Effect of Soil Washing Solutions on Simultaneous Removal of Heavy Metals and Arsenic from Contaminated Soil
Abstract
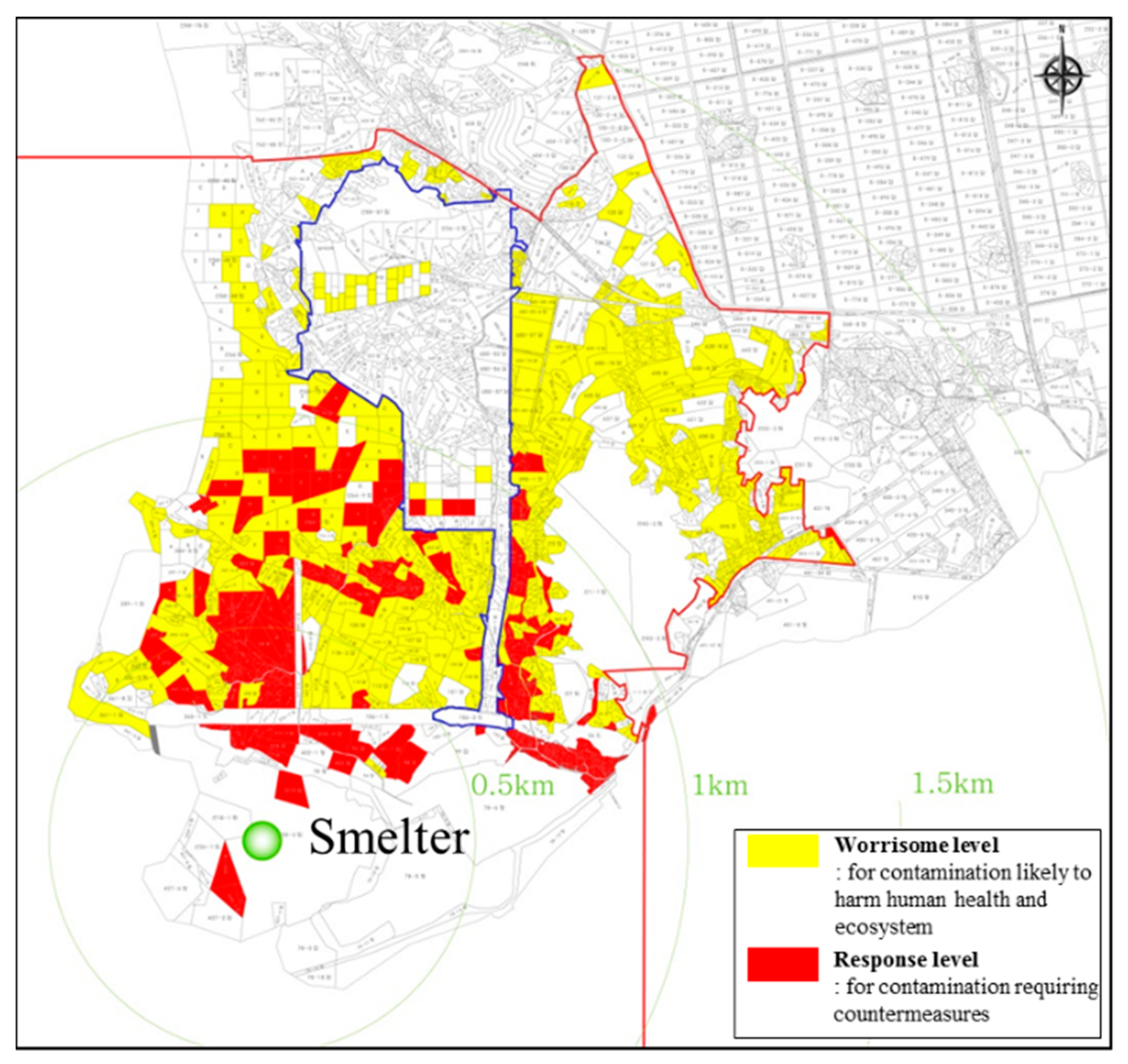
1. Introduction
2. Materials and Methods
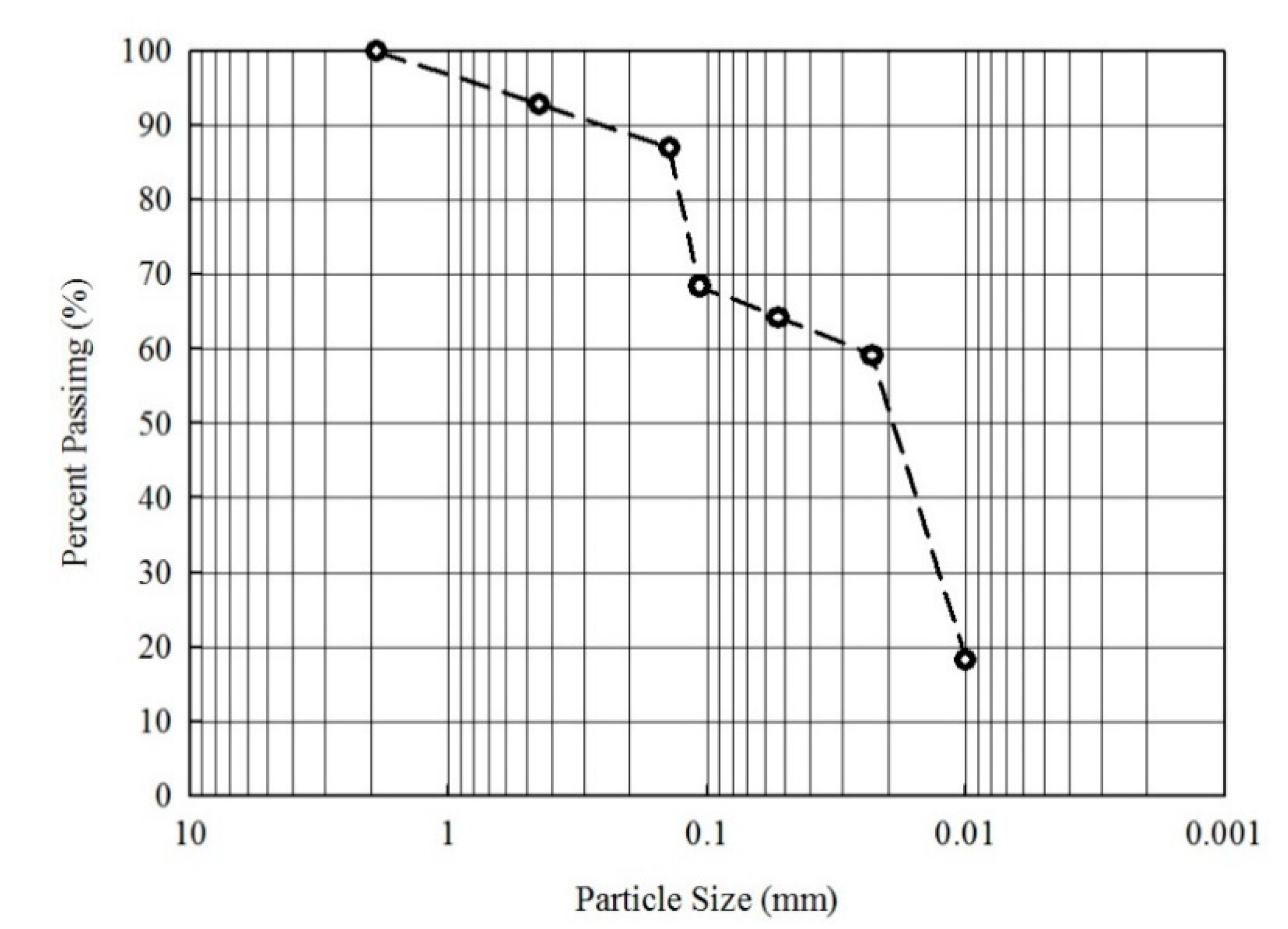
2.1. Soil Sampling and Characterization
2.2. Batch Experiment: Effect of Soil Characteristics and Washing Solution
2.3. Analysis Method
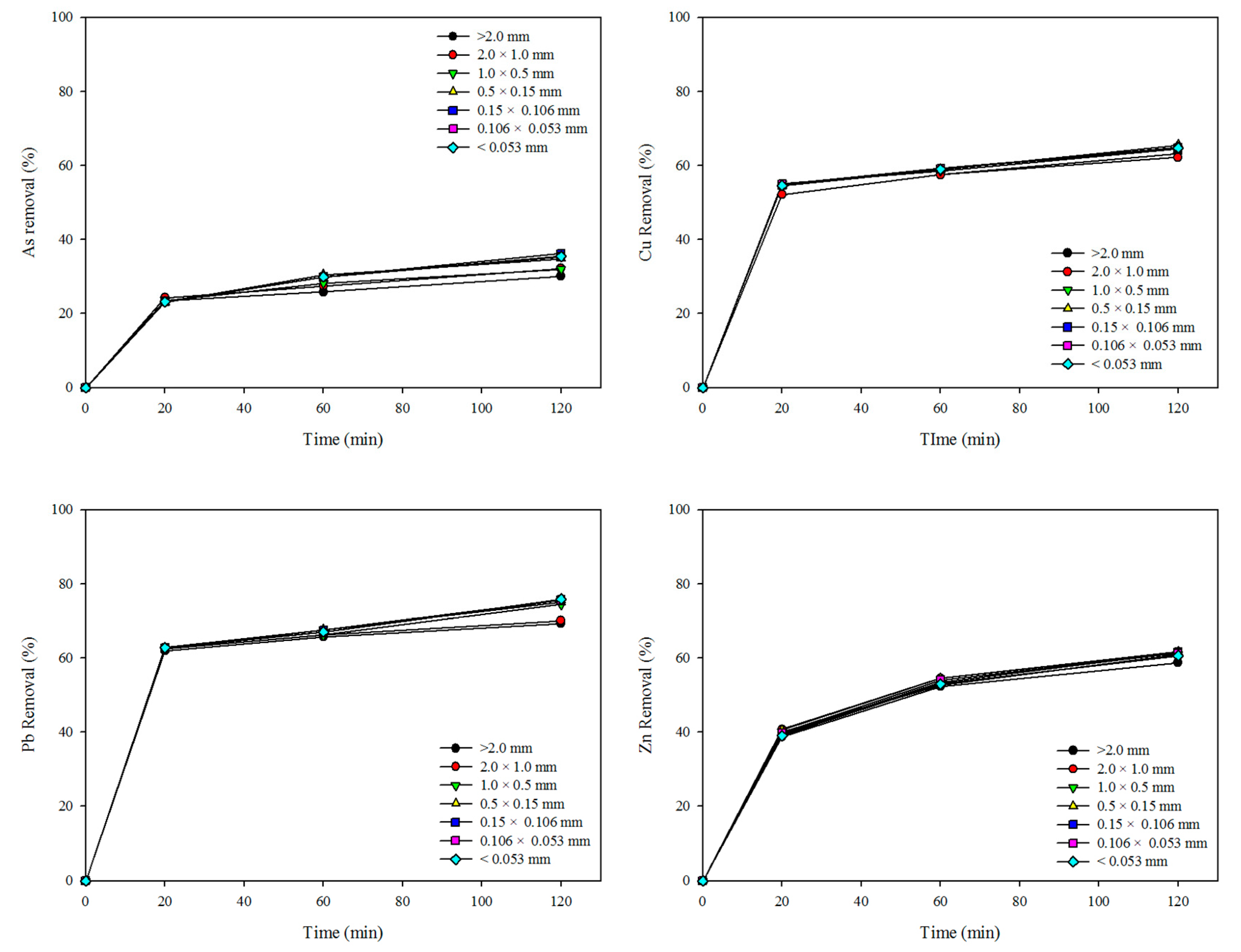
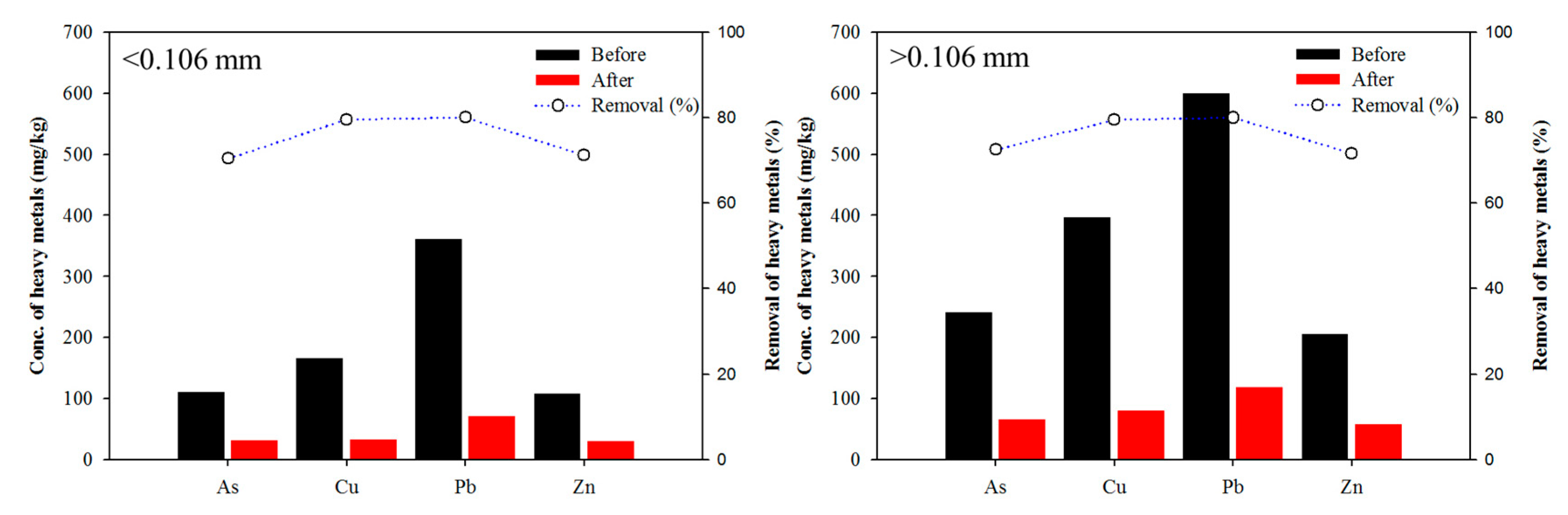
3. Results and Discussion
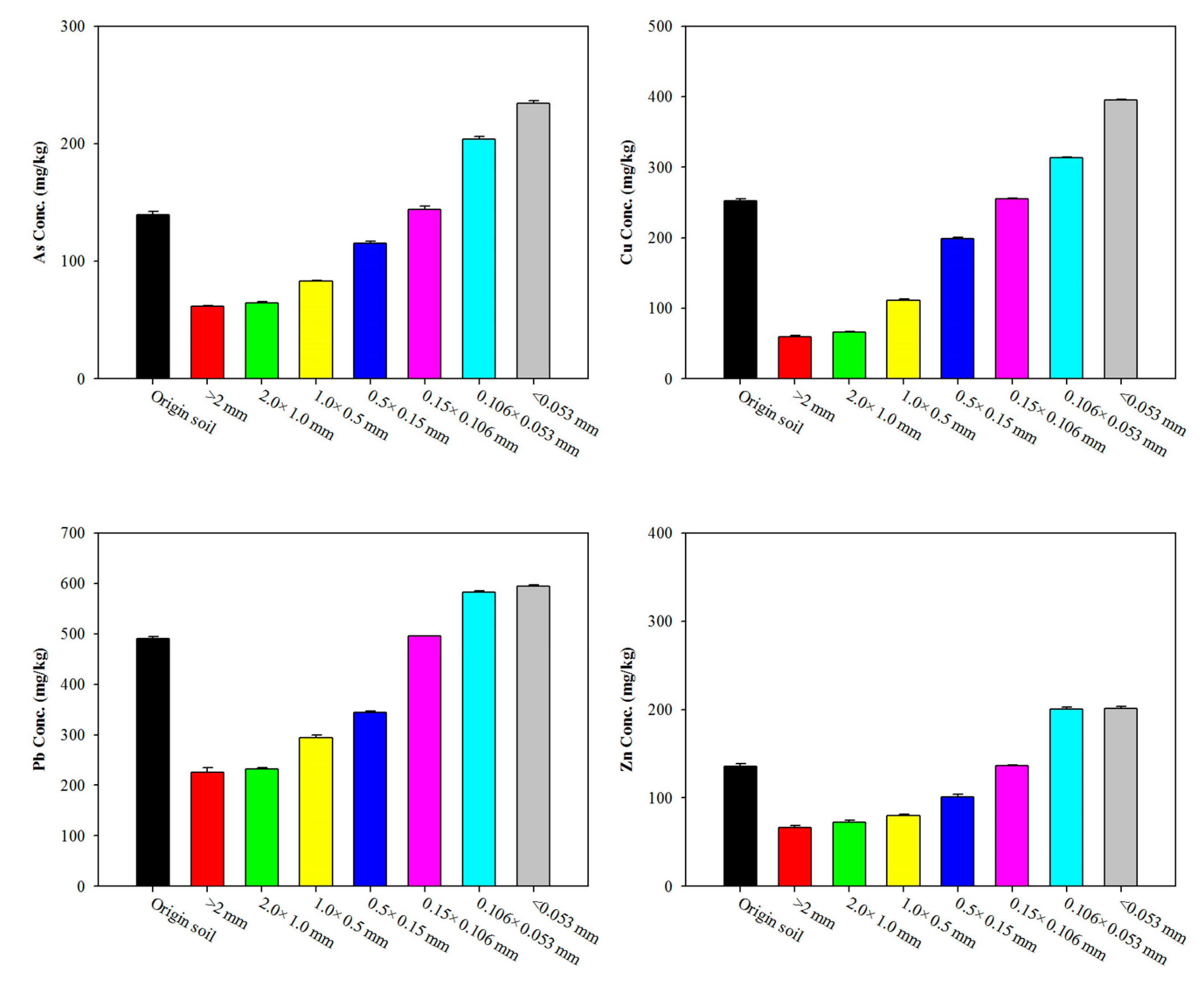
3.1. Characteristics of Soil: Effect of Particle-Size Fraction
3.2. Effect of Washing Solution: Sulfuric Acid, Phosphoric Acid, and Mixing Washing Solution
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Elements. | Size Particle Fraction | EI (%) | K (min−1) | R2 |
|---|---|---|---|---|
| As | >2 mm | 30.14 | 0.0873 | 0.9840 |
| 2.0 × 1.0 mm | 32.20 | 0.0801 | 0.9830 | |
| 1.0 × 0.5 mm | 31.96 | 0.0723 | 0.9908 | |
| 0.5 × 0.15 mm | 34.79 | 0.0558 | 0.9923 | |
| 0.15 × 0.106 mm | 36.19 | 0.0534 | 0.9813 | |
| 0.106 × 0.053 mm | 35.31 | 0.0550 | 0.9864 | |
| <0.053 mm | 35.44 | 0.0548 | 0.9861 | |
| Cu | >2 mm | 63.29 | 0.0977 | 0.9938 |
| 2.0 × 1.0 mm | 62.22 | 0.1011 | 0.9958 | |
| 1.0 × 0.5 mm | 64.55 | 0.1120 | 0.9935 | |
| 0.5 × 0.15 mm | 65.54 | 0.1027 | 0.9921 | |
| 0.15 × 0.106 mm | 64.88 | 0.1048 | 0.9939 | |
| 0.106 × 0.053 mm | 64.72 | 0.1076 | 0.9945 | |
| <0.053 mm | 64.73 | 0.1056 | 0.9943 | |
| Pb | >2 mm | 69.33 | 0.1240 | 0.9980 |
| 2.0 × 1.0 mm | 70.10 | 0.1238 | 0.9977 | |
| 1.0 × 0.5 mm | 74.43 | 0.1081 | 0.9906 | |
| 0.5 × 0.15 mm | 75.01 | 0.1032 | 0.9927 | |
| 0.15×0.106 mm | 75.74 | 0.1025 | 0.9910 | |
| 0.106 × 0.053 mm | 75.59 | 0.1034 | 0.9900 | |
| <0.053 mm | 75.85 | 0.1036 | 0.9895 | |
| Zn | >2 mm | 58.75 | 0.0550 | 0.9951 |
| 2.0 × 1.0 mm | 61.42 | 0.0557 | 0.9943 | |
| 1.0 × 0.5 mm | 60.96 | 0.0549 | 0.9906 | |
| 0.5 × 0.15 mm | 61.73 | 0.0548 | 0.9938 | |
| 0.15 × 0.106 mm | 61.56 | 0.0521 | 0.9922 | |
| 0.106 × 0.053 mm | 61.29 | 0.0534 | 0.9940 | |
| <0.053 mm | 60.64 | 0.0523 | 0.9932 |
References
- Lee, P.-K.; Yu, S.; Jeong, Y.-J.; Seo, J.; Choi, S.; Yoon, B.-Y. Source identification of arsenic contamination in agricultural soils surrounding a closed Cu smelter, South Korea. Chemosphere 2019, 217, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Ettler, V. Soil contamination near non-ferrous metal smelters: A review. Appl. Geochem. 2016, 64, 56–74. [Google Scholar] [CrossRef]
- Jeong, S.; Hong, J.K.; Jho, E.H.; Nam, K. Interaction among soil physicochemical properties, bacterial community structure, and arsenic contamination: Clay-induced change in long-term arsenic contaminated soils. J. Hazard. Mater. 2019, 378, 120729. [Google Scholar] [CrossRef] [PubMed]
- Sterckeman, T.; Douay, F.; Proix, N.; Fourrier, H.; Perdrix, E. Assessment of the Contamination of Cultivated Soils by Eighteen Trace Elements Around Smelters in the North of France. Water Air Soil Pollut. 2002, 135, 173–194. [Google Scholar] [CrossRef]
- An, J.; Jeong, B.; Nam, K. Evaluation of the effectiveness of in situ stabilization in the field aged arsenic-contaminated soil: Chemical extractability and biological response. J. Hazard. Mater. 2019, 367, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Li, Y.; Yan, X. Removal of heavy metals and arsenic from a co-contaminated soil by sieving combined with washing process. J. Environ. Sci. 2016, 41, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-C.; Kim, E.J.; Kim, H.-W.; Baek, K. Oxalate-based remediation of arsenic bound to amorphous Fe and Al hydrous oxides in soil. Geoderma 2016, 270, 76–82. [Google Scholar] [CrossRef]
- Im, J.; Yang, K.; Jho, E.H.; Nam, K. Effect of different soil washing solutions on bioavailability of residual arsenic in soils and soil properties. Chemosphere 2015, 138, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Im, J.; Yang, K.; Moon, S.; Kim, Y.-J.; Nam, K. Role of phosphate and Fe-oxides on the acid-aided extraction efficiency and readsorption of As in field-aged soil. J. Hazard. Mater. 2015, 300, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Jho, E.H.; Im, J.; Yang, K.; Kim, Y.-J.; Nam, K. Changes in soil toxicity by phosphate-aided soil washing: Effect of soil characteristics, chemical forms of arsenic, and cations in washing solutions. Chemosphere 2015, 119, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Chen, J.; Wang, X. Removal of arsenic and cadmium with sequential soil washing techniques using Na 2 EDTA, oxalic and phosphoric acid: Optimization conditions, removal effectiveness and ecological risks. Chemosphere 2016, 156, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Kim, M.-J. Reuse of washing effluent containing oxalic acid by a combined precipitation–acidification process. Chemosphere 2013, 90, 1526–1532. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-K.; Kang, M.-J.; Yu, S.; Kwon, Y.K. Assessment of trace metal pollution in roof dusts and soils near a large Zn smelter. Sci. Total. Environ. 2020, 713, 136536. [Google Scholar] [CrossRef] [PubMed]
- Soil Environment Conservation Act: 11464; Korean Ministry of Environment (KMOE): Sejong, Korea, 2013.
- Michálková, Z.; Komárek, M.; Šillerová, H.; Della Puppa, L.; Joussein, E.; Bordas, F.; Vaněk, A.; Vaněk, O.; Ettler, V. Evaluating the potential of three Fe- and Mn-(nano)oxides for the stabilization of Cd, Cu and Pb in contaminated soils. J. Environ. Manag. 2014, 146, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Nejad, Z.D.; Jung, M.C.; Kim, K.-H. Remediation of soils contaminated with heavy metals with an emphasis on immobilization technology. Environ. Geochem. Heal. 2017, 40, 927–953. [Google Scholar] [CrossRef] [PubMed]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Official Test Methods of Soil Quality: 2009-255; Korean Ministry of Environment (KMOE): Gwacheon, Korea, 2009.
- Oh, S.-Y.; Yoon, M.-K.; Kim, I.-H.; Kim, J.Y.; Bae, W. Chemical extraction of arsenic from contaminated soil under subcritical conditions. Sci. Total. Environ. 2011, 409, 3066–3072. [Google Scholar] [CrossRef] [PubMed]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Jeon, E.-K.; Baek, K. Role of reducing agent in extraction of arsenic and heavy metals from soils by use of EDTA. Chemosphere 2016, 152, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-C.; Lee, C.; Lee, J.-S.; Baek, K. Simultaneous application of chemical oxidation and extraction processes is effective at remediating soil Co-contaminated with petroleum and heavy metals. J. Environ. Manag. 2017, 186, 314–319. [Google Scholar] [CrossRef] [PubMed]
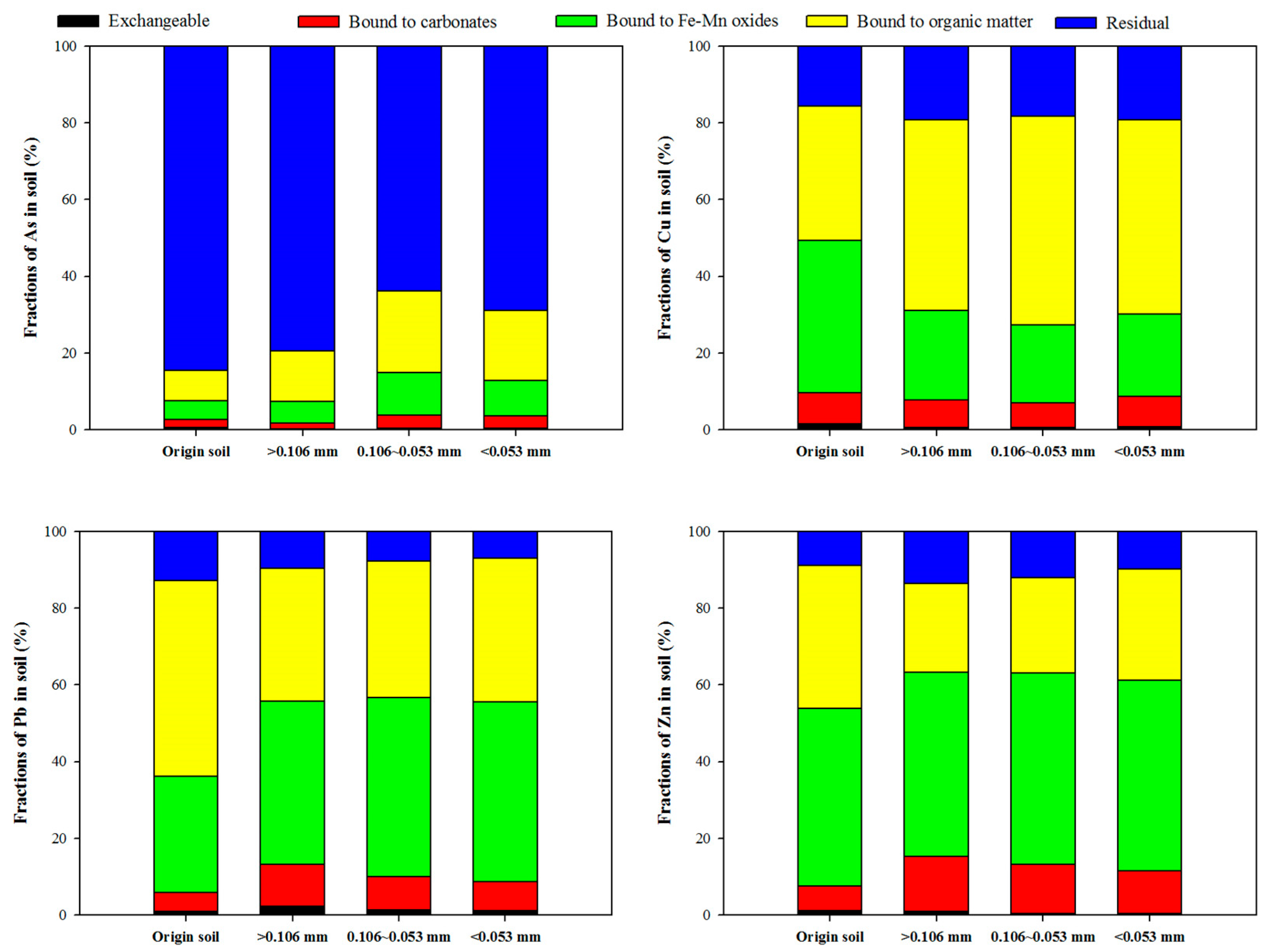
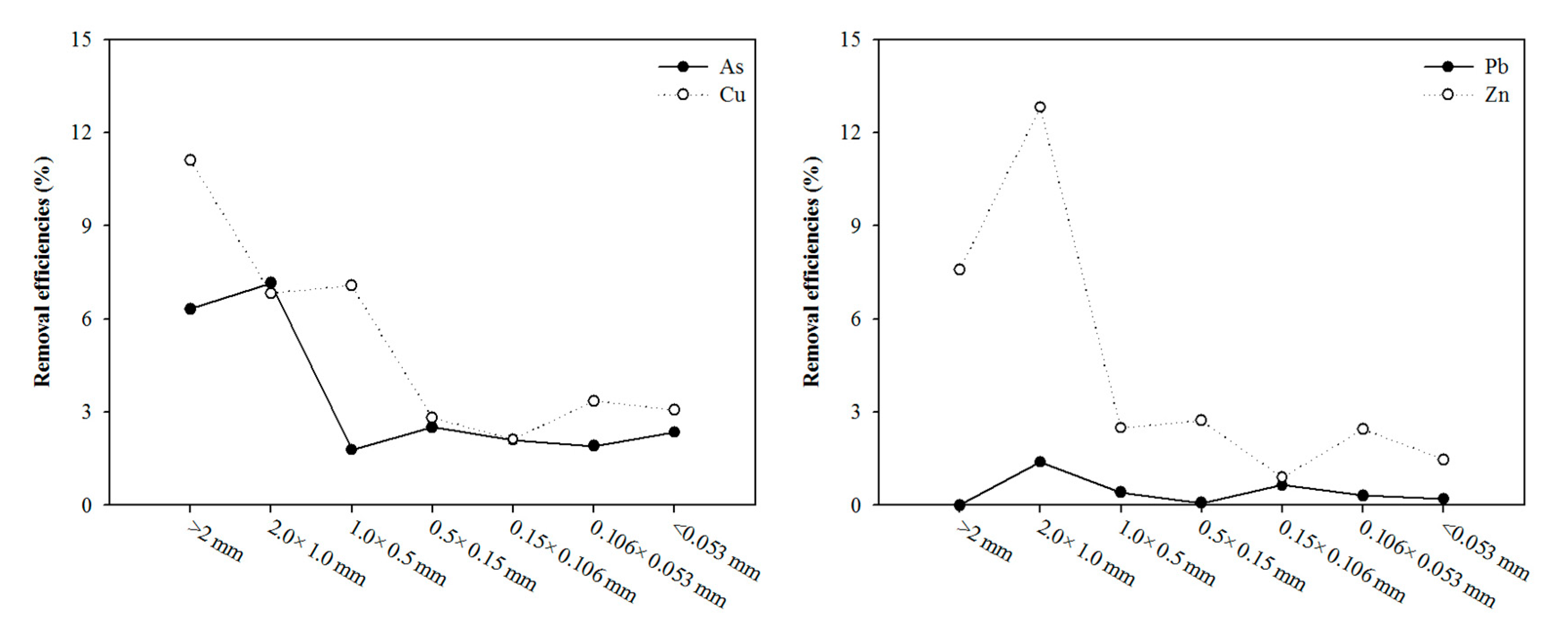
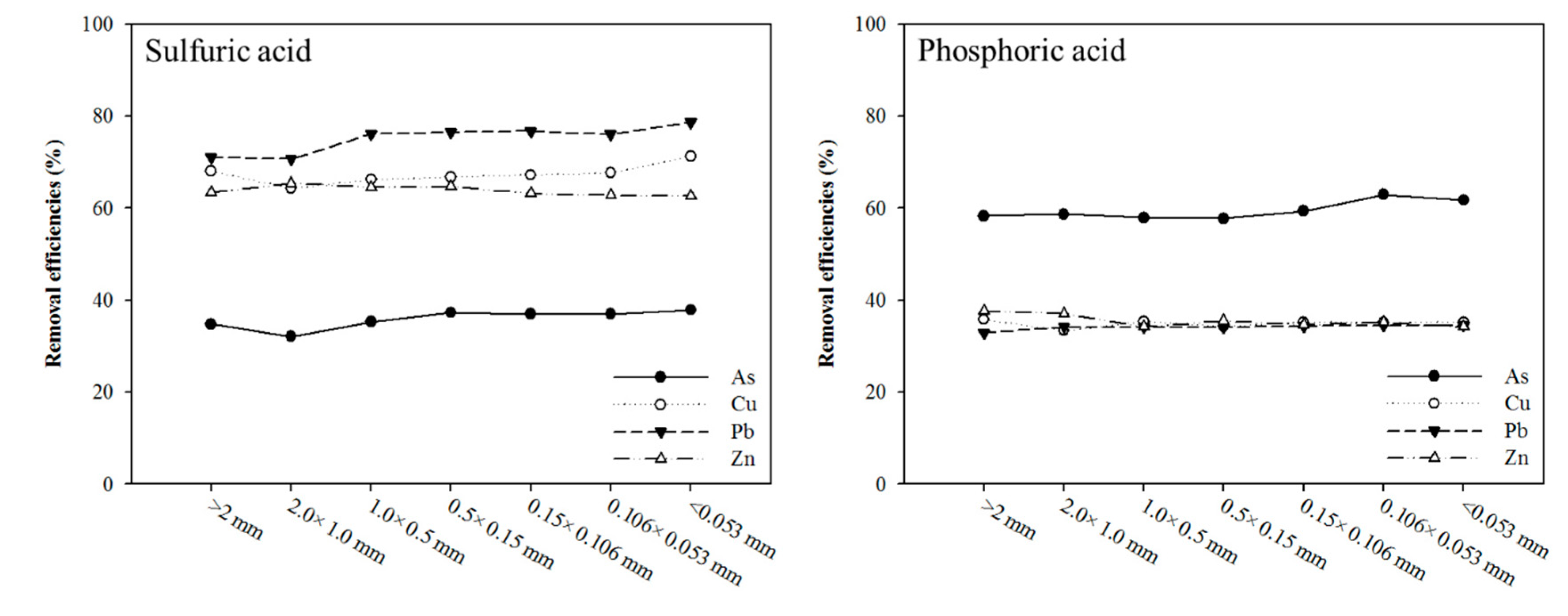
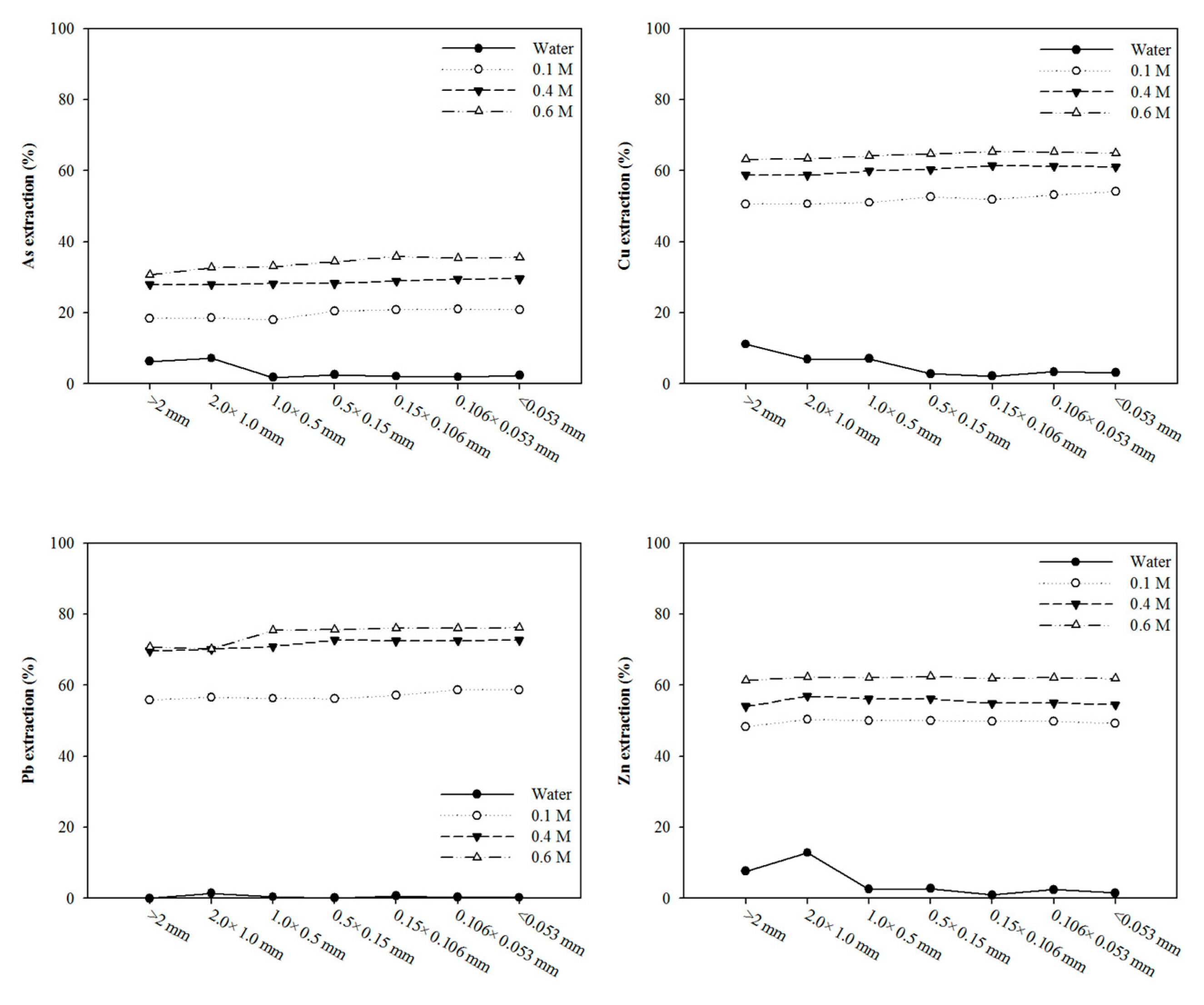

| pH | Organic Matter (%) | CEC (cmol/kg) | Sand (%) | Silt (%) | Clay (%) |
|---|---|---|---|---|---|
| 6.40 | 2.74 | 19.65 | 37.60 | 43.72 | 21.68 |
| Elements | As (mg/kg) | Cu (mg/kg) | Ni (mg/kg) | Pb (mg/kg) | Zn (mg/kg) |
|---|---|---|---|---|---|
| Korean limit (1) | 25 | 150 | 100 | 200 | 300 |
| Original soil | 139.53 ± 2.65 | 252.40 ± 2.55 | 17.86 ± 0.62 | 490.74 ± 3.55 | 135.37 ± 3.58 |
| +0.106 mm | 112.10 | 167.64 | 14.24 | 362.50 | 109.46 |
| −0.106 mm | 241.94 | 394.36 | 34.75 | 600.61 | 207.09 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, K.; Myung, E.; Kim, H.; Park, C.; Choi, N.; Park, C. Effect of Soil Washing Solutions on Simultaneous Removal of Heavy Metals and Arsenic from Contaminated Soil. Int. J. Environ. Res. Public Health 2020, 17, 3133. https://doi.org/10.3390/ijerph17093133
Cho K, Myung E, Kim H, Park C, Choi N, Park C. Effect of Soil Washing Solutions on Simultaneous Removal of Heavy Metals and Arsenic from Contaminated Soil. International Journal of Environmental Research and Public Health. 2020; 17(9):3133. https://doi.org/10.3390/ijerph17093133
Chicago/Turabian StyleCho, Kanghee, Eunji Myung, Hyunsoo Kim, Cheonyoung Park, Nagchoul Choi, and Cheol Park. 2020. "Effect of Soil Washing Solutions on Simultaneous Removal of Heavy Metals and Arsenic from Contaminated Soil" International Journal of Environmental Research and Public Health 17, no. 9: 3133. https://doi.org/10.3390/ijerph17093133
APA StyleCho, K., Myung, E., Kim, H., Park, C., Choi, N., & Park, C. (2020). Effect of Soil Washing Solutions on Simultaneous Removal of Heavy Metals and Arsenic from Contaminated Soil. International Journal of Environmental Research and Public Health, 17(9), 3133. https://doi.org/10.3390/ijerph17093133




