Tunnelled Haemodialysis Catheter Removal: An Underappreciated Problem, Not Always Simple and Safe
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
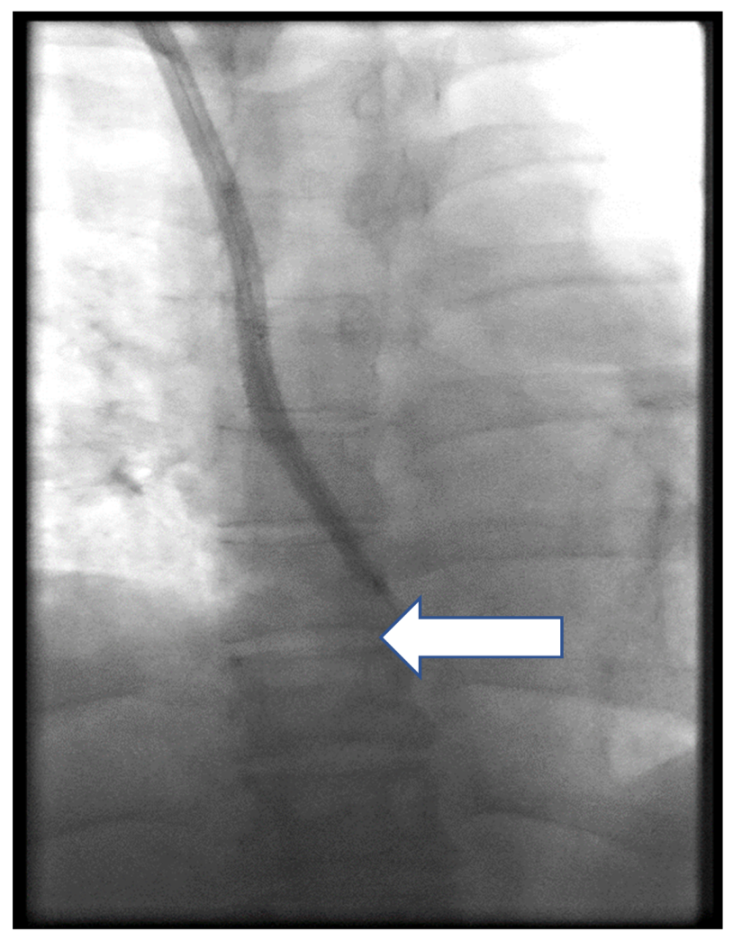
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- USRDS 2004 annual data report. Am. J. Kidney Dis. 2005, 45, 8–280. [CrossRef]
- Kohli, M.D.; Trerotola, S.O.; Namyslowski, J.; Stecker, M.S.; McLennan, G.; Patel, N.H.; Johnson, M.S.; Shah, H.; Seshadri, R. Outcome of Polyester Cuff Retention Following Traction Removal of Tunneled Central Venous Catheters. Radiology 2001, 219, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Fülöp, T.; Rodriguez, B.; Kosztaczky, B.A.; Gharaibeh, K.A.; Lengvárszky, Z.; Dossabhoy, N.; Tapolyai, M. Tunneled Hemodialysis Catheter Removals by Non-Interventional Nephrologists: The University of Mississippi Experience. Semin. Dial. 2015, 28, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Semadi, I.N.; Koerniawan, H.S.; Irawan, H. Retrieval of Intravascular Fractured Fragment of Tunnelled Double Lumen Catheter in Hemodialysis Patient. Open Access Maced. J. Med Sci. 2019, 7, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Bjeletich, J.; Hickman, R.O. The Hickman indwelling catheter. Am. J. Nurs. 1980, 80, 62–65. [Google Scholar] [PubMed]
- Reed, W.P.; Newman, K.A.; Tenney, J.H. An improved technique for the removal of long term implantable central venous lines. Surg. Gynecol. Obstet. 1985, 161, 479–480. [Google Scholar]
- Letachowicz, K.; Gołębiowski, T.; Kusztal, M.; Penar, J.; Letachowicz, W.; Weyde, W.; Klinger, M. Over-catheter tract suture to prevent bleeding and air embolism after tunnelled catheter removal. J. Vasc. Access 2017, 18, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Niyyar, V.D.; Work, J. Avoiding a cutdown--use of the transcatheter extractor in removal of tunneled dialysis catheters. Semin. Dial. 2011, 24, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H. A Breakthrough Technique for the Removal of a Hemodialysis Catheter Stuck in the Central Vein: Endoluminal Balloon Dilatation of the Stuck Catheter. J. Vasc. Access 2011, 12, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Garcarek, J.; Gołębiowski, T.; Letachowicz, K.; Kusztal, M.; Szymczak, M.; Katarzyna, M.; Jakuszko, K.; Zmonarski, S.; Guziński, M.; Weyde, W.; et al. Balloon Dilatation for Removal of an Irretrievable Permanent Hemodialysis Catheter: The Safest Approach. Artif. Organs 2015, 40, E84–E88. [Google Scholar] [CrossRef] [PubMed]
- Fisher, W.B. Complication of a Hickman catheter. Cutaneous erosion of the Dacron cuff. JAMA 1985, 254, 2934. [Google Scholar] [CrossRef] [PubMed]
- Beyer, G.A.; Thorsen, M.K.; Shaffer, K.A.; Walker, A.P. Mammographic appearance of the retained Dacron cuff of a Hickman catheter. Am. J. Roentgenol. 1990, 155, 1203–1204. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ellis, R.L.; Dempsey, P.J.; Rubin, E.; Pile, N.S.; Bernreuter, W.K. Mammography of breasts in which catheter cuffs have been retained: Normal, infected, and postoperative appearances. Am. J. Roentgenol. 1997, 169, 713–715. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stecker, M.S.; Johnson, M.S.; Ying, J.; McLennan, G.; Agarwal, D.M.; Namyslowski, J.; Ahmad, I.; Shah, H.; Butty, S.; Casciani, T. Time to Hemostasis after Traction Removal of Tunneled Cuffed Central Venous Catheters. J. Vasc. Interv. Radiol. 2007, 18, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-J.; Liang, Z.-A.; Fu, P.; Feng, Y. Removal of a Fractured Tunneled Cuffed Catheter from the Right Atrium and Inferior Vena Cava by Percutaneous Snare Technique. J. Vasc. Access 2016, 17, e42–e43. [Google Scholar] [CrossRef] [PubMed]
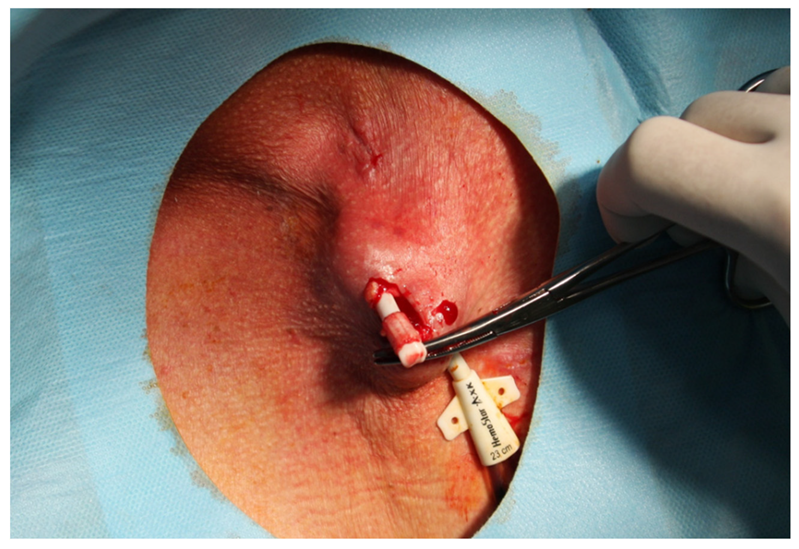
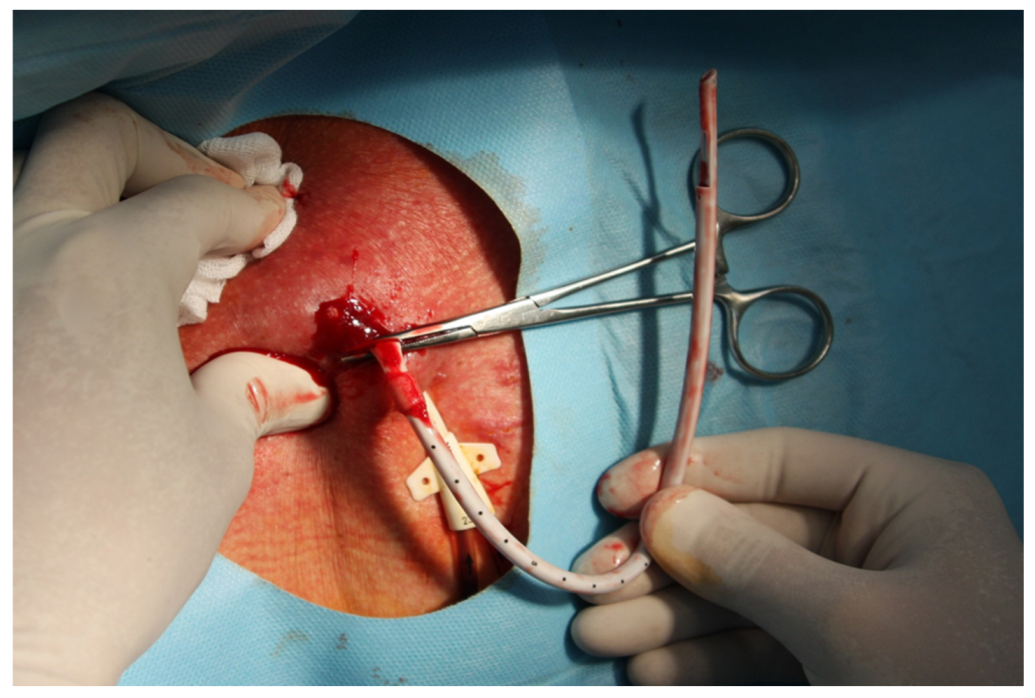

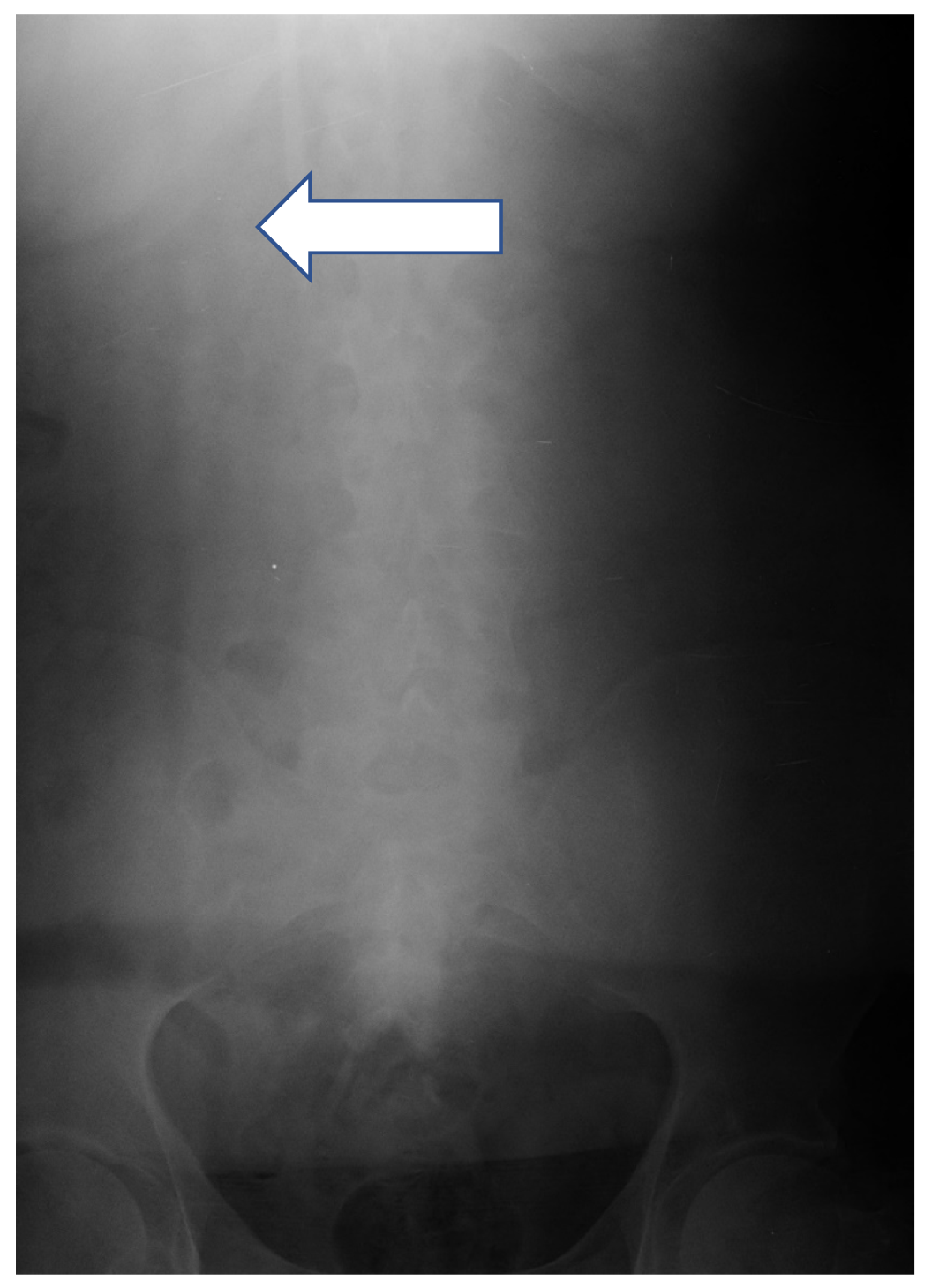
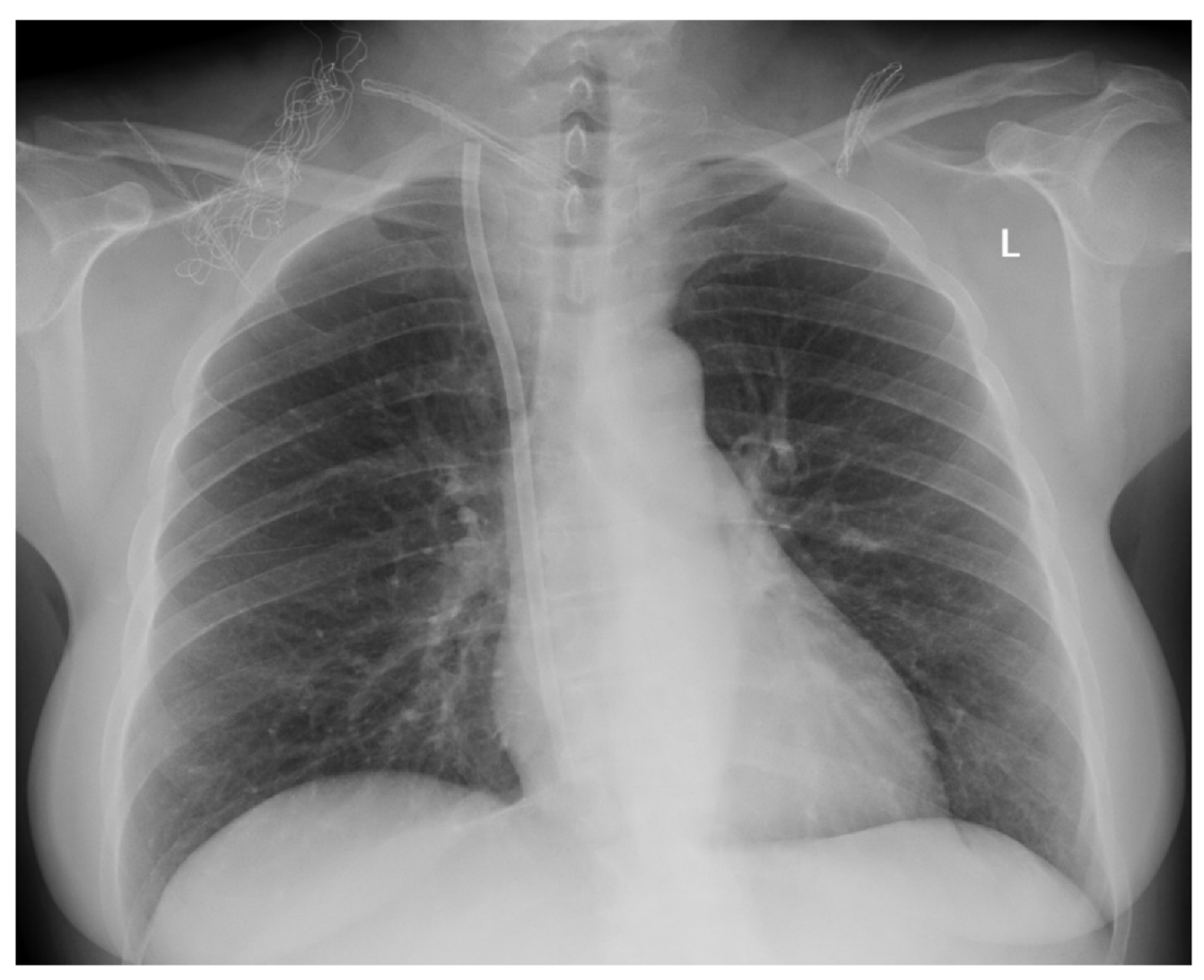
| Characteristic | MCDM Group | CDM Group | p |
|---|---|---|---|
| N | 67 | 76 | |
| Sex, n (%) | |||
| Female | 53 (79.1) | 49 (64.5) | 0.081 |
| Male | 14 (20.9) | 27 (35.5) | |
| Age, years, mean ± SD | 65.72 ± 16.04 | 63.16 ± 13.33 | 0.299 |
| ESRD cause, n (%) | |||
| ADKPD | 3 (4.5) | 7 (9.2) | 0.464 |
| DM | 29 (43.3) | 37 (48.7) | |
| GN | 12 (17.9) | 8 (10.5) | |
| HTN | 5 (7.5) | 7 (9.2) | |
| NPL | 15 (22.4) | 11 (14.5) | |
| UNKN | 3 (4.5) | 6 (7.9) | |
| Reason for removal, n (%) | |||
| AVF | 21 (31.3) | 33 (43.4) | 0.118 |
| AVG | 2 (3.0) | 7 (9.2) | |
| CRI | 13 (19.4) | 11 (14.5) | |
| DYS | 31 (46.3) | 24 (31.6) | |
| PD | 0 (0.0) | 1 (1.3) | |
| CTC removal site, n (%) | |||
| LFV | 4 (6.0) | 1 (1.3) | 0.487 |
| LJV | 14 (20.9) | 23 (30.3) | |
| LSV | 1 (1.5) | 1 (1.3) | |
| RFV | 2 (3.0) | 1 (1.3) | |
| RJV | 44 (65.7) | 49 (64.5) | |
| RSV | 2 (3.0) | 1 (1.3) | |
| CTC time at removal, weeks, median (Q1; Q3) | 57.00 (42.00; 71.50) | 55.00 (22.25; 94.00) | 0.433 |
| CTC Removal Complication | MCDM Group | CDM Group | RR (95% CI) | p |
|---|---|---|---|---|
| n | 67 | 76 | ||
| Any complication | 9 (13.4) | 11 (14.5) | 1.08 (0.47; 2.44) | >0.999 |
| Bleeding | 5 (7.5) | 6 (7.9) | 1.06 (0.34; 3.31) | >0.999 |
| Wound infection | 4 (6.0) | 3 (3.9) | 0.66 (0.15; 2.85) | 0.706 |
| Air embolism | 0 (0.0) | 1 (1.3) | n/a | >0.999 |
| CTC migration | 0 (0.0) | 2 (2.6) | n/a | 0.499 |
| Characteristic | MCDM Group: No Bleeding | MCDM Group: Bleeding | p |
|---|---|---|---|
| N | 62 | 5 | |
| Sex, n (%) | |||
| Female | 48 (77.4) | 5 (100.0) | 0.576 |
| Male | 14 (22.6) | 0 (0.0) | |
| Age, years, median (Q1; Q3) | 66.50 (57.00; 74.25) | 83.00 (73.00; 85.00) | 0.023 |
| ESRD cause, n (%) | |||
| ADKPD | 3 (4.8) | 0 (0.0) | 0.192 |
| Diabetes | 28 (45.2) | 1 (20.0) | |
| Glomerulonephritis | 12 (19.4) | 0 (0.0) | |
| Hypertension | 4 (6.5) | 1 (20.0) | |
| Neoplasm | 12 (19.4) | 3 (60.0) | |
| Unknown | 3 (4.8) | 0 (0.0) | |
| Reason for removal, n (%) | |||
| Patent AVF | 19 (30.6) | 2 (40.0) | 0.877 |
| Patent AVG | 2 (3.2) | 0 (0.0) | |
| Catheter related infection | 13 (21.0) | 0 (0.0) | |
| Cathteter dysfunction | 28 (45.2) | 3 (60.0) | |
| Conversion to PD | 19 (30.6) | 2 (40.0) | |
| CTC removal site, n (%) | |||
| Left femoral vein | 4 (6.5) | 0 (0.0) | >0.999 |
| Left jugular vein | 13 (21.0) | 1 (20.0) | |
| Left subclavian vein | 1 (1.6) | 0 (0.0) | |
| Right femoral vein | 2 (3.2) | 0 (0.0) | |
| Right jugular vein | 40 (64.5) | 4 (80.0) | |
| Right subclavian vein | 2 (3.2) | 0 (0.0) | |
| CTC time at removal, weeks, median (Q1; Q3) | 57.00 (42.50; 71.75) | 37.00 (21.00; 59.00) | 0.202 |
| Characteristic | MCDM Group: No Infection | MCDM Group: Infection | p |
|---|---|---|---|
| N | 63 | 4 | |
| Sex, n (%) | |||
| Female | 50 (79.4) | 3 (75.0) | >0.999 |
| Male | 13 (20.6) | 1 (25.0) | |
| Age, years, median (Q1; Q3) | 68.00 (57.00; 76.00) | 69.00 (64.25; 73.75) | 0.741 |
| ESRD cause, n (%) | |||
| ADKPD | 3 (4.8) | 0 (0.0) | 0.392 |
| Diabetes | 28 (44.4) | 1 (25.0) | |
| Glomerulonephritis | 11 (17.5) | 1 (25.0) | |
| Hypertension | 5 (7.9) | 0 (0.0) | |
| Neoplasm | 14 (22.2) | 1 (25.0) | |
| Unknown | 2 (3.2) | 1 (25.0) | |
| Reason for removal, n (%) | |||
| AVF | 20 (31.7) | 1 (25.0) | >0.999 |
| AVG | 2 (3.2) | 0 (0.0) | |
| CRI | 12 (19.0) | 1 (25.0) | |
| DYS | 29 (46.0) | 2 (50.0) | |
| PD | 20 (31.7) | 1 (25.0) | |
| CTC removal site, n (%) | |||
| Left femoral vein | 3 (4.8) | 1 (25.0) | 0.095 |
| Left jugular vein | 14 (22.2) | 0 (0.0) | |
| Left subclavian vein | 1 (1.6) | 0 (0.0) | |
| Right femoral vein | 2 (3.2) | 0 (0.0) | |
| Right jugular vein | 42 (66.7) | 2 (50.0) | |
| Right subclavian vein | 1 (1.6) | 1 (25.0) | |
| CTC time at removal, weeks, median (Q1; Q3) | 57.00 (42.00; 71.50) | 58.50 (43.00; 110.25) | 0.741 |
| Characteristic | CDM Group: No Bleeding | CDM Group: Bleeding | p |
|---|---|---|---|
| N | 70 | 6 | |
| Sex, n (%) | |||
| Female | 46 (65.7) | 3 (50.0) | 0.660 |
| Male | 24 (34.3) | 3 (50.0) | |
| Age, years, median (Q1; Q3) | 65.00 (56.25; 71.25) | 71.50 (63.00; 78.50) | 0.244 |
| ESRD cause, n (%) | |||
| ADKPD | 7 (10.0) | 0 (0.0) | 0.704 |
| Diabetes | 34 (48.6) | 3 (50.0) | |
| Glomerulonephritis | 7 (10.0) | 1 (16.7) | |
| Hypertension | 7 (10.0) | 0 (0.0) | |
| Neoplasm | 9 (12.9) | 2 (33.3) | |
| Unknown | 6 (8.6) | 0 (0.0) | |
| Reason for removal, n (%) | |||
| AVF | 32 (45.7) | 1 (16.7) | 0.340 |
| AVG | 7 (10.0) | 0 (0.0) | |
| CRI | 10 (14.3) | 1 (16.7) | |
| DYS | 20 (28.6) | 4 (66.7) | |
| PD | 1 (1.4) | 0 (0.0) | |
| CTC removal site, n (%) | |||
| Left femoral vein | 1 (1.4) | 0 (0.0) | >0.999 |
| Left jugular vein | 21 (30.0) | 2 (33.3) | |
| Left subclavian vein | 1 (1.4) | 0 (0.0) | |
| Right femoral vein | 1 (1.4) | 0 (0.0) | |
| Right jugular vein | 45 (64.3) | 4 (66.7) | |
| Right subclavian vein | 1 (1.4) | 0 (0.0) | |
| CTC time at removal, weeks, median (Q1; Q3) | 60.00 (37.50; 87.00) | 46.50 (34.50; 71.25) | 0.294 |
| Characteristic | CDM Group: No Infection | CDM Group: Infection | p |
|---|---|---|---|
| N | 73 | 3 | |
| Sex, n (%) | |||
| Female | 46 (63.0) | 3 (100.0) | 0.548 |
| Male | 27 (37.0) | 0 (0.0) | |
| Age, years, median (Q1; Q3) | 65.00 (57.00; 72.00) | 56.00 (46.50; 61.00) | 0.182 |
| ESRD cause, n (%) | |||
| ADKPD | 7 (9.6) | 0 (0.0) | >0.999 |
| Diabetes | 34 (46.6) | 3 (100.0) | |
| Glomerulonephritis | 8 (11.0) | 0 (0.0) | |
| Hypertension | 7 (9.6) | 0 (0.0) | |
| Neoplasm | 11 (15.1) | 0 (0.0) | |
| Unknown | 6 (8.2) | 0 (0.0) | |
| Reason for removal, n (%) | |||
| AVF | 32 (43.8) | 1 (33.3) | 0.690 |
| AVG | 7 (9.6) | 0 (0.0) | |
| CRI | 10 (13.7) | 1 (33.3) | |
| DYS | 23 (31.5) | 1 (33.3) | |
| PD | 1 (1.4) | 0 (0.0) | |
| CTC removal site, n (%) | |||
| Left femoral vein | 1 (1.4) | 0 (0.0) | >0.999 |
| Left jugular vein | 22 (30.1) | 1 (33.3) | |
| Left subclavian vein | 1 (1.4) | 0 (0.0) | |
| Right femoral vein | 1 (1.4) | 0 (0.0) | |
| Right jugular vein | 47 (64.4) | 2 (66.7) | |
| Right subclavian vein | 1 (1.4) | 0 (0.0) | |
| CTC time at removal, weeks, median (Q1; Q3) | 59.00 (36.50; 87.00) | 75.00 (48.50; 84.00) | 0.947 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porazko, T.; Hobot, J.; Ziembik, Z.; Klinger, M. Tunnelled Haemodialysis Catheter Removal: An Underappreciated Problem, Not Always Simple and Safe. Int. J. Environ. Res. Public Health 2020, 17, 3027. https://doi.org/10.3390/ijerph17093027
Porazko T, Hobot J, Ziembik Z, Klinger M. Tunnelled Haemodialysis Catheter Removal: An Underappreciated Problem, Not Always Simple and Safe. International Journal of Environmental Research and Public Health. 2020; 17(9):3027. https://doi.org/10.3390/ijerph17093027
Chicago/Turabian StylePorazko, Tomasz, Jacek Hobot, Zbigniew Ziembik, and Marian Klinger. 2020. "Tunnelled Haemodialysis Catheter Removal: An Underappreciated Problem, Not Always Simple and Safe" International Journal of Environmental Research and Public Health 17, no. 9: 3027. https://doi.org/10.3390/ijerph17093027
APA StylePorazko, T., Hobot, J., Ziembik, Z., & Klinger, M. (2020). Tunnelled Haemodialysis Catheter Removal: An Underappreciated Problem, Not Always Simple and Safe. International Journal of Environmental Research and Public Health, 17(9), 3027. https://doi.org/10.3390/ijerph17093027





