Child Salivary SIgA and Its Relationship to Enteric Infections and EED Biomarkers in Maputo, Mozambique
Abstract
1. Background
2. Materials and Methods
2.1. Study Setting and Participants
2.2. Procedures
2.3. Statistical Analysis
2.4. Ethics
3. Results
3.1. Summary Characteristics
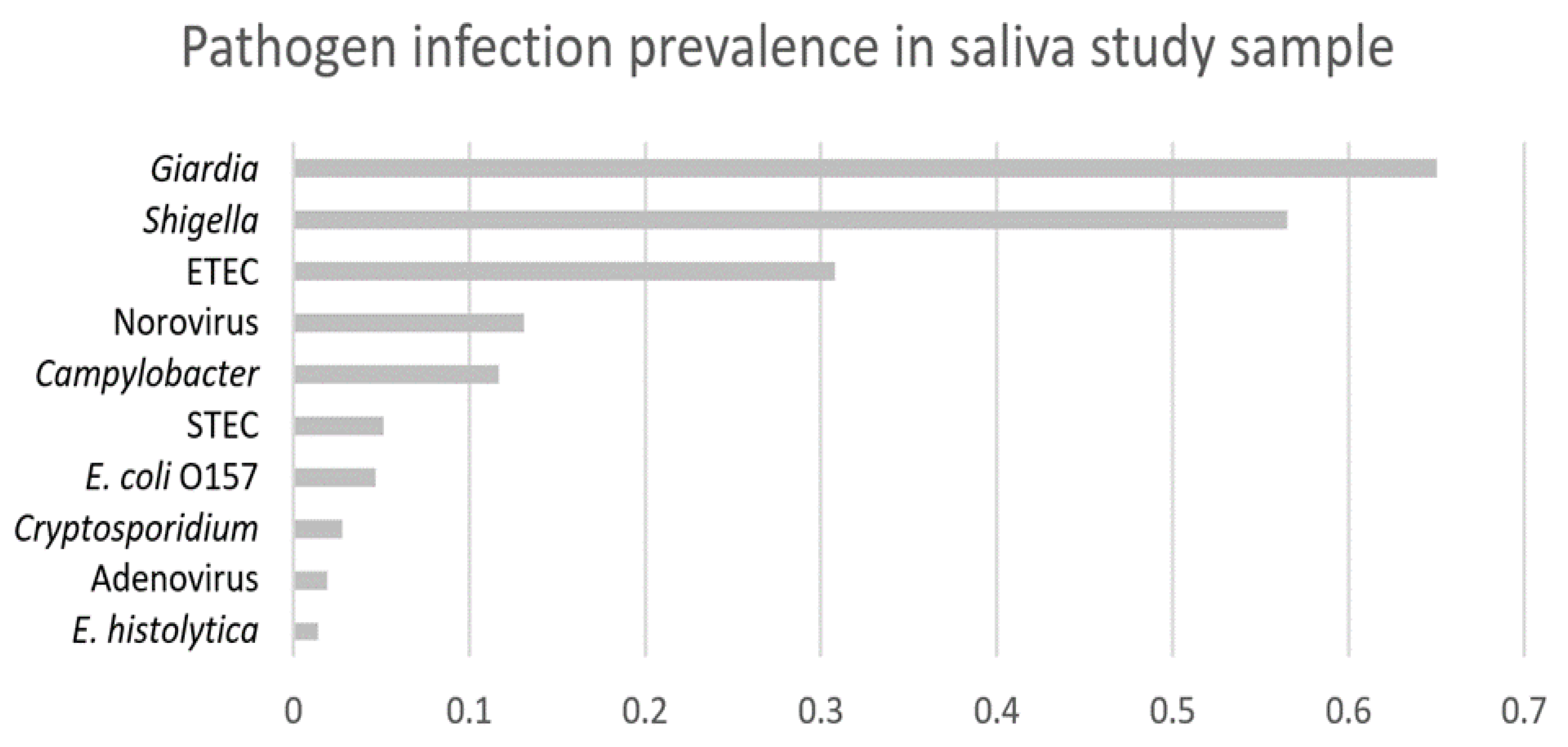
3.2. Secretory Immunoglobulin A (SIgA) and Enteric Infections
3.3. SIgA and Environmental Enteric Dysfunction (EED) Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Platts-Mills, J.A.; Babji, S.; Bodhidatta, L.; Gratz, J.; Haque, R.; Havt, A.; McCormick, B.J.; McGrath, M.; Olortegui, M.P.; Samie, A.; et al. Pathogen-specific burdens of community diarrhoea in developing countries: A multisite birth cohort study (MAL-ED). Lancet Glob. Health 2015, 3, e564–e575. [Google Scholar] [CrossRef]
- GBD Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. Infect. Dis. 2018, 18, 1211–1228. [CrossRef]
- Kosek, M.N.; The MAL-ED Network Investigators. Causal Pathways from Enteropathogens to Environmental Enteropathy: Findings from the MAL-ED Birth Cohort Study. EBioMedicine 2017, 18, 109–117. [Google Scholar] [CrossRef]
- Crane, R.J.; Kelsey, J.D.J.; Berkley, J.A. Environmental enteric dysfunction: An Overview. Food Nutr. Bull. 2015, 36, 576–587. [Google Scholar] [CrossRef]
- Harper, K.M.; Mutasa, M.; Prendergast, A.J.; Humphrey, J.; Manges, A.R. Environmental enteric dysfunction pathways and child stunting: A systematic review. PLoS Negl. Trop. Dis. 2018, 12, e0006205. [Google Scholar] [CrossRef]
- UNICEF; WHO; W.B.G. Levels and Trends in Child Malnutrition; WHO: Geneva, Switzerland, 2018.
- Binnicker, M.J. Multiplex Molecular Panels for Diagnosis of Gastrointestinal Infection: Performance, Result Interpretation, and Cost-Effectiveness. J. Clin. Microbiol. 2015, 53, 3723–3728. [Google Scholar] [CrossRef]
- Ghoshal, U.; Jain, V.; Dey, A.; Ranjan, P. Evaluation of enzyme linked immunosorbent assay for stool antigen detection for the diagnosis of cryptosporidiosis among HIV negative immunocompromised patients in a tertiary care hospital of northern India. J. Infect. Public Health 2018, 11, 115–119. [Google Scholar] [CrossRef]
- Brown, J.; Cumming, O. Stool-Based Pathogen Detection Offers Advantages as an Outcome Measure for Water, Sanitation, and Hygiene Trials. Am. J. Trop. Med. Hyg. 2019, 102, 260–261. [Google Scholar] [CrossRef]
- Arnold, B.F.; Scobie, H.M.; Priest, J.W.; Lammie, P.J. Integrated serologic surveillance of population immunity and disease transmission. Emerg. Infect. Dis. 2018, 24, 1188–1194. [Google Scholar] [CrossRef]
- McMurtry, C.M.; Pillai Riddell, R.; Taddio, A.; Racine, N.; Asmundson, G.J.G.; Noel, M.; Chambers, C.T.; Shah, V. HELPinKids&Adults Team Far From “Just a Poke”. Clin. J. Pain 2015, 31, S3–S11. [Google Scholar] [PubMed]
- Beltrami, E.M.; Williams, I.T.; Shapiro, C.N.; Chamberland, M.E. Risk and Management of Blood-Borne Infections in Health Care Workers. Clin. Microbiol. Rev. 2000, 13, 385–407. [Google Scholar] [CrossRef] [PubMed]
- Campisi, G.; Di Fede, O.; Roccia, P.; Di Nicola, F.; Falaschinp, S.; Lo Muzio, L. Saliva: Its value as a biological matrix and current methods of sampling. Eur. J. Inflamm. 2006, 4, 11–19. [Google Scholar] [CrossRef]
- Koni, A.C.; Scott, R.A.; Wang, G.; Bailey, M.E.S.; Peplies, J.; Bammann, K.; Pitsiladis, Y.P. DNA yield and quality of saliva samples and suitability for large-scale epidemiological studies in children. Int. J. Obes. 2011, 35, S113–S118. [Google Scholar] [CrossRef] [PubMed]
- Zabriskie, J.B. Essential Clinical Immunology; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Brandtzaeg, P. Do Salivary Antibodies Reliably Reflect Both Mucosal and Systemic Immunity? Ann. N. Y. Acad. Sci. 2007, 1098, 288–311. [Google Scholar] [CrossRef]
- Tsujita, S.; Morimoto, K. Secretory IgA in saliva can be a useful stress marker. Environ. Health Prev. Med. 1999, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.K.; Hollander, G.A.; McMichael, A. Evolution of the immune system in humans from infancy to old age. Proc. R. Soc. B 2015, 282. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimmons, S.P.; Evans, M.K.; Pearce, C.L.; Sheridan, M.J.; Wientzen, R.; Cole, M.F. Immunoglobulin A subclasses in infants’ saliva and in saliva and milk from their mothers. J. Pediatr. 1994, 124, 566–573. [Google Scholar] [CrossRef]
- Ben-Aryeh, H.; Fisher, M.; Szargel, R.; Laufer, D. Composition of whole unstimulated saliva of healthy children: Changes with age. Arch. Oral Biol. 1990, 35, 929–931. [Google Scholar] [CrossRef]
- Mantis, N.J.; Forbes, S.J. Secretory IgA: Arresting microbial pathogens at epithelial borders. Immunol. Investig. 2010, 39, 383–406. [Google Scholar] [CrossRef]
- Wells, J.M.; Brummer, R.J.; Derrien, M.; MacDonald, T.T.; Troost, F.; Cani, P.D.; Theodorou, V.; Dekker, J.; Méheust, A.; de Vos, W.M.; et al. Homeostasis of the gut barrier and potential biomarkers. Am. J. Physiol. Liver Physiol. 2017, 312, G171–G193. [Google Scholar] [CrossRef] [PubMed]
- Mantis, N.J.; Rol, N.; Corth, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Rojas, R.; Apodaca, G. Immunoglobulin transport across polarized epithelial cells. Nat. Rev. Mol. Cell Biol. 2002, 3, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Kaetzel, C.S.; Robinson, J.K.; Chintalacharuvu, K.R.; Vaerman, J.-P.; Lamm, M.E. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: A local defense function for IgA (epitheal mncous/nual immuniy). Proc. Nati. Acad. Sci. USA 1991, 88, 8796–8800. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Cumming, O.; Bartram, J.; Cairncross, S.; Ensink, J.; Holcomb, D.; Knee, J.; Kolsky, P.; Liang, K.; Liang, S.; et al. A controlled, before-and-after trial of an urban sanitation intervention to reduce enteric infections in children: Research protocol for the Maputo Sanitation (MapSan) study, Mozambique. BMJ Open 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Egorov, A.I.; Griffin, S.M.; Ward, H.D.; Reilly, K.; Fout, G.S.; Wade, T.J. Application of a salivary immunoassay in a prospective community study of waterborne infections. Water Res. 2018, 142, 289–300. [Google Scholar] [CrossRef]
- Instituto Nacional de Estatística (Mozambique); Ministério da Saúde. Moçambique Inquérito Demográfi co e de Saúde; MEASURE DHS/ICF International (Assistência Técnica): Calverton, MD, USA, 2011.
- Knee, J.; Sumner, T.; Adriano, Z.; Berendes, D.; de Bruijn, E.; Schmidt, W.-P.; Nalá, R.; Cumming, O.; Brown, J. Risk factors for childhood enteric infection in urban Maputo, Mozambique: A cross-sectional study. PLoS Negl. Trop. Dis. 2018, 12, e0006956. [Google Scholar] [CrossRef]
- Sheetal, A.; Hiremath, V.K.; Patil, A.G.; Sajjansetty, S.; Kumar, S.R. Malnutrition and its Oral Outcome—A Review. J. Clin. Diagn. Res. 2013, 7, 178–180. [Google Scholar] [CrossRef]
- Lehmann, F.S.; Burri, E.; Beglinger, C. The role and utility of faecal markers in inflammatory bowel disease. Therap. Adv. Gastroenterol. 2015, 8, 23–36. [Google Scholar] [CrossRef]
- de Serres, F.; Blanco, I. Role of alpha-1 antitrypsin in human health and disease. J. Intern. Med. 2014, 276, 311–335. [Google Scholar] [CrossRef]
- Murr, C.; Widner, B.; Wirleitner, B.; Fuchs, D. Neopterin as a Marker for Immune System Activation. Curr. Drug Metab. 2005, 3, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Sonesson, M.; Hamberg, K.; Lundin Wallengren, M.-L.; Matsson, L.; Ericson, D. Salivary IgA in minor-gland saliva of children, adolescents, and young adults. Eur. J. Oral Sci. 2011, 119, 15–20. [Google Scholar] [CrossRef]
- Eliasson, L.; Birkhed, D.; Österberg, T.; Carlén, A. Minor salivary gland secretion rates and immunoglobulin A in adults and the elderly. Eur. J. Oral Sci. 2006, 114, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P. Secretory immunity with special reference to the oral cavity. J. Oral Microbiol. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Glennie, S.J.; Nyirenda, M.; Williams, N.A.; Heyderman, R.S. Do multiple concurrent infections in African children cause irreversible immunological damage? Immunology 2011, 135, 125–132. [Google Scholar] [CrossRef]
- Katona, P.; Katona-Apte, J. The Interaction between Nutrition and Infection. Clin. Infect. Dis. 2008, 46, 1582–1588. [Google Scholar] [CrossRef]
- Platts-Mills, J.A.; Taniuchi, M.; Uddin, M.J.; Sobuz, S.U.; Mahfuz, M.; Gaffar, S.M.A.; Mondal, D.; Hossain, M.I.; Islam, M.M.; Ahmed, A.M.S.; et al. Association between enteropathogens and malnutrition in children aged 6-23 mo in Bangladesh: A case-control study. Am. J. Clin. Nutr. 2017, 105, 1132–1138. [Google Scholar] [CrossRef]
- Watson, R.R.; McMurray, D.N.; Martin, P.; Reyes, M.A. Effect of age, malnutrition and renutrition on free secretory component and IgA in secretions. Am. J. Clin. Nutr. 1985, 42, 281–288. [Google Scholar] [CrossRef]
- Fagerås, M.; Tomičić, S.; Voor, T.; Björkstén, B.; Jenmalm, M.C. Slow Salivary Secretory IgA Maturation May Relate to Low Microbial Pressure and Allergic Symptoms in Sensitized Children. Pediatr. Res. 2011, 70, 572–577. [Google Scholar] [CrossRef]
- Weemaes, C.; Klasen, I.; Göertz, J.; Beldhuis-Valkis, M.; Olafssonz, O.; Haraldsson, A. Development of Immunoglobulin A in Infancy and Childhood. Scand. J. Immunol. 2003, 58, 642–648. [Google Scholar] [CrossRef]
- Knee, J. Quantifying the Impact of an Urban Onsite Shared Sanitation Intervention on Child Health in Maputo, Mozambique: The Mapsan Trial. Ph. D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2019. [Google Scholar]
- Engeland, C.G.; Hugo, F.N.; Hilgert, J.B.; Nascimento, G.G.; Junges, R.; Lim, H.-J.; Marucha, P.T.; Bosch, J.A.; Engeland, C.G. The effects of psychological distress on salivary secretory immunity HHS Public Access. Brain Behav. Immun. 2016, 52, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.W.; Nambiar, S.; Muhardi, L.; Abdul Kader, U.H.; Garssen, J.; Sandalova, E. Young Children Display Diurnal Patterns of Salivary IgA and Alpha-Amylase Expression Which Are Independent of Food Intake and Demographic Factors. BioMed Res. Int. 2019, 2019, 3687416. [Google Scholar] [CrossRef] [PubMed]
- Tenovuo, J.; Lehtonen, O.-P.J.; Aaltonen, A.S.; Vilja, P.; Tuohimaa, P. Antimicrobial Factors in Whole Saliva of Human Infants. Infect. Immun. 1986, 51, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Marcotte, H.; Lavoie, M.C. Oral Microbial Ecology and the Role of Salivary Immunoglobulin A. Microbiol. Mol. Biol. Rev. 1998, 62, 71–109. [Google Scholar] [CrossRef]
- Exum, N.G.; Pisanic, N.; Granger, D.A.; Schwab, K.J.; Detrick, B.; Kosek, M.; Egorov, A.I.; Griffin, S.M.; Heaney, C.D. Use of Pathogen-Specific Antibody Biomarkers to Estimate Waterborne Infections in Population-Based Settings. Curr. Environ. Health Rep. 2016, 3, 322–334. [Google Scholar] [CrossRef]
- Wade, T.J.; Augustine, S.A.J.; Griffin, S.M.; Sams, E.A.; Oshima, K.H.; Egorov, A.I.; Simmons, K.J.; Eason, T.N.; Dufour, A.P. Asymptomatic norovirus infection associated with swimming at a tropical beach: A prospective cohort study. PLoS ONE 2018, 13, e0195056. [Google Scholar] [CrossRef]
| Characteristic | |
|---|---|
| Number of saliva samples | |
| Extracted | 244 |
| Excluded due to insufficient volume | 13 |
| Excluded due to visible serum | 15 |
| Excluded due to replicate rejection | 2 |
| Included in analysis | 214 |
| Male child (%) | 50 |
| Child age in years—Median (inter-quartile range (IQR)) | 2.5 (1.8, 3.7) |
| Difference in days between saliva and stool sample collection—Median (IQR) | 0 (−1, 1) |
| Sample volume available in μL—Median (IQR) | 175 (100, 300) |
| Salivary SIgA levels in μg/mL—Median (IQR) | 54 (34, 85) |
| Coefficient of variation between duplicate samples (%) | 6.4 |
| All Samples (N = 214) | After Removing Outliers (N = 206) | |||||
|---|---|---|---|---|---|---|
| Difference in SIgA (log μg/mL) | 95% Confidence Interval | p-Value | Difference in SIgA (log μg/mL) | 95% Confidence Interval | p-Value | |
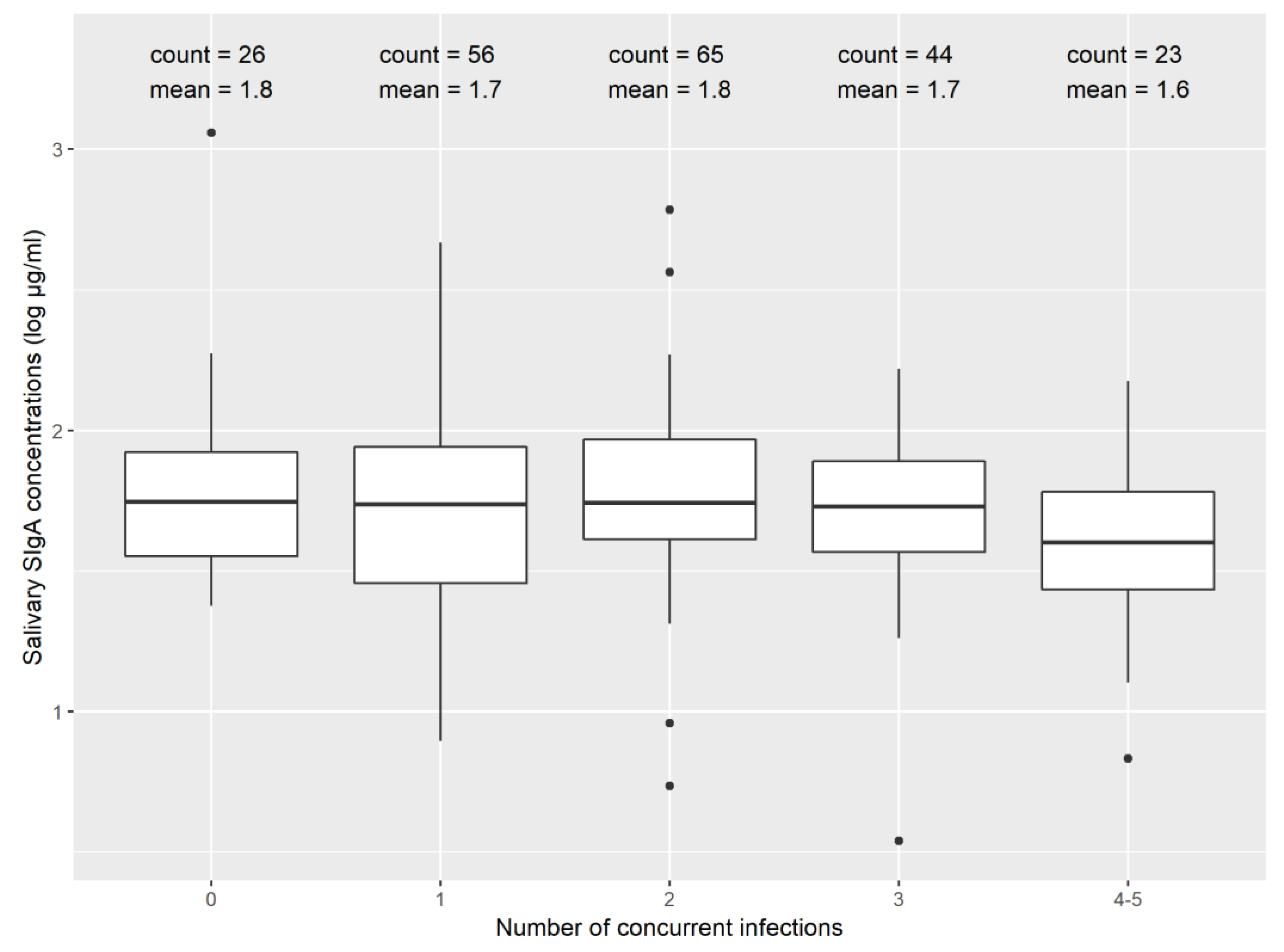
| Number of infections | −0.04 | (−0.08, −5 × 10−3) | 0.03 | −0.03 | (−0.06, 2 × 10−3) | 0.07 |
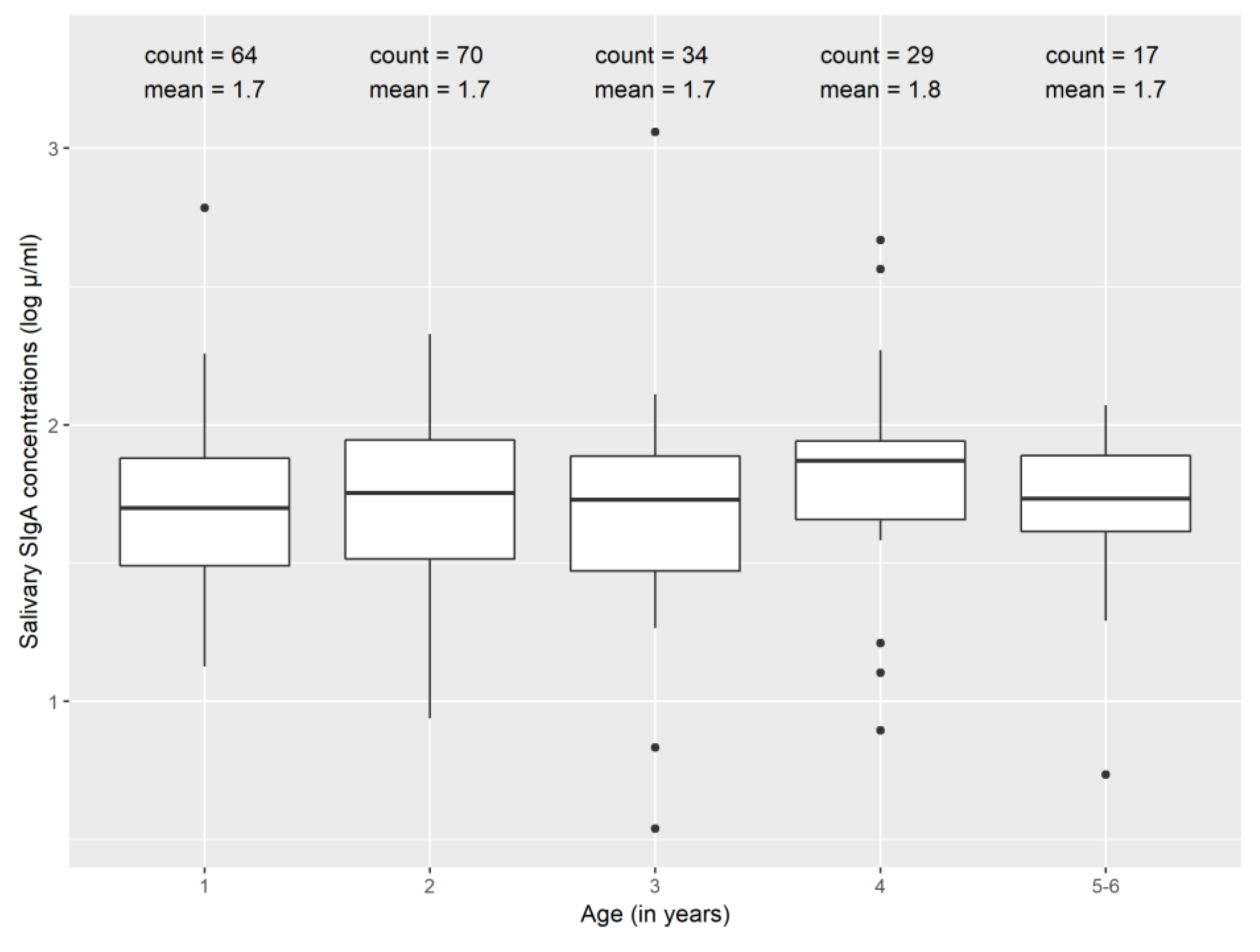
| Age (in months) | 4 × 10−4 | (−2 × 10−3, 3 × 10−3) | 0.79 | 1 × 10−3 | (−1 × 10−3, 3 × 10−3) | 0.31 |
| Sample volume (in μL) | −1 × 10−3 | (−9 × 10−4, −3 × 10−4) | <0.001 | −6 × 10−4 | (−8 × 10−4, −3 × 10−4) | <0.001 |
| Rainfall (terciles) | 0.03 | (−0.02, 0.08) | 0.29 | 0.04 | (−4 × 10−3, 0.08) | 0.07 |
| All Samples | After Removing Outliers | |||||||
|---|---|---|---|---|---|---|---|---|
| EED Biomarker | N | Difference in SIgA (log μg/mL) | 95% Confidence Interval | p-Value | N | Difference in SIgA (log μg/mL) | 95% Confidence Interval | p-Value |
| Neopterin (log nmol/L) | 188 | 0.02 | (−0.09, 0.13) | 0.75 | 180 | −0.02 | (−0.12, 0.07) | 0.61 |
| Myeloperoxidase (log ng/mL) | 213 | 0.02 | (−0.07, 0.12) | 0.64 | 201 | 0.04 | (−0.05, 0.12) | 0.39 |
| Calprotectin (log ng/mL) | 211 | 0.02 | (−0.06, 0.10) | 0.68 | 202 | 4 × 10−3 | (−0.07, 0.07) | 0.91 |
| Alpha-1 antitrypsin (log ng/mL) | 207 | −0.08 | (−0.17, 4 × 10−3) | 0.06 | 196 | −0.02 | (−0.1, 0.06) | 0.62 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goddard, F.G.B.; Knee, J.; Sumner, T.; Nalá, R.; Clasen, T.; Brown, J. Child Salivary SIgA and Its Relationship to Enteric Infections and EED Biomarkers in Maputo, Mozambique. Int. J. Environ. Res. Public Health 2020, 17, 3035. https://doi.org/10.3390/ijerph17093035
Goddard FGB, Knee J, Sumner T, Nalá R, Clasen T, Brown J. Child Salivary SIgA and Its Relationship to Enteric Infections and EED Biomarkers in Maputo, Mozambique. International Journal of Environmental Research and Public Health. 2020; 17(9):3035. https://doi.org/10.3390/ijerph17093035
Chicago/Turabian StyleGoddard, Frederick G. B., Jacqueline Knee, Trent Sumner, Rassul Nalá, Thomas Clasen, and Joe Brown. 2020. "Child Salivary SIgA and Its Relationship to Enteric Infections and EED Biomarkers in Maputo, Mozambique" International Journal of Environmental Research and Public Health 17, no. 9: 3035. https://doi.org/10.3390/ijerph17093035
APA StyleGoddard, F. G. B., Knee, J., Sumner, T., Nalá, R., Clasen, T., & Brown, J. (2020). Child Salivary SIgA and Its Relationship to Enteric Infections and EED Biomarkers in Maputo, Mozambique. International Journal of Environmental Research and Public Health, 17(9), 3035. https://doi.org/10.3390/ijerph17093035





