Hypocholesterolaemic and Anti-Atherogenic Effects of Palm-Based Oils (NoveLin I and NoveLin II) in Cholesterol-Fed Rabbits
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. NoveLin I and NoveLin II Preparation
2.3. Animal Feeding
2.4. Determination of Vitamin E Content
2.5. Analysis of Plasma Lipid
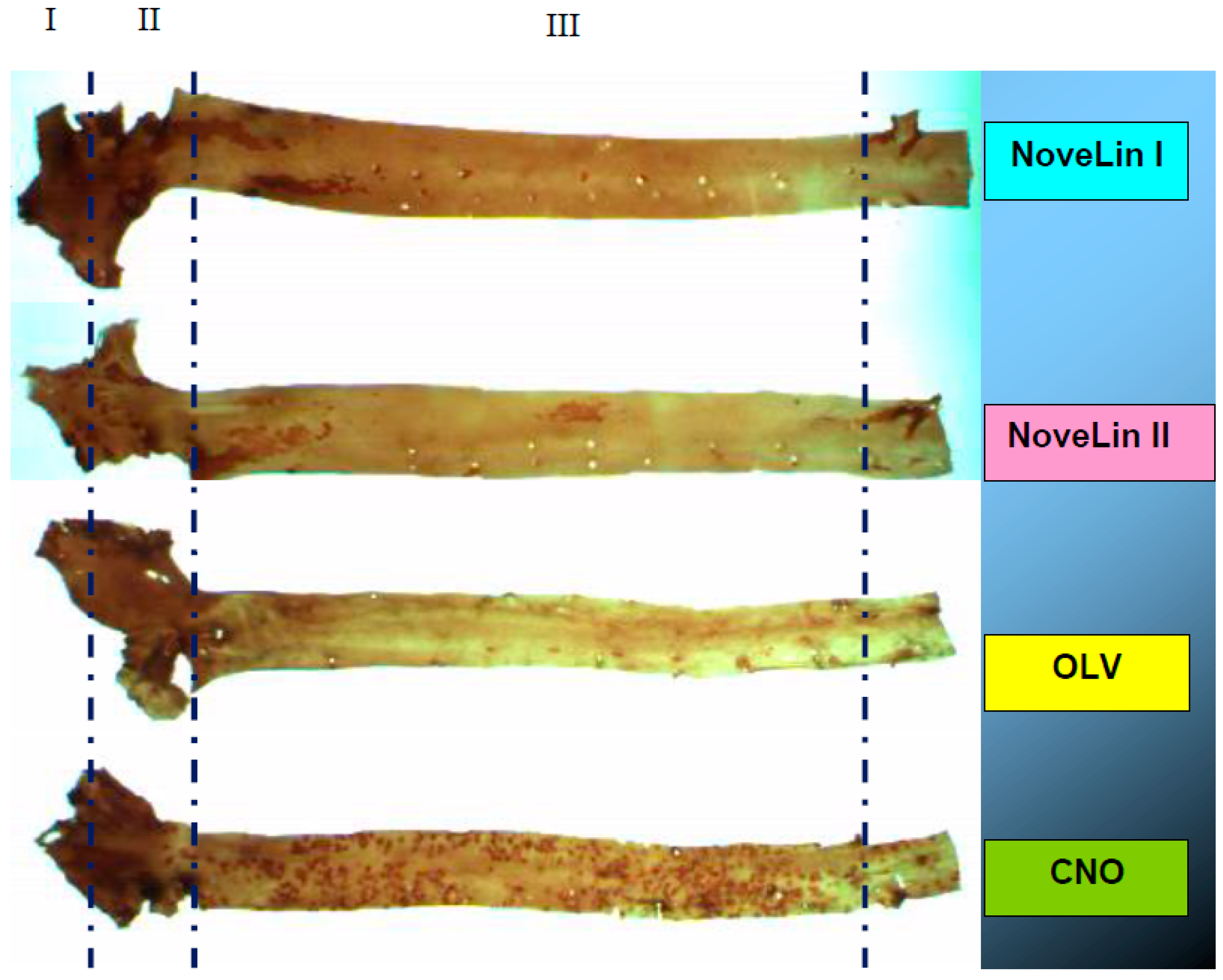
2.6. Measurement of Aortic Lesion Area
- Fibrous plaques: Raised nodular lesions, continuous, intense red (when unstained, appear as white hard nodules visible with the naked eye)
- Fatty plaques: Raised distinct lesions, intensely stained red
- Fatty streaks: Lipid accumulation, stained light red
- Lesion free: No plaques or streaks
2.7. Statistical Analysis
3. Results
3.1. Fatty Acid Composition of Diets
3.2. Vitamin E Content of Test Diets
3.3. Animal Body and Organ Weights
3.4. Lipid Profile of Tested Animals
3.5. Aortic Lesions Measurement
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Viles-Gonzalez, J.F.; Fuster, V.; Badimon, J.J. Atherothrombosis: A widespread disease with unpredictable and life-threatening consequences. Eur. Heart J. 2004, 25, 1197–1207. [Google Scholar] [CrossRef]
- Douglas, G.; Channon, K.M. The pathogenesis of atherosclerosis. Medicine 2010, 38, 397–402. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Levin, S.M.; Barnard, N.D. Association between plant-based diets and plasma lipids: A systematic review and meta-analysis. Nutr. Rev. 2017, 75, 683–698. [Google Scholar] [CrossRef] [PubMed]
- Sundram, K.; Pathmanathan, R.; Wong, K.T.; Baskaran, G. Impact of saturated and trans fatty acid enriched oil blends on atherosclerosis in rabbits fed cholesterol-free diets. Asia Pac. J. Clin. Nutr. 1996, 6, 31–35. [Google Scholar]
- Chiu, S.; Williams, P.T.; Krauss, R.M. Effects of a very high saturated fat diet on LDL particles in adults with atherogenic dyslipidemia: A randomized controlled trial. PLoS ONE 2017, 12, e0170664. [Google Scholar] [CrossRef]
- Sundram, K. Modulation of human lipids and lipoproteins by dietary palm oil and palm olein: A review. Asia Pac. J. Clin. Nutr. 1997, 6, 12–16. [Google Scholar]
- Sundram, K.; Hayes, K.C.; Siru, O.H. Both dietary 18:2 and 16:0 may be required to improve the serum LDL/HDL cholesterol ratio in normocholesterolemic men. J. Nutrl. Biochem. 1995, 4, 179–187. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Bogensberger, B.; Benčič, A.; Knüppel, S.; Boeing, H.; Hoffmann, G. Effects of oils and solid fats on blood lipids: A systematic review and network meta-analysis. J. Lipid Res. 2018, 59, 1771–1782. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.K.W.; Hayes, K.C.; Dewitt, G.F.; Jegathesan, M.; Satgunasingham, N.; Ong, A.S.H.; Tan, D.T.S. Palmitic and oleic acids exert similar effects on lipid profiles in normocholesterolemic humans. J. Am. Coll. Nutr. 1992, 11, 383–390. [Google Scholar] [CrossRef]
- Lv, C.; Wang, Y.; Zhou, C.; Ma, W.; Yang, Y.; Xiao, R.; Yu, H. Effects of dietary palm olein on the cardiovascular risk factors in healthy young adults. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Franklin, B.; Kris-Etherton, P.; Harris, W.S.; Howard, B.; et al. Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation 2006, 114, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Sundram, K.; Perlman, D.; Hayes, K.C. U.S. Patent No. 5,578,334; Patent and Trademark Office: Washington, DC, USA, 1996. [Google Scholar]
- Siew, W.L.; Idris, N.A.B.H.; Cheah, K.Y. U.S. Patent No. 7,785,645; Patent and Trademark Office: Washington, DC, USA, 2010. [Google Scholar]
- Idris, C.; Sundram, K.; Razis, A. Effect of Consumption Heated Oils with or without Dietary Cholesterol on the Development of Atherosclerosis. Nutrients 2018, 10, 1527. [Google Scholar] [CrossRef] [PubMed]
- Pasias, I.; Kiriakou, I.; Papakonstantinou, L.; Proestos, C. Determination of Vitamin E in Cereal Products and Biscuits by GC-FID. Foods 2018, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Sundram, K.; Pathmanathan, R.; Rosnah, M.N. Dietary cholesterol addition to hydrogenated or saturated fat blends enhances atherosclerosis in the rabbit model. In Proceedings of the International Oil Palm Conference (PIPOC), Kuala Lumpur, Malaysia, 1–6 February 1999; p. 129. [Google Scholar]
- Idris, C.A.; Karupaiah, T.; Sundram, K.; Tan, Y.A.; Balasundram, N.; Leow, S.S.; Nasruddin, N.S.; Sambanthamurthi, R. Oil palm phenolics and vitamin E reduce atherosclerosis in rabbits. J. Funct. Foods. 2014, 7, 541–550. [Google Scholar] [CrossRef]
- Idris, C.A.C.; Sundram, K. Effect of dietary cholesterol, trans and saturated fatty acids on serum lipoproteins in non-human primates. Asia Pac. J. Clin. Nutr. 2002, 11, S408–S415. [Google Scholar] [CrossRef][Green Version]
- Wilson, T.A.; Nicolosi, R.J.; Handelman, G.; Yoganathan, S.; Kotyla, T.; Orthoefer, F.; Binford, P. Comparative effects of emu and olive oil on aortic early atherosclerosis and associated risk factors in hypercholesterolemic hamsters. Nutr. Res. 2004, 24, 395–406. [Google Scholar] [CrossRef]
- Kritchevsky, D.; Tepper, S.A.; Wright, S.; Czarnecki, S.K.; Wilson, T.A.; Nicolosi, R.J. Cholesterol vehicle in experimental atherosclerosis 24: Avocado oil. J. Am. Coll. Nutr. 2003, 22, 52–55. [Google Scholar] [CrossRef]
- Pang, K.L.; Chin, K.Y. The role of tocotrienol in protecting against metabolic diseases. Molecules 2019, 24, 923. [Google Scholar] [CrossRef]
- Brandhorst, S.; Longo, V.D. Dietary restrictions and nutrition in the prevention and treatment of cardiovascular disease. Circ. Res. 2019, 124, 952–965. [Google Scholar] [CrossRef]
- Liu, A.G.; Ford, N.A.; Hu, F.B.; Zelman, K.M.; Mozaffarian, D.; Kris-Etherton, P.M. A healthy approach to dietary fats: Understanding the science and taking action to reduce consumer confusion. Nutr. J. 2017, 16, 53. [Google Scholar] [CrossRef]
- Eyres, L.; Eyres, M.F.; Chisholm, A.; Brown, R.C. Coconut oil consumption and cardiovascular risk factors in humans. Nutr. Rev. 2016, 74, 267–280. [Google Scholar] [CrossRef] [PubMed]
| Fatty Acid | NoveLin I | NoveLin II | OLV | CNO | Palm Olein [12] | AHA Diet [11] |
|---|---|---|---|---|---|---|
| C8:0 | 7.51 | - | ||||
| C10:0 | 6.27 | - | ||||
| C12:0 | 46.42 | 0.44 | ||||
| C14:0 | 0.6 | 0.66 | 18.45 | 1.02 | 0.2 | |
| C16:0 | 25.97 | 23.41 | 11.97 | 9.45 | 40.49 | 25.7 |
| C18:0 | 3.58 | 2.82 | 3.96 | 2.83 | 3.99 | 4.1 |
| C20:0 | 0.4 | 0.87 | - | 0.79 | - | |
| SFA | 30.55 | 27.76 | 15.93 | 91.72 | 45.94 | 30.0 |
| C18:1 | 38.22 | 52.11 | 76.01 | 6.76 | 43.81 | 37.4 |
| MUFA | 38.22 | 52.11 | 76.01 | 6.76 | 43.81 | 37.4 |
| C18:2 | 28.86 | 17.07 | 6.78 | 1.51 | 10.25 | 29.3 |
| C18:3 | 2.37 | 3.05 | 0.58 | - | - | 3.3 |
| PUFA | 31.23 | 20.12 | 7.36 | 1.51 | 10.25 | 32.6 |
| S:M:P Ratio | 1:1:1 | 0.5:1:0.4 | 0.2:1:0.1 | 13.6:1:0.2 | 1:1:0.2 | 1:1:1 |
| Isomers | NoveLin I | NoveLin II | OLV | CNO |
|---|---|---|---|---|
| alpha-t | 94.28 | 109.96 | 114.56 | 163.71 |
| beta-t | 10.42 | 30.88 | 8.29 | 0.00 |
| delta-t | 64.42 | 5.87 | 0.00 | 12.13 |
| gamma-t | 188.48 | 104.31 | 11.77 | 0.00 |
| Total t | 357.60 | 251.02 | 134.62 | 175.84 |
| alpha-t3 | 59.33 | 65.12 | 0.00 | 0.00 |
| delta-t3 | 57.11 | 65.24 | 0.00 | 0.00 |
| gamma-t3 | 120.17 | 165.15 | 0.00 | 0.00 |
| Total t3 | 236.61 | 295.51 | 0.00 | 0.00 |
| Total vitamin E | 594.21 | 546.51 | 134.62 | 175.84 |
| Body Weights | NoveLin I n = 10 | NoveLin II n = 10 | OLV n = 10 | CNO n = 10 |
|---|---|---|---|---|
| Initial (g) | 2081.92 ± 107.89 a | 2044.92 ± 96.04 a | 2016.21 ± 53.17 a | 1956.59 ± 69.66 a |
| Final (g) | 2570.00 ± 128.91 a | 2550.53 ± 139.41 a | 2421.76 ± 156.16 a | 2516.84 ± 98.24 a |
| Organ Weight (g) | NoveLin I n = 10 | NoveLin II n = 10 | OLV n = 10 | CNO n = 10 |
|---|---|---|---|---|
| Heart | 4.34 ± 0.20 a | 4.24 ± 0.31 a | 4.50 ± 0.28 a | 4.30 ± 0.34 a |
| Lungs | 11.41 ± 1.31 a | 10.26 ± 0.96 a | 16.33 ± 1.99 a | 13.44 ± 1.62 a |
| Liver | 55.26 ± 4.65 a | 55.86 ± 4.27 a | 56.78 ± 5.24 a | 52.58 ± 4.35 a |
| Kidneys | 13.63 ± 0.83 a | 12.54 ± 1.05 a | 13.02 ± 0.58 a | 13.33 ± 0.75 a |
| Spleen | 0.62 ± 0.10 a | 0.54 ± 0.07 a | 0.69 ± 0.05 a | 0.45 ± 0.05 a |
| Plasma Lipid (mmol/L) | NoveLin I n = 10 | NoveLin II n = 10 | OLV n = 10 | CNO n = 10 |
|---|---|---|---|---|
| TC | 15.53 ± 2.84 a | 13.93 ± 2.49 a | 13.16 ± 2.14 a | 18.67 ± 2.36 b |
| TG | 2.29 ± 1.22 | 1.61 ± 1.02 | 2.03 ± 0.98 | 2.18 ± 0.47 |
| LDL–C | 13.15 ± 2.54 a | 12.14 ± 2.21 a | 12.74 ± 1.98 a | 16.60 ± 2.08 b |
| HDL–C | 1.86 ± 0.29 | 1.67 ± 0.17 | 1.77 ± 0.25 | 2.11 ± 0.15 |
| LDL/HDL | 5.08 ± 1.63 a | 3.91 ± 1.65 b | 4.22 ± 2.01 a,b | 8.43 ± 2.10 c |
| Diet | Fibrous Plaque | Fatty Plaque | Fatty Streak | Free Lesion |
|---|---|---|---|---|
| NoveLin I | 1.57 ± 0.77 b | 3.68 ± 1.57 b | 8.37 ± 1.89 a,b | 86.3 ± 3.92 b |
| NoveLin II | 0.64 ± 0.40 c | 1.47 ± 0.66 c | 3.87 ± 1.32 c | 93.86 ± 6.49 b |
| OLV | 0.30 ± 0.15 d | 3.51 ± 1.92 b | 7.06 ± 1.98 b | 88.95 ± 1.55 b |
| CNO | 6.45 ± 3.36 a | 5.78 ± 1.84 b | 9.99 ± 1.56 a | 77.47 ± 2.28 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che Idris, C.A.; Wai Lin, S.; Abdull Razis, A.F. Hypocholesterolaemic and Anti-Atherogenic Effects of Palm-Based Oils (NoveLin I and NoveLin II) in Cholesterol-Fed Rabbits. Int. J. Environ. Res. Public Health 2020, 17, 3226. https://doi.org/10.3390/ijerph17093226
Che Idris CA, Wai Lin S, Abdull Razis AF. Hypocholesterolaemic and Anti-Atherogenic Effects of Palm-Based Oils (NoveLin I and NoveLin II) in Cholesterol-Fed Rabbits. International Journal of Environmental Research and Public Health. 2020; 17(9):3226. https://doi.org/10.3390/ijerph17093226
Chicago/Turabian StyleChe Idris, Che Anishas, Siew Wai Lin, and Ahmad Faizal Abdull Razis. 2020. "Hypocholesterolaemic and Anti-Atherogenic Effects of Palm-Based Oils (NoveLin I and NoveLin II) in Cholesterol-Fed Rabbits" International Journal of Environmental Research and Public Health 17, no. 9: 3226. https://doi.org/10.3390/ijerph17093226
APA StyleChe Idris, C. A., Wai Lin, S., & Abdull Razis, A. F. (2020). Hypocholesterolaemic and Anti-Atherogenic Effects of Palm-Based Oils (NoveLin I and NoveLin II) in Cholesterol-Fed Rabbits. International Journal of Environmental Research and Public Health, 17(9), 3226. https://doi.org/10.3390/ijerph17093226






