activator for Phenol Degradation. International Journal of Environmental Research and Public Health, 17(9), 3286. https://doi.org/10.3390/ijerph17093286





