High Prevalence of Gestational Diabetes Mellitus in Rural Tanzania—Diagnosis Mainly Based on Fasting Blood Glucose from Oral Glucose Tolerance Test
Abstract
1. Introduction
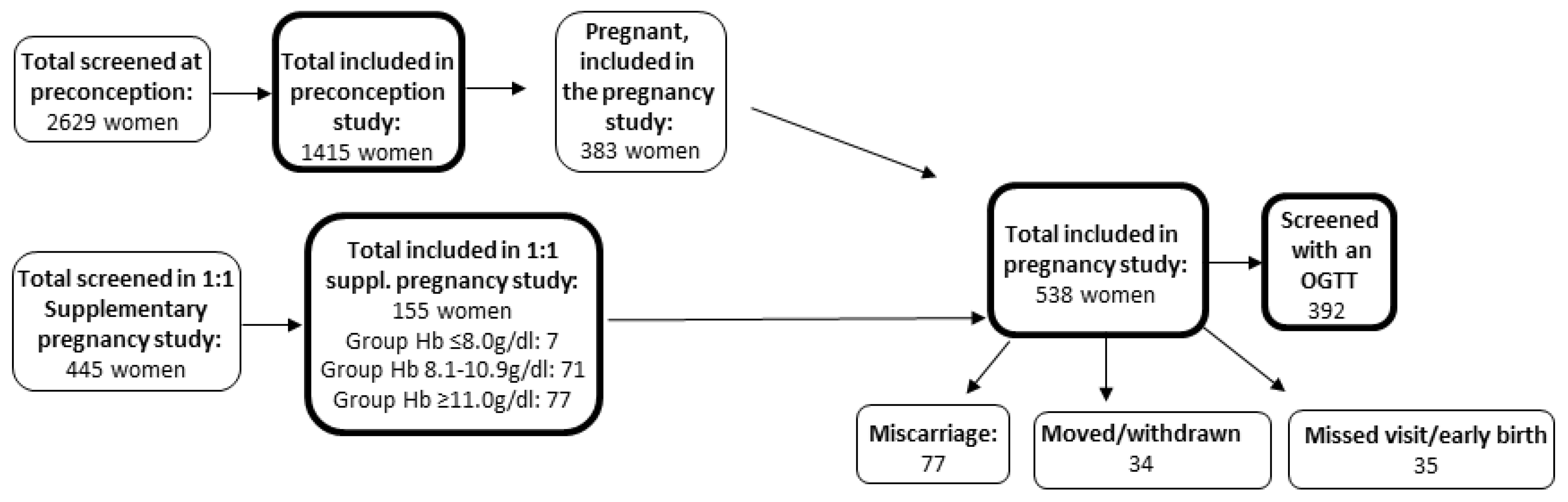
2. Subjects
3. Materials and Methods
4. Statistics and Ethics
5. Results
6. Discussion
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Drivsholm, T.; Ibsen, H.; Schroll, M.; Davidsen, M.; Borch-Johnsen, K. Increasing prevalence of diabetes mellitus and impaired glucose tolerance among 60-year-old Danes. Diabetic Med. 2001, 18, 126–132. [Google Scholar] [CrossRef]
- Grunnet, L.G.; Hansen, S.; Hjort, L.; Madsen, C.M.; Kampmann, F.B.; Thuesen AC, B.; Damm, P. Adiposity, dysmetabolic traits, and earlier onset of female puberty in adolescent offspring of women with gestational diabetes mellitus: A clinical study within the Danish national birth cohort. Diabetes Care 2017, 40, 1746–1755. [Google Scholar] [CrossRef]
- Kim, C.; Newton, K.M.; Knopp, R.H. Gestational diabetes and the incidence of type 2 diabetes: A systematic review. Diabetes Care 2002, 25, 1862–1868. [Google Scholar] [CrossRef]
- Pastakia, S.D.; Njuguna, B.; Ajwang’Onyango, B.; Washington, S.; Christoffersen-Deb, A.; Kosgei, W.K.; Saravanan, P. Prevalence of gestational diabetes mellitus based on various screening strategies in western Kenya: A prospective comparison of point of care diagnostic methods. BMC Pregnancy Childbirth 2017, 17, 226. [Google Scholar] [CrossRef]
- Mwanri, A.W.; Kinabo, J.; Ramaiya, K.; Feskens, E.J.M. Gestational diabetes mellitus in sub-Saharan Africa: Systematic review and metaregression on prevalence and risk factors. Trop. Med. Int. Heal. 2015, 20, 983–1002. [Google Scholar] [CrossRef]
- Muche, A.A.; Olayemi, O.O.; Gete, Y.K. Prevalence and determinants of gestational diabetes mellitus in Africa based on the updated international diagnostic criteria: A systematic review and meta-analysis. Arch. Pub. Heal. 2019, 77, 36. [Google Scholar] [CrossRef]
- Swai AB, M.; Kitange, H.M.; McLarty, D.G.; Kilima, P.M.; Masuki, G.; Mtinangi, B.L.; Alberti, K.G.M.M. No Deterioration of Oral Glucose Tolerance During Pregnancy in Rural Tanzania. Diabetes Med. 1991, 8, 254–257. [Google Scholar] [CrossRef]
- Njete, H.I.; John, B.; Mlay, P.; Mahande, M.J.; Msuya, S.E. Prevalence, predictors and challenges of gestational diabetes mellitus screening among pregnant women in northern Tanzania. Trop. Med. Int. Health 2018, 23, 236–242. [Google Scholar] [CrossRef]
- Mwanri, A.W.; Kinabo, J.; Ramaiya, K.; Feskens, E.J.M. Prevalence of gestational diabetes mellitus in urban and rural Tanzania. Diabetes Res. Clin. Pract. 2014, 103, 71–78. [Google Scholar] [CrossRef]
- Zhang, C.; Rawal, S. Dietary iron intake, iron status, and gestational diabetes. Am. J. Clin. Nutr. 2017, 106, 1672S–1680S. [Google Scholar] [CrossRef]
- Global, Regional, and National Trends in Haemoglobin Concentration and Prevalence of Total and Severe Anaemia in Children and Pregnant and Non-Pregnant Women for 1995–2011: A Systematic Analysis of Population-Representative Data-ClinicalKey. Available online: https://www-clinicalkey-com.ep.fjernadgang.kb.dk/#!/content/playContent/1-s2.0-S2214109X13700019?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS2214109X13700019%3Fshowall%3Dtrue&referrer=https:%2F%2Ffs.regionh.dk%2Fadfs%2Fls%2F%3FSAMLRe (accessed on 28 April 2020).
- World Health Organisation. The Global Prevalence of Anaemia in 2011; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Namaste, S.M.; Rohner, F.; Huang, J.; Bhushan, N.L.; Flores-Ayala, R.; Kupka, R.; Northrop-Clewes, C.A. Adjusting ferritin concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2017, 106 (Suppl. 1), 359S–371S. [Google Scholar] [CrossRef] [PubMed]
- Hjort, L.; Møller, S.L.; Minja, D.; Msemo, O.; Nielsen, B.B.; Christensen, D.L.; Groop, L. Cohort profile: FOETAL for NCD—Foetal exposure and Epidemiological Transition: The role of Anemia in early Life for Non-Communicable Diseases in later life—A prospective preconception study in rural Tanzania. BMJ Open 2019, 9, e024861. [Google Scholar] [CrossRef] [PubMed]
- Papageorghiou, A.T.; Kennedy, S.H.; Salomon, L.J.; Ohuma, E.O.; Cheikh Ismail, L.; Barros, F.C.; Gravett, M.G. International standards for early fetal size and pregnancy dating based on ultrasound measurement of crown-rump length in the first trimester of pregnancy. Ultrasound Obs. Gynecol. 2014, 44, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Bricker, L. Re: Ultrasound-based gestational-age estimation in late pregnancy. A. T. Papageorghiou, B. Kemp, W. Stones, E. O. Ohuma, S. H. Kennedy, M. Purwar, L. J. Salomon, D. G. Altman, J. A. Noble, E. Bertino, M. G. Gravett, R. Pang, L. Cheikh Ismail, F. C. Barros, A. Lambert, Y. A. Jaffer, C. G. Victora, Z. A. Bhutta and J. Villar, for the International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st). Ultrasound Obstet. Gynecol. 2016, 48, 693. [Google Scholar] [CrossRef] [PubMed]
- Msemo, O.A.; Schmiegelow, C.; Nielsen, B.B.; Kousholt, H.; Grunnet, L.G.; Christensen, D.L.; Bygbjerg, I.C. Risk factors of pre-hypertension and hypertension among non-pregnant women of reproductive age in northeastern Tanzania: A community based cross-sectional study. Trop. Med. Int. Health 2018, 23, 1176–1187. [Google Scholar] [CrossRef]
- Rajadhyaksha, A.; Rodriguez, M.; Nichols, J.H. Evaluation of the HemoCue Glucose 201 Room Temperature Microcuvettes. J. Near-Patient Test. Technol 2008, 7, 12–15. [Google Scholar] [CrossRef]
- Fogh-Andersen, N.; D’Orazio, P.; Kuwa, K.; Külpmann, W.R.; Mager, G.; Larsson, L. Recommendation on Reporting Results for Blood Glucose (From an IFCC Stage 1 Document) IFCC Scientific Division Working Group on Selective Electrodes. EJIFCC 2000, 12, 114–116. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30479594 (accessed on 28 April 2020).
- Colagiuri, S.; Sandbaek, A.; Carstensen, B.; Christensen, J.; Glumer, C.; Lauritzen, T.; Borch-Johnsen, K. Comparability of venous and capillary glucose measurements in blood. Diabetes Med. 2003, 20, 953–956. [Google Scholar] [CrossRef]
- Jamieson, E.L.; Spry, E.P.; Kirke, A.B.; Atkinson, D.N.; Marley, J.V. Real-World Gestational Diabetes Screening: Problems with the Oral Glucose Tolerance Test in Rural and Remote Australia. Int. J. Environ. Res. Public Health 2019, 16, 4488. [Google Scholar] [CrossRef]
- Wilson, E.B. Probable inference, the law of succession, and statistical inference. J. Am. Stat. Assoc. 1927, 22, 209–212. [Google Scholar] [CrossRef]
- Schindhelm, R.K.; Diamant, M.; Bakker, S.J.; Van Dijk, R.A.; Scheffer, P.G.; Teerlink, T.; Heine, R.J. Liver alanine aminotransferase, insulin resistance and endothelial dysfunction in normotriglyceridaemic subjects with type 2 diabetes mellitus. Eur. J. Clin. Investig. 2005, 35, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Takei, R.; Inoue, T.; Sonoda, N.; Kohjima, M.; Okamoto, M.; Sakamoto, R.; Ogawa, Y. Bilirubin reduces visceral obesity and insulin resistance by suppression of inflammatory cytokines. PLoS ONE 2019, 14, e0223302. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, H.D.; Jensen, D.M.; Jensen, R.C.; Kyhl, H.B.; Jensen, T.K.; Glintborg, D.; Andersen, M. Gestational Diabetes Mellitus: Does One Size Fit All? A Challenge to Uniform Worldwide Diagnostic Thresholds. Diabetes Care 2018, 41, 1339–1342. [Google Scholar] [CrossRef] [PubMed]
- Arora, G.P.; Thaman, R.G.; Prasad, R.B.; Almgren, P.; Brøns, C.; Groop, L.C.; Vaag, A.A. Prevalence and risk factors of gestational diabetes in Punjab, North India: Results from a population screening program. Eur. J. Endocrinol. 2015, 173, 257–267. [Google Scholar] [CrossRef]
- Sacks, D.A.; Hadden, D.R.; Maresh, M.; Deerochanawong, C.; Dyer, A.R.; Metzger, B.E.; Persson, B. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Diabetes Care 2012, 35, 526–528. [Google Scholar] [CrossRef]
- Olagbuji, B.N.; Atiba, A.S.; Olofinbiyi, B.A.; Akintayo, A.A.; Awoleke, J.O.; Ade-Ojo, I.P.; Group-Nigeria, G.D.S. Prevalence of and risk factors for gestational diabetes using 1999, 2013 WHO and IADPSG criteria upon implementation of a universal one-step screening and diagnostic strategy in a sub-Saharan African population. Eur J. Obs. Gynecol. Reprod. Biol. 2015, 189, 27–32. [Google Scholar] [CrossRef]
- Jensen, D.M.; Mølsted-Pedersen, L.; Beck-Nielsen, H.; Westergaard, J.G.; Ovesen, P.; Damm, P. Screening for gestational diabetes mellitus by a model based on risk indicators: A prospective study. Am. J. Obstet. Gynecol. 2003, 189, 1383–1388. Available online: http://www.ncbi.nlm.nih.gov/pubmed/14634573 (accessed on 28 April 2020).
- Macaulay, S.; Ngobeni, M.; Dunger, D.B.; Norris, S.A. The prevalence of gestational diabetes mellitus amongst black South African women is a public health concern. Diabetes Res. Clin. Pract. 2018, 139, 278–287. [Google Scholar] [CrossRef]
- Tiongco, R.E.; Arceo, E.; Clemente, B.; Ruth Pineda-Cortel, M. Association of maternal iron deficiency anemia with the risk of gestational diabetes mellitus: A meta-analysis. Arch. Gynecol. Obstet. 2019, 299, 89–95. [Google Scholar] [CrossRef]
- Hurrle, S.; Hsu, W.H. The etiology of oxidative stress in insulin resistance. Biomed. J. 2017, 40, 257–262. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, L.; Tan, Y.; Wang, G.; Lin, X.; Cai, L. Role of iron deficiency and overload in the pathogenesis of diabetes and diabetic complications. Curr. Med. Chem. 2009, 16, 113–129. Available online: http://www.ncbi.nlm.nih.gov/pubmed/19149565 (accessed on 28 April 2020). [CrossRef]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Prim. 2019, 5, 47. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019; Available online: https://www.diabetesatlas.org (accessed on 28 April 2020).
- Thurnham, D.I.; McCabe, L.D.; Haldar, S.; Wieringa, F.T.; Northrop-Clewes, C.A.; McCabe, G.P. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: A meta-analysis. Am. J. Clin. Nutr. 2010, 92, 546–555. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, E.G.; Reynolds, C.M.E.; O’Kelly, R.; Killalea, A.; Sheehan, S.R.; Turner, M.J. A Prospective Evaluation of Point-of-Care Measurements of Maternal Glucose for the Diagnosis of Gestational Diabetes Mellitus. Clin. Chem. 2020, 66, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Schmiegelow, C.; Minja, D.; Oesterholt, M.; Pehrson, C.; Suhrs, H.E.; Boström, S.; Lusingu, J. Malaria and Fetal Growth Alterations in the 3rd Trimester of Pregnancy: A Longitudinal Ultrasound Study. PLoS ONE 2013, 8, e53794. [Google Scholar] [CrossRef] [PubMed]
| GDM Cases | Controls | p-Value | |
|---|---|---|---|
| n = 153 | n = 239 | ||
| Age (years) * | 27 (22;35) | 26 (22;33) | 0.07 |
| Parity (n: nulliparity/1 child/≥2 children) # | 15/34/104 | 38/46/155 | 0.21 |
| Ethnicity (n (%)) # | |||
| Sambaa | 61 (39.9) | 83 (34.7) | |
| Zigua | 47 (30.7) | 81 (33.9) | |
| Pare | 11 (7.2) | 15 (6.3) | |
| Bondei | 2 (1.3) | 8 (3.3) | |
| Other | 32 (20.9) | 52 (21.8) | 0.65 |
| Education (n (%)) # | |||
| None | 11 (7.2) | 24 (10.0) | |
| Incomplete primary | 23 (15.0) | 32 (13.4) | |
| Complete primary | 103 (67.3) | 161 (67.4) | |
| Secondary or higher | 16 (10.5) | 22 (9.2) | 0.76 |
| Source of domestic water (n (%)) # | |||
| Tap | 95 (62.1) | 129 (54.0) | |
| Well | 22 (14.4) | 37 (15.5) | |
| River | 33 (21.5) | 65 (27.2) | |
| Pond/pool | 3 (2.0) | 8 (3.3) | 0.42 |
| Type of toilet facility (n (%)) # | |||
| Flush | 46 (30.3) | 61 (25.5) | |
| Pit | 105 (69.1) | 175 (73.2) | |
| None | 1 (0.6) | 3 (1.3) | 0.55 |
| Family history of diabetes | 12 (8.7) | 19 (9.0) | 0.93 |
| BMI at inclusion (kg/m2) * | 23.4 (20.9;26.9) | 22.7 (20.5;25.6) | 0.14 |
| MUAC at inclusion (cm) * | 27.5 (25.9;30.8) | 27.5 (25.1;30.2) | 0.27 |
| Fasting glucose (OGTT) (mmol/L) | 5.5 (0.6) | 4.4 (0.4) | - |
| 1-h glucose (OGTT) (mmol/L) | 7.5 (1.4) | 6.6 (1.1) | - |
| 2-h glucose (OGTT) (mmol/L) | 6.8 (1.1) | 6.3 (0.9) | - |
| Systolic BP (mmHg) | 103.9 (9.97) | 104.4 (11.75) | 0.64 |
| Diastolic BP (mmHg) | 66.1 (8.0) | 66.3 (9.3) | 0.84 |
| Pulse (beats per min.) | 92.2 (12.1) | 90.8 (11.2) | 0.25 |
| Hb (g/dL) | 10.63 (1.32) | 10.72 (1.27) | 0.49 |
| Albumin (g/dL) | 28.0 (2.15) | 27.72 (2.37) | 0.29 |
| Ferritin (ng/mL) * | 9.3 (6.0;17.5) | 8.5 (6.2;12) | 0.19 |
| CRP (mg/L) * | 5.0 (5.0;5.0) | 5.0 (5.0;5.0) | 0.65 |
| ALAT (U/L) * | 13.9 (11.4;17.4) | 14.0 (11.8;16.9) | 0.90 |
| Vitamin B12 (pmol/L) * | 277 (216;370) | 274 (205;340) | 0.28 |
| Folic acid (nmol/L) * | 24.7 (16.6;35.6) | 25.4 (16.1;38.4) | 0.61 |
| Bilirubin (µmol/L) * | 6.0 (4.6;9.1) | 5.7 (4.3;8.4) | 0.28 |
| Malaria | 10 (6.5) | 12 (5.1) | 0.65 |
| HIV seropositive | 5 (3.3) | 4 (1.7) | 0.14 |
| Gestational age at birth (weeks+days) | 39+6 (1+4) | 40+0 (1+4) | 0.26 |
| Birth weight (g) | 3012 (539) | 3014 (455) | 0.97 |
| GDM Diagnosis Based upon: | n | % (95%-CI) |
|---|---|---|
| Fasting sample | 144 | 94.1 (94.0;94.2) |
| 2-h OGTT | 5 | 3.3 (3.2;3.4) |
| Fasting sample and 1-h OGTT | 3 | 2.0 (1.9;2.0) |
| Fasting sample and 1-h OGTT and 2-h OGTT | 1 | 0.65 (0.61;0.70) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grunnet, L.G.; Hjort, L.; Minja, D.T.; Msemo, O.A.; Møller, S.L.; Prasad, R.B.; Groop, L.; Lusingu, J.; Nielsen, B.B.; Schmiegelow, C.; et al. High Prevalence of Gestational Diabetes Mellitus in Rural Tanzania—Diagnosis Mainly Based on Fasting Blood Glucose from Oral Glucose Tolerance Test. Int. J. Environ. Res. Public Health 2020, 17, 3109. https://doi.org/10.3390/ijerph17093109
Grunnet LG, Hjort L, Minja DT, Msemo OA, Møller SL, Prasad RB, Groop L, Lusingu J, Nielsen BB, Schmiegelow C, et al. High Prevalence of Gestational Diabetes Mellitus in Rural Tanzania—Diagnosis Mainly Based on Fasting Blood Glucose from Oral Glucose Tolerance Test. International Journal of Environmental Research and Public Health. 2020; 17(9):3109. https://doi.org/10.3390/ijerph17093109
Chicago/Turabian StyleGrunnet, Louise Groth, Line Hjort, Daniel Thomas Minja, Omari Abdul Msemo, Sofie Lykke Møller, Rashmi B. Prasad, Leif Groop, John Lusingu, Birgitte Bruun Nielsen, Christentze Schmiegelow, and et al. 2020. "High Prevalence of Gestational Diabetes Mellitus in Rural Tanzania—Diagnosis Mainly Based on Fasting Blood Glucose from Oral Glucose Tolerance Test" International Journal of Environmental Research and Public Health 17, no. 9: 3109. https://doi.org/10.3390/ijerph17093109
APA StyleGrunnet, L. G., Hjort, L., Minja, D. T., Msemo, O. A., Møller, S. L., Prasad, R. B., Groop, L., Lusingu, J., Nielsen, B. B., Schmiegelow, C., Bygbjerg, I. C., & Christensen, D. L. (2020). High Prevalence of Gestational Diabetes Mellitus in Rural Tanzania—Diagnosis Mainly Based on Fasting Blood Glucose from Oral Glucose Tolerance Test. International Journal of Environmental Research and Public Health, 17(9), 3109. https://doi.org/10.3390/ijerph17093109





