A Qualitative Evaluation of Adverse Drug Reaction Reporting System in Pakistan: Findings from the Nurses’ Perspective
Abstract
1. Introduction
2. Methods
2.1. Ethical Approval
2.2. Study Design
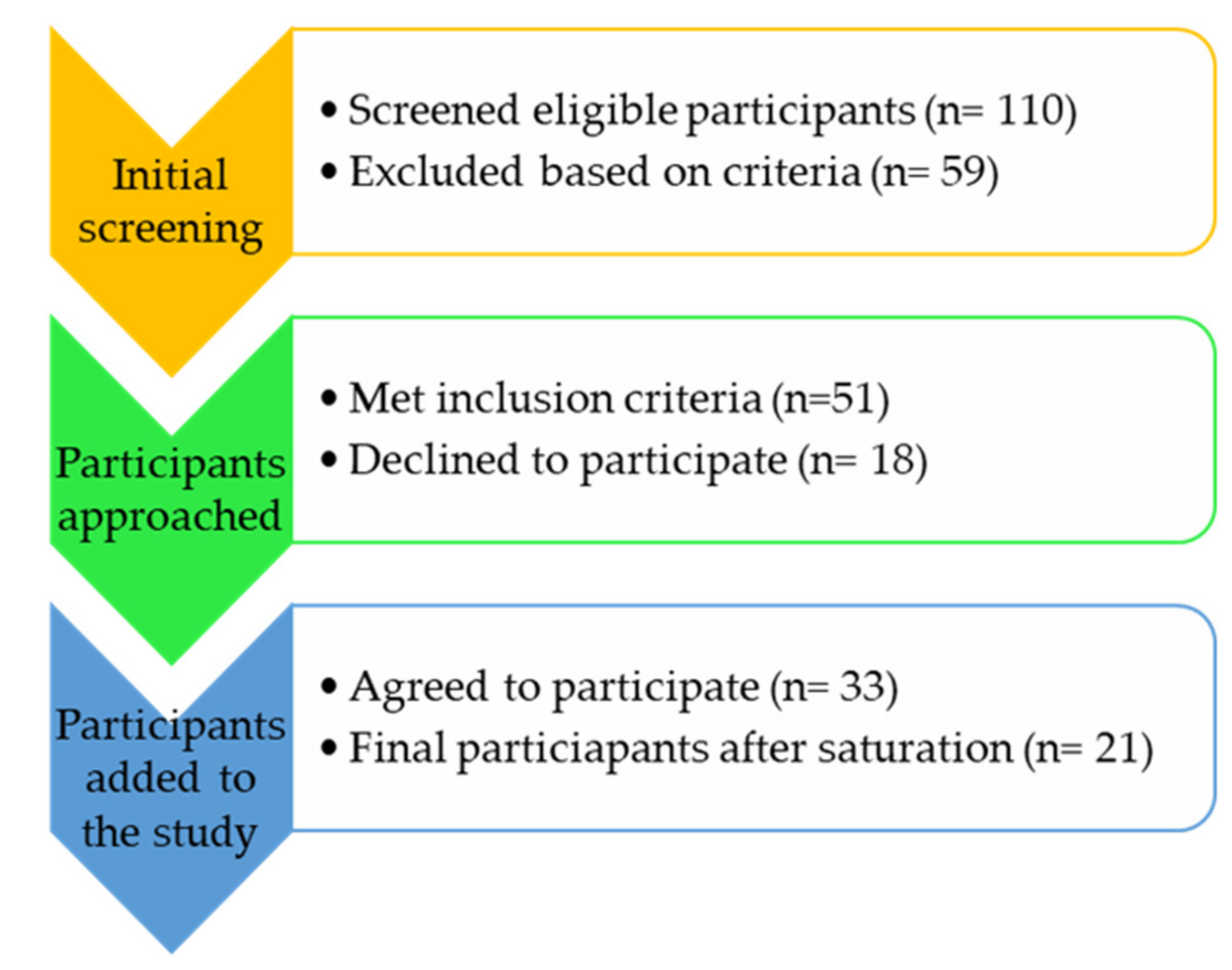
2.3. Study Sampling
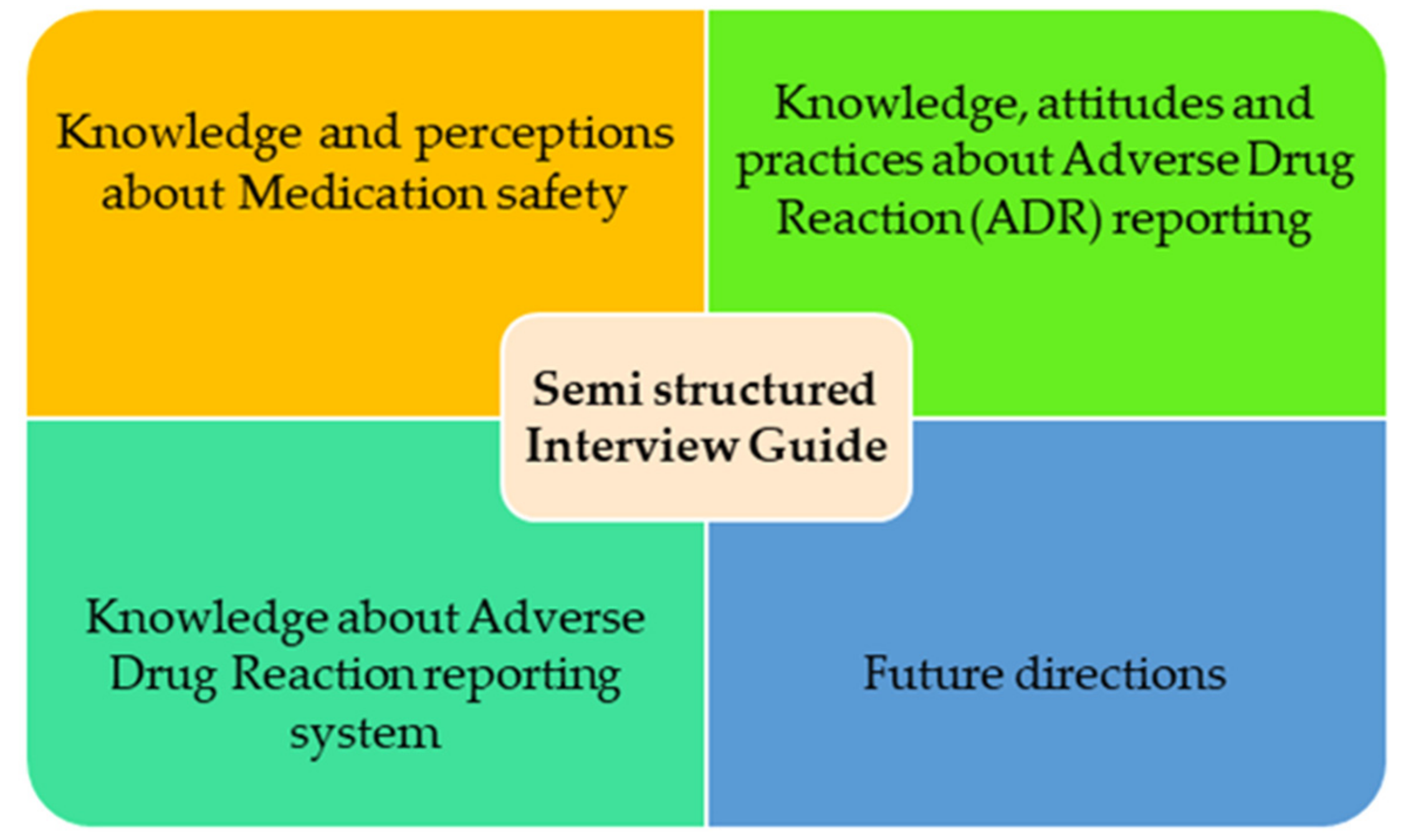
2.4. Data Collection
2.5. Data Analysis
2.6. Reporting
3. Results
3.1. Demographic Details of the Participants
3.2. Thematic Analysis of the Content
3.2.1. Theme 1: Knowledge about the Concept of the Medication Safety & the ADR
Subtheme 1: Knowledge about the Definition of Medicine Safety and ADR
“We have five rights of patients, right route, right drug, time, patient, and administration. According to these rules, patient safety can be ensured.”(N1)
“Medication safety means medicine free of harm.”(N2)
“Medicine safe from all possible side effects.”(N13)
“I know adverse drug reaction, its deviated from the pre-mentioned side effects and they are not expected as such and happen at normal doses.”(N6)
“ADR is a drug reaction which can endanger the life of a patient at doses which are normally given to the patients to treat.”(N19)
Subtheme 2: Perceptions towards Types of ADR Need to be Reported
“I think every type of reaction should be reported. because my personal experience says that we need to be careful and should not ignore even the minor one.”(N3)
“Major one should be reported, because minor can be tackled but those which cannot be managed, obviously should be reported.”(N11)
3.2.2. Theme 2: Knowledge Regarding Pharmacovigilance Activities
“Not at all, no basic guidance means such responsibility have not been assigned to us, we have not been taught this way, we are just supposed to inform the doctors. There is no such activity in my hospital.”(N11)
“I will tell this to my senior nurse, and she will convey this to the doctor. The rest, I don’t know.”(N15)
3.2.3. Theme 3: Willingness to Report
“For me, it’s easy, but due to burden and lack of a system, I cannot report, otherwise its easy and once it’s part of duty.”(N3)
“Well, simply, its motivation to do something new, to implement and to follow something important as it will improve my practice.”(N10)
“I am a competent authority, I have knowledge. I know that my voice will be heard, I have experience and I have a duty to report, so I think it’s very easy for me to report an ADR.”(N17)
3.2.4. Theme 4: Practices Related to ADR Reporting
“Yes, we have basic guidance, in that if a reaction happens, we have to inform the pharmacist by filling the ADR form. So, basic guidance we do have. The rest, we do not know.”(N2)
“I did not have any, because I never saw any system here to help us in reporting. So, we discuss this verbally. We report to doctors and pharmacists verbally. If they need anything written, we provide them. But usually, we inform this to pharmacist.”(N5)
“I just wrote with red pen and resume my whole scenario with blue pen. Actually, I have a responsibility just to document, because nobody tells how to highlight it.”(N17)
“I have seen one in my training. We report to doctor but do not report in a systematic manner.”(N21)
3.2.5. Theme 5: Barriers to the ADR Reporting
Subtheme 1: Lack of Time to Report an ADR
“Here, we have lot of patients, so the main problem is patient load. The willingness is there, and once a system is established, then we can do the reporting.”(N3)
“For me, it’s easy, but due to the burden and lack of a system, I cannot report. Otherwise, its easy and once it’s part of duty, everybody will follow that.”(N19)
“Most importantly, the workload is too much for us. This is a major burden. More patients, fewer staff.”(N21)
Subtheme 2: Lack of a Proper Reporting System for the ADRs
“Here, we do not have system, but if a system is here, then we can report. It’s our professional obligation, to save the patient from the adverse drug reaction.”(N13)
“I think system is the main reason, we do not have system. These systems should be there and implemented from top management, SOPs should be there, and every department should be informed about the reporting guidelines, and the reporting should involve healthcare professionals and patient or their caregivers. Lack of training is not as such, if there is no system, there’s no training.”(N16)
Subtheme 3: Legal Liability
“No, legal liability is not there, until we did administer the right drug.”(N4)
“Well, reporting saves us from legal consequences. Rather, it sometimes helps us to see the mistakes in administration as we attended, noted, and mentioned the things. So, it’s a good thing rather.”(N7)
“Legal consequences matter as they will affect your job.”(N19)
3.2.6. Theme 6: Facilitators to the ADR Reporting
Subtheme 1: Incentives
“Nurses do not go for monetary incentives. It depends upon awareness only.”(N6)
“Yes, job incentives will also motivate, but if the system is proper, genuine reporting will be there.”(N14)
Subtheme 2: The Need of an Online System
“It should. Medication should be online. A paper system is very unethical. I’ve never gone for it. But, unfortunately, we only have papers system. Electronic medication report (EMR) has been used and it has its own limitation.”(N7)
“A possible suggestion is that there must be a system for ADR reporting even at minor level.”(N18)
Subtheme 3: Availability of Pharmacist
“I think clinical pharmacist should be employed, so we need them in wards to work with us. If they are there, then we will not be confused, and we can ask them if we experience any problem.”(N4)
“We need good pharmacist, so that they can train us.”(N11)
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Pharmacovigilance: Ensuring the Safe Use of Medicines; WHO: Geneva, Switzerland, 2014; p. 6. [Google Scholar]
- United States Agency for International Development. Supporting Pharmacovigilance in Developing Countries: The Systems Perspective; USAID: Arlington, VA, USA, 2009. [Google Scholar]
- Praus, M.; Schindel, F.; Fescharek, R.; Schwarz, S. Alert systems for post-marketing surveillance of adverse drug reactions. Stat. Med. 1993, 12, 2383–2393. [Google Scholar] [CrossRef] [PubMed]
- Wadivkar, P.; Zad, V.; Vakharia, M.; Shah, K. Assessment of knowledge of pharmacovigilance among the nurses of tertiary care hospital. Int. J. Basic Clin. Pharmacol. 2015, 4, 1194–1197. [Google Scholar] [CrossRef]
- Food and Drug Administration. Good Pharmacovigilance Practices and Pharmacoepidemiologic Assessment; FDA: Washington, DC, USA, 2005. [Google Scholar]
- Morrison-Griffiths, S.; Walley, T.J.; Park, B.K.; Breckenridge, A.M.; Pirmohamed, M. Reporting of adverse drug reactions by nurses. Lancet 2003, 361, 1347–1348. [Google Scholar] [CrossRef]
- Bäckström, M.; Ekman, E.; Mjörndal, T. Adverse drug reaction reporting by nurses in Sweden. Eur. J. Clin. Pharmacol. 2007, 63, 613–618. [Google Scholar] [CrossRef]
- Hanafi, S.; Torkamandi, H.; Hayatshahi, A.; Gholami, K.; Shahmirzadi, N.A.; Javadi, M.R. An educational intervention to improve nurses’ knowledge, attitude, and practice toward reporting of adverse drug reactions. Iran. J. Nurs. Midwifery Res. 2014, 19, 101. [Google Scholar]
- Gul, R. The image of nursing from nurses’ and non-nurses’ perspective in Pakistan. Silent Voice 2008, 1, 4–17. [Google Scholar]
- World Bank. Data, Land Area sq.km. Available online: https://data.worldbank.org/indicator/AG.LND.TOTL.K2 (accessed on 30 September 2019).
- World Health Organization. Primary Health Care Systems (PRIMASYS): Comprehensive Case Study from Pakistan; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Pakistan Nursing Council. What is Pakistan Nursing Council (PNC)? Available online: https://www.pnc.org.pk/ (accessed on 30 January 2020).
- Upvall, M.J.; Karmaliani, R.; Pirani, F.; Gul, R.; Khalid, F. Developing nursing leaders through graduate education in Pakistan. Int. J. Nurs. Educ. Scholarsh. 2004, 1. [Google Scholar] [CrossRef]
- Parveen, S. Acute Shortage of Nursing Professional in Pakistan. Texila Int. J. Nurs. 2016, 2, 1–6. [Google Scholar] [CrossRef]
- Bryant, N.H. Women in Nursing in Islamic Societies; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Arabi, A.; Rafii, F.; Cheraghi, M.A.; Ghiyasvandian, S. Nurses’ policy influence: A concept analysis. Iranian J. Nurs. Midwifery Res. 2014, 19, 315. [Google Scholar]
- Yilmaz, K. Comparison of quantitative and qualitative research traditions: Epistemological, theoretical, and methodological differences. Eur. J. Educ. 2013, 48, 311–325. [Google Scholar] [CrossRef]
- Punch, K.F. Introduction to Social Research: Quantitative and Qualitative Approaches; Sage: Los Angeles, CA, USA, 2013. [Google Scholar]
- Saunders, B.; Sim, J.; Kingstone, T.; Baker, S.; Waterfield, J.; Bartlam, B.; Burroughs, H.; Jinks, C. Saturation in qualitative research: Exploring its conceptualization and operationalization. Qual. Quant. 2018, 52, 1893–1907. [Google Scholar] [CrossRef] [PubMed]
- Morse, J.M. Data Were Saturated; Sage Publications Sage CA: Los Angeles, CA, USA, 2015. [Google Scholar]
- Hussain, R.; Hassali, M.A.; Hashmi, F.; Farooqui, M. A qualitative exploration of knowledge, attitudes and practices of hospital pharmacists towards adverse drug reaction reporting system in Lahore, Pakistan. J. Pharm. Policy Pract. 2018, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Cresswell, J.; Plano Clark, V. Designing and Conducting Mixed Method Research, 2nd ed.; Sage: Thousand Oaks, CA, USA, 2011; p. 201. [Google Scholar]
- Patton, M. Qualitative Research: Wiley Online Library; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Charmaz, K.; Belgrave, L. Qualitative interviewing and grounded theory analysis. In The SAGE Handbook of Interview Research: The Complexity of the Craft; Sage: Los Angeles, CA, USA, 2012; Volume 2, pp. 347–365. [Google Scholar]
- Conforti, A.; Opri, S.; D’Incau, P.; Sottosanti, L.; Moretti, U.; Ferrazin, F.; Leone, R. Adverse drug reaction reporting by nurses: Analysis of Italian pharmacovigilance database. Pharmacoepidemiol. Drug Saf. 2012, 21, 597–602. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, A.; Giusti, A.; Colaceci, S.; Vellone, E.; Alvaro, R. Nurses’ reporting of suspect adverse drug reactions: A mixed-methods study. Ann. Dell’istituto Super. Sanita 2015, 51, 277–283. [Google Scholar]
- Eisenhauer, L.A.; Hurley, A.C.; Dolan, N. Nurses’ reported thinking during medication administration. J. Nurs. Scholarsh. 2007, 39, 82–87. [Google Scholar] [CrossRef]
- Hajebi, G.; Mortazavi, S.A.; Salamzadeh, J.; Zian, A. A survey of knowledge, attitude and practice of nurses towards pharamacovigilance in Taleqani Hospital. Iran. J. Pharm. Res. 2010, 9, 199. [Google Scholar]
- Hamedivafa, F.P. A survey of knowledge, attitude and practice of nurses towards pharmacovigilance in teaching hospital, Qazvin-Iran. Res. Pharm. Sci. 2012, 7, S863. [Google Scholar]
- Johansson-Pajala, R.-M.; Jorsäter Blomgren, K.; Bastholm-Rahmner, P.; Fastbom, J.; Martin, L. Nurses in municipal care of the elderly act as pharmacovigilant intermediaries: A qualitative study of medication management. Scand. J. Prim. Health Care 2016, 34, 37–45. [Google Scholar] [CrossRef][Green Version]
- Mendes Marques, J.I.O.; Polónia, J.M.J.; Figueiras, A.G.; Costa Santos, C.M.N.; Herdeiro, M.T.F. Nurses’ attitudes and spontaneous adverse drug reaction reporting: A case–control study in Portugal. J. Nurs. Manag. 2016, 24, 409–416. [Google Scholar] [CrossRef]
- Rajalakshmi, R.; Devi, B.V.; Prasad, T.D.; Swetha, S.; Dharini, B. Knowledge, attitude and practice towards pharmacovigilance and adverse drug reaction reporting among nurses in a tertiary care hospital, tirupati. Int. J. Pharm. Clin. Res. 2017, 9, 683–689. [Google Scholar]
- Vural, F.; Çiftçi, S.; Vural, B. The knowledge, attitude and behaviours of nurses about pharmacovigilance, adverse drug reaction and adverse event reporting in a state hospital. North. Clin. Istanb. 2014, 1, 147. [Google Scholar] [CrossRef] [PubMed]
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef]
- Tong, A.; Sainsbury, P.; Craig, J. Consolidated criteria for reporting qualitative research (COREQ): A 32-item checklist for interviews and focus groups. Int. J. Qual. Health Care 2007, 19, 349–357. [Google Scholar] [CrossRef] [PubMed]
- World Bank. World Development Indicators database: Population 2018. Available online: https://databank.worldbank.org/data/download/POP.pdf (accessed on 30 September 2019).
- Choo, J.; Hutchinson, A.; Bucknall, T. Nurses’ role in medication safety. J. Nurs. Manag. 2010, 18, 853–861. [Google Scholar] [CrossRef]
- Huda, S.U.; Alisbinati, A.S.A. Nursing Education in Pakistan: Challenges and Trends in Degree Program. Int. J. Nurs. Educ. 2015, 7, 59–62. [Google Scholar] [CrossRef]
- Agha Khan University-SONAM. Post RN Bachelor of Science in Nursing (Two-Year) Programme. Available online: https://www.aku.edu/sonampk/programmes/Pages/postrn-bscn.aspx (accessed on 30 August 2019).
- Nilsson, K.; Lundgren, S.; Furåker, C. Registered nurses’ everyday activities in municipal health care: A study of diaries. Int. J. Nurs. Pract. 2009, 15, 543–552. [Google Scholar] [CrossRef]
- Thomson, M.S.; Gruneir, A.; Lee, M.; Baril, J.; Field, T.S.; Gurwitz, J.H.; Rochon, P.A. Nursing time devoted to medication administration in long-term care: Clinical, safety, and resource implications. J. Am. Geriatr. Soc. 2009, 57, 266–272. [Google Scholar] [CrossRef]
- Fadare, J.O.; Enwere, O.O.; Afolabi, A.; Chedi, B.; Musa, A. Knowledge, attitude and practice of adverse drug reaction reporting among healthcare workers in a tertiary centre in Northern Nigeria. Trop. J. Pharm. Res. 2011, 10, 235–242. [Google Scholar] [CrossRef]
- Hanafi, S.; Torkamandi, H.; Hayatshahi, A.; Gholami, K.; Javadi, M. Knowledge, attitudes and practice of nurse regarding adverse drug reaction reporting. Iran. J. Nurs. Midwifery Res. 2012, 17, 21. [Google Scholar]
- Ahsin, S.; Abbas, S.; Zaidi, N.; Azad, N.; Kaleem, F. Reciprocal benefit to senior and junior peers: An outcome of a pilot research workshop at medical university. J. Pak. Med Assoc. 2015, 65, 882–884. [Google Scholar]
- Beament, T.; Mercer, S. Speak up! Barriers to challenging erroneous decisions of seniors in anaesthesia. Anaesthesia 2016, 71, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Copping, C. Preventing and reporting drug administration errors. Nurs. Times 2005, 101, 32–34. [Google Scholar] [PubMed]
- Valente, S.; Murray, L.P. Creative strategies to improve patient safety: Allergies and adverse drug reactions. J. Nurses Prof. Dev. 2011, 27, E1–E5. [Google Scholar] [CrossRef] [PubMed]
- Drug Regulatory Authority of Pakistan. Pakistan National Pharmacovigilance Guidelines (Draft), 1st ed.; DRAP: Islamabad, Pakistan, 2019; p. 95. [Google Scholar]
- Dilles, T.; Elseviers, M.M.; Van Rompaey, B.; Van Bortel, L.M.; Stichele, R.R.V. Barriers for nurses to safe medication management in nursing homes. J. Nurs. Scholarsh. 2011, 43, 171–180. [Google Scholar] [CrossRef]
- Caplan, L.; Haverhals, L.M. Barriers and facilitators for preventing adverse drug reactions of long latency: A qualitative study. Int. J. Risk Saf. Med. 2012, 24, 81–94. [Google Scholar] [CrossRef]
- Jordan, S.; Vaismoradi, M.; Griffiths, P. Adverse Drug Reactions, Nursing and Policy: A Narrative Review. Ann. Nurs. Pract. 2016, 3, 1050. [Google Scholar]
- Ellis, W.; Kaasalainen, S.; Baxter, P.; Ploeg, J. Medication management for nurses working in long-term care. Can. J. Nurs. Res. 2012, 44, 128–149. [Google Scholar]
- World Health Organization. Primary Care Systems Profiles & Performance (PRIMASYS). Available online: https://www.who.int/alliance-hpsr/projects/AHPSR-Pakistan-061016.pdf (accessed on 15 April 2020).
- Agha Khan University. The Agha Khan University Hospitals. Available online: https://hospitals.aku.edu/Pages/default.aspx (accessed on 15 September 2019).
- Shaukat Khanum Memorial Cancer Hospital and Research Centre (SKMCH). Available online: https://shaukatkhanum.org.pk/ (accessed on 20 September 2019).
- Shamim, S.; Sharib, S.M.; Malhi, S.M.; Muntaha, S.-U.; Raza, H.; Ata, S.; Farooq, A.S.; Hussain, M. Adverse drug reactions (ADRS) reporting: Awareness and reasons of under-reporting among health care professionals, a challenge for pharmacists. Springer Plus 2016, 5, 1778. [Google Scholar] [CrossRef]
- Iffat, W.; Shakeel, S.; Rahim, N.; Anjum, F.; Nesar, S.; Ghayas, S. Pakistani physicians knowledge and attitude towards reporting adverse drug reactions. Afr. J. Pharm. Pharmacol. 2014, 8, 379–385. [Google Scholar]
- Nisa, Z.U.; Zafar, A.; Sher, F. Assessment of knowledge, attitude and practice of adverse drug reaction reporting among healthcare professionals in secondary and tertiary hospitals in the capital of Pakistan. Saudi Pharm. J. 2018, 26, 453–461. [Google Scholar] [CrossRef]
- Hussain, R.; Hassali, M.A. Current status and future prospects of pharmacovigilance in Pakistan. J. Pharm. Policy Pract. 2019, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Khademian, Z.; Nikandish, R. The effect of nurse empowerment educational program on patient safety culture: A randomized controlled trial. BMC Med Educ. 2018, 18, 158. [Google Scholar] [CrossRef] [PubMed]
- Lee, O. How to Create a Culture of Patient Safety. Available online: https://www.policymedical.com/how-to-create-a-culture-of-patient-safety/ (accessed on 15 January 2020).
- Sammer, C.E.; Lykens, K.; Singh, K.P.; Mains, D.A.; Lackan, N.A. What is patient safety culture? A review of the literature. J. Nurs. Scholarsh. 2010, 42, 156–165. [Google Scholar] [CrossRef]
- Sayre, M.M. Improving Collaboration and Patient Safety by Encouraging Nurses to Speak-up: Overcoming Personal and Organizational Obstacles through Self-Reflection and Collaboration; University of California: Los Angeles, CA, USA, 2010. [Google Scholar]
- Mardon, R.E.; Khanna, K.; Sorra, J.; Dyer, N.; Famolaro, T. Exploring relationships between hospital patient safety culture and adverse events. J. Patient Saf. 2010, 6, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, K.; You, L.-M.; Xiang, J.-G.; Hu, H.-G.; Zhang, L.-F.; Zheng, J.; Zhu, X.-W. The relationship between patient safety culture and adverse events: A questionnaire survey. Int. J. Nurs. Stud. 2014, 51, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Ulfvarson, J.; Mejyr, S.; Bergman, U. Nurses are increasingly involved in pharmacovigilance in Sweden. Pharmacoepidemiol. Drug Saf. 2007, 16, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Costello, J.L.; Torowicz, D.L.; Yeh, T.S. Effects of a pharmacist-led pediatrics medication safety team on medication-error reporting. Am. J. Health-Syst. Pharm. 2007, 64, 1422–1426. [Google Scholar] [CrossRef]
- Tong, E.; Roman, C.; Mitra, B.; Yip, G.; Gibbs, H.; Newnham, H.; Smit, D.; Galbraith, K.; Dooley, M. Partnered pharmacist charting on admission in the General Medical and Emergency Short-stay Unit—A cluster-randomised controlled trial in patients with complex medication regimens. J. Clin. Pharm. Ther. 2016, 41, 414–418. [Google Scholar] [CrossRef]
- De, J.O.G.; Castro-Alves, L.J.; Kendall, M.C.; McCarthy, R. Effectiveness of Pharmacist Intervention to Reduce Medication Errors and Health-Care Resources Utilization After Transitions of Care: A Meta-analysis of Randomized Controlled Trials. J. Patient Saf. 2017. [Google Scholar] [CrossRef]
- Kelly, M.; Kaye, K.I.; Davis, S.R.; Shenfield, G.M. Factors influencing adverse drug reaction reporting in New South Wales teaching hospitals. J. Pharm. Pract. Res. 2004, 34, 32–35. [Google Scholar] [CrossRef]
- Dilles, T.; Stichele, R.V.; Van Rompaey, B.; Van Bortel, L.; Elseviers, M. Nurses’ practices in pharmacotherapy and their association with educational level. J. Adv. Nurs. 2010, 66, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
| Phase of Analysis. | Tasks Completed | Research Team Member Involved |
|---|---|---|
| Phase 1: Data familiarization | Transcription, reading and re-reading of interview transcripts. | RH |
| Phase 2: Initial codes generation | Initial, open coding of entire data set | RH and MAH |
| Phase 3: Search for themes | Categorization of codes into potential themes | RH and MAH |
| Phase 4: Review of themes | Confirming themes—ensuring the internal homogeneity and external heterogeneity of themes. | RH, discussed with MAH. |
| Phase 5: Defining and naming themes | Further refinement of themes | RH, confirmed with MAH. |
| Phase 6: Report finalization | Production of the manuscript, selection of illustrative quotes | RH, reviewed by and discussed with MAH and ZB. |
| Characteristics | Frequency |
|---|---|
| Gender | |
| Male | 0 |
| Females | 21 |
| Age (Years) | |
| 20–30 | 12 |
| 31–40 | 9 |
| Education | |
| Basic nursing | 6 |
| Specialization | 15 |
| Experience (Years) | |
| 1–5 | 2 |
| 6–10 | 11 |
| >10 | 8 |
| ADR reporting | |
| Yes | 4 |
| No | 17 |
| Category | Themes and Subthemes |
|---|---|
| Knowledge |
|
| Attitudes |
|
| Practices |
|
| Barriers |
|
| Facilitators |
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, R.; Hassali, M.A.; ur Rehman, A.; Muneswarao, J.; Atif, M.; Babar, Z.-U.-D. A Qualitative Evaluation of Adverse Drug Reaction Reporting System in Pakistan: Findings from the Nurses’ Perspective. Int. J. Environ. Res. Public Health 2020, 17, 3039. https://doi.org/10.3390/ijerph17093039
Hussain R, Hassali MA, ur Rehman A, Muneswarao J, Atif M, Babar Z-U-D. A Qualitative Evaluation of Adverse Drug Reaction Reporting System in Pakistan: Findings from the Nurses’ Perspective. International Journal of Environmental Research and Public Health. 2020; 17(9):3039. https://doi.org/10.3390/ijerph17093039
Chicago/Turabian StyleHussain, Rabia, Mohamed Azmi Hassali, Anees ur Rehman, Jaya Muneswarao, Muhammad Atif, and Zaheer-Ud-Din Babar. 2020. "A Qualitative Evaluation of Adverse Drug Reaction Reporting System in Pakistan: Findings from the Nurses’ Perspective" International Journal of Environmental Research and Public Health 17, no. 9: 3039. https://doi.org/10.3390/ijerph17093039
APA StyleHussain, R., Hassali, M. A., ur Rehman, A., Muneswarao, J., Atif, M., & Babar, Z.-U.-D. (2020). A Qualitative Evaluation of Adverse Drug Reaction Reporting System in Pakistan: Findings from the Nurses’ Perspective. International Journal of Environmental Research and Public Health, 17(9), 3039. https://doi.org/10.3390/ijerph17093039







