The Effects of Cyanobacterial Bloom Extracts on the Biomass, Chl-a, MC and Other Oligopeptides Contents in a Natural Planktothrix agardhii Population
Abstract
1. Introduction
2. Materials and Methods
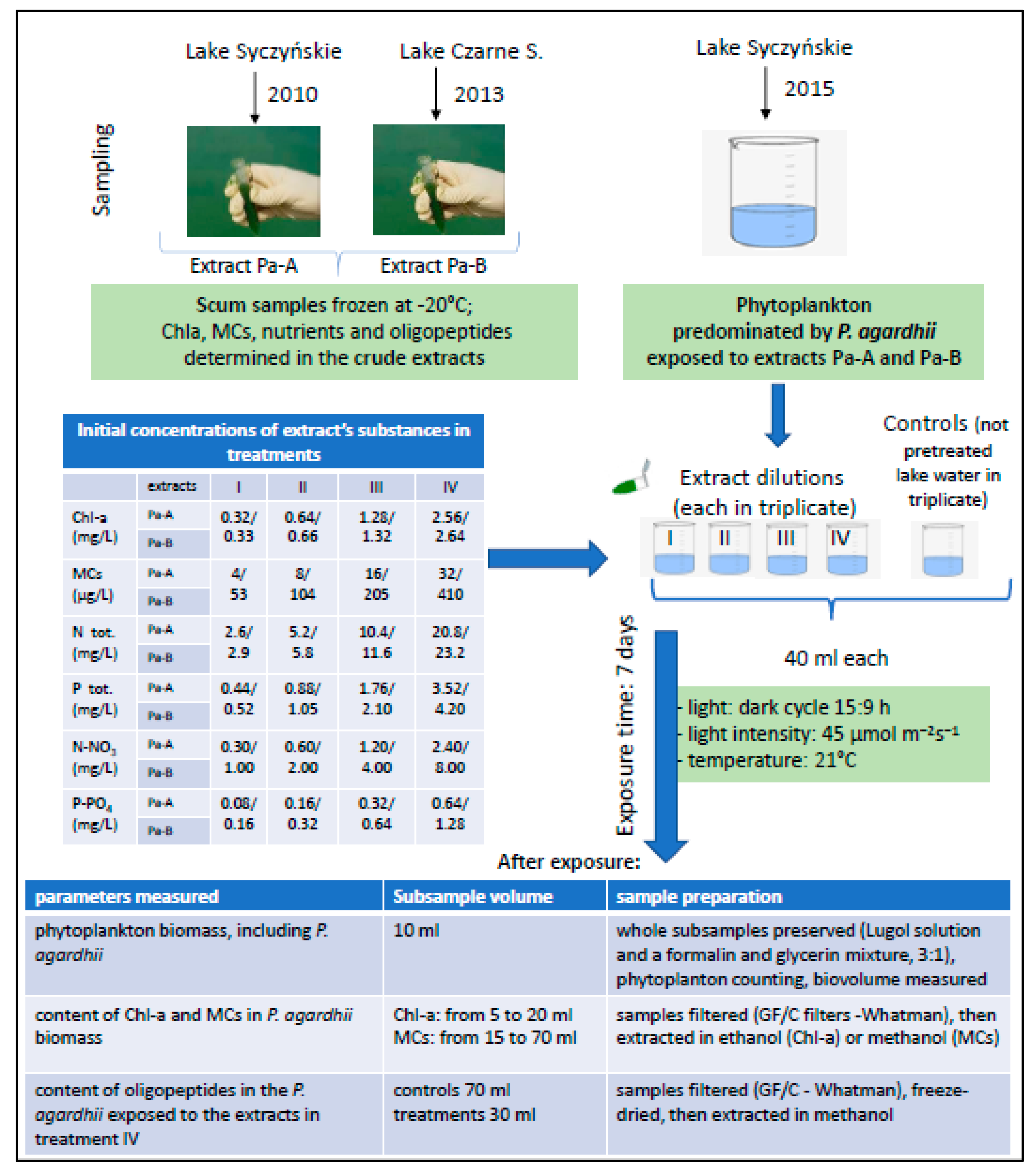
2.1. Sampling
2.2. Experiments
2.3. Phytoplankton Enumeration, Chlorophyll-a, Nitrogen and Phosphorus Analyses
2.4. Extraction and HPLC Analysis
HPLC-PDA Analysis of Microcystins
2.5. Extraction and LC-MS/MS Analysis of Oligopeptides
2.6. Data Analysis
3. Results
3.1. Characteristics of Two Planktothrix-Dominated Scum Samples and Their Crude Extracts Used in Experiments
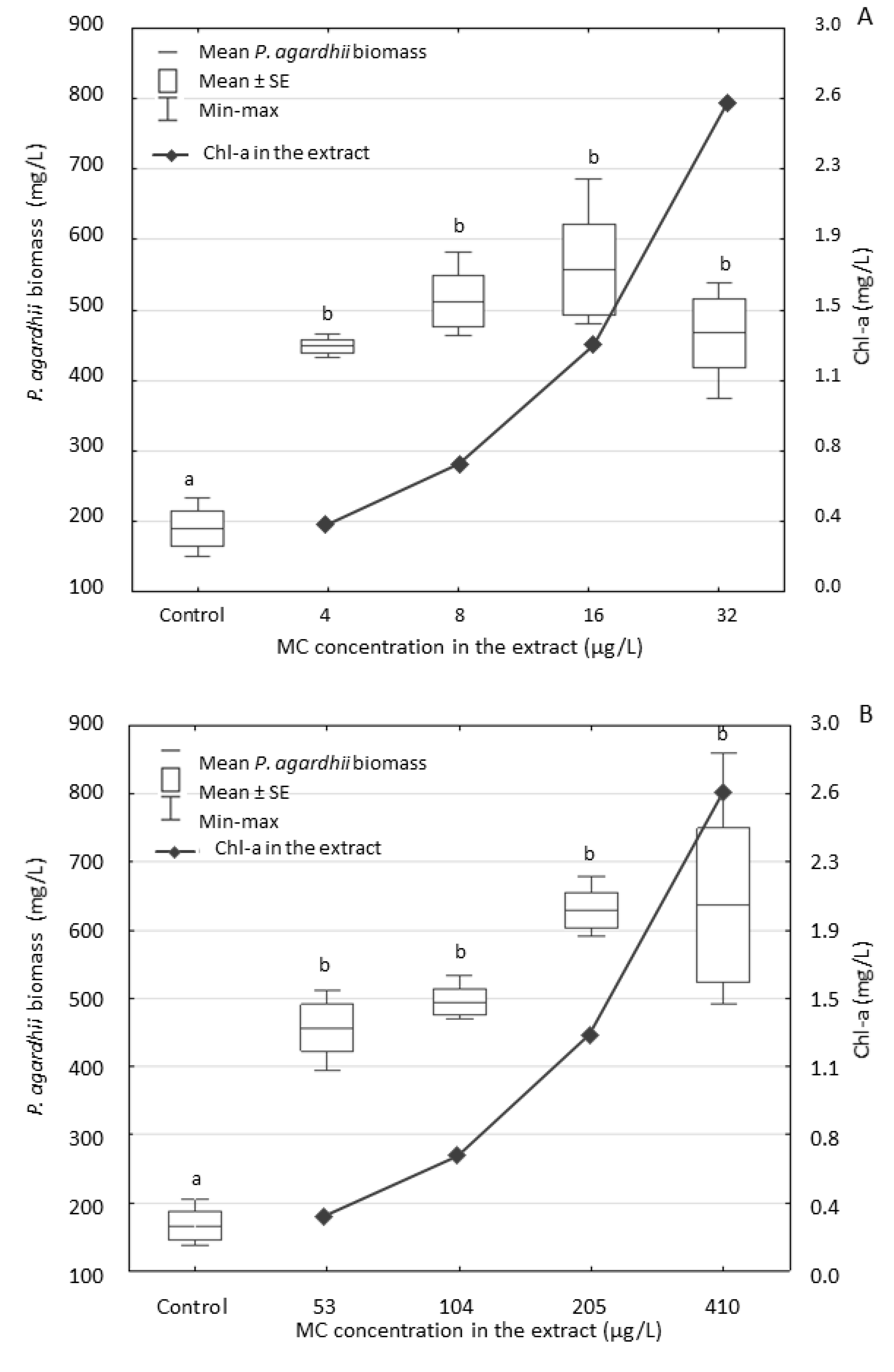
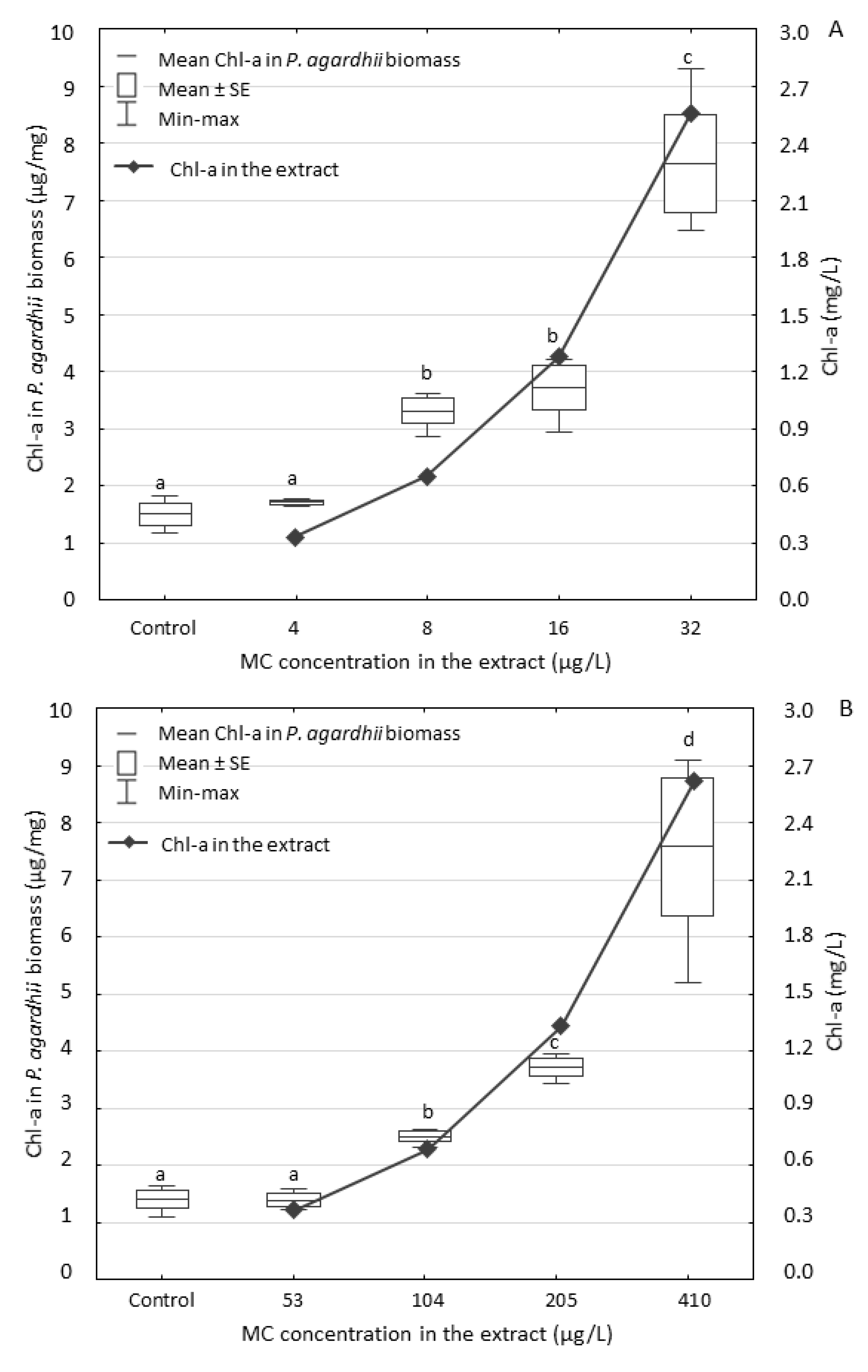
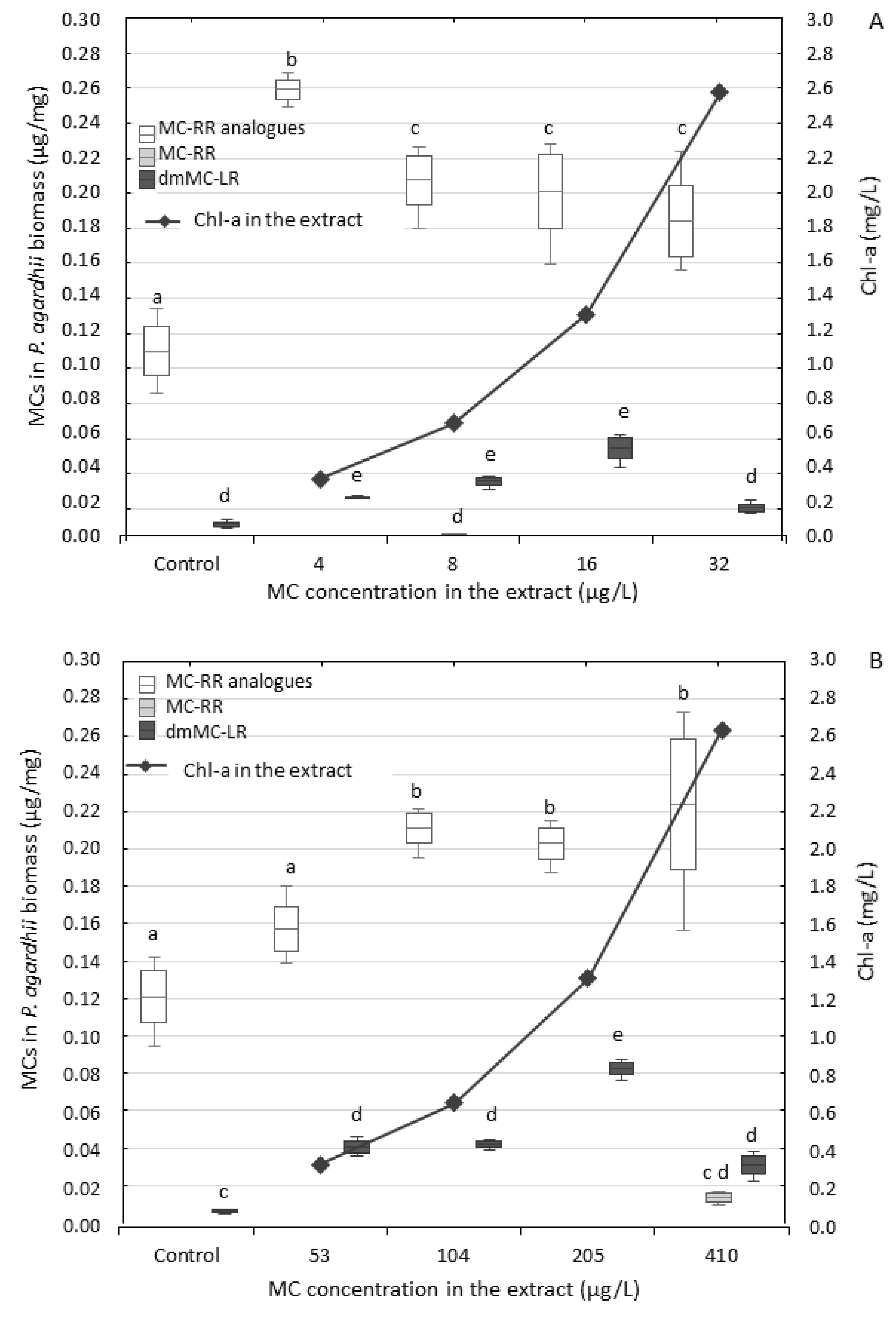
3.2. Effect of the Extracts on P. agardhii Biomass and Production of Intracellular MCs
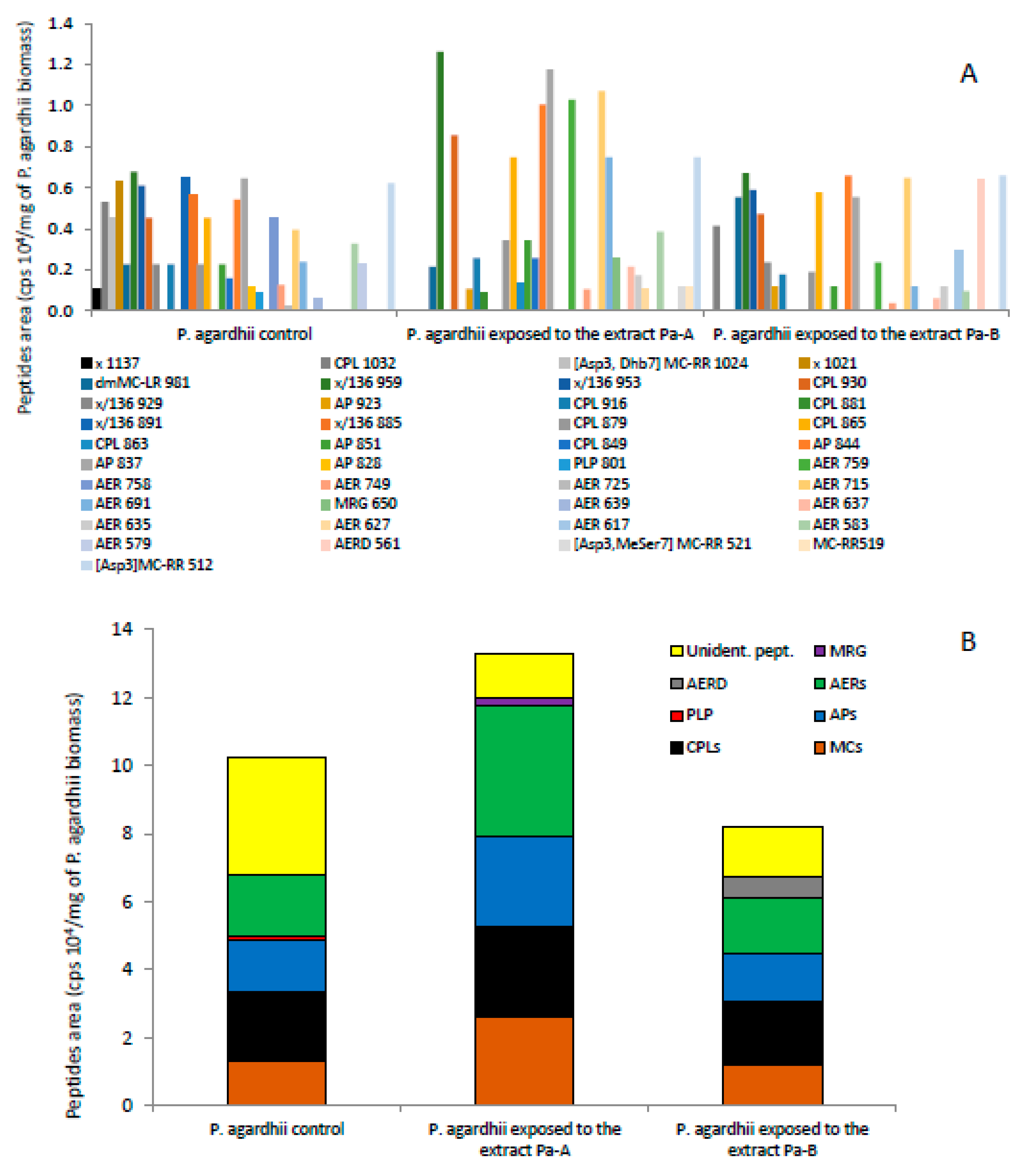
3.3. Effect of the Extracts on the Production of Oligopeptides by P. agardhii
4. Discussion
4.1. Higher P. agardhii Productivity: The Role of Nutrient Availability
4.2. Changes in Content of MCs in the Exposed P. agardhii
4.3. Changes in Oligopeptide Profiles in P. agardhii Population Exposed to the Extracts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacterial blooms: The potential roles in eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Verspagen, J.M.; Van de Waal, D.B.; Rost, B.; Huisman, J. Phenotypic plasticity of carbon fixation stimulates cyanobacterial blooms at elevated CO2. Sci. Adv. 2020, 6, eaax2926. [Google Scholar] [CrossRef] [PubMed]
- Czarnecki, O.; Henning, M.; Lippert, I.; Welker, M. Identification of peptide metabolites of Microcystis (Cyanobacteria) that inhibit trypsin-like activity in planktonic herbivorous Daphnia (Cladocera). Environ. Microbiol. 2006, 8, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Toporowska, M.; Pawlik-Skowrońska, B.; Kalinowska, R. Accumulation and effects of cyanobacterial microcystins and anatoxin-a on benthic larvae of Chironomus spp. (Diptera: Chironomidae). Eur. J. Entomol. 2016, 111, 83–90. [Google Scholar] [CrossRef]
- Carmichael, W.W. Cyanobacteria secondary metabolites—The cyanotoxins. J. Appl. Bacterial. 1992, 72, 445–459. [Google Scholar] [CrossRef]
- Welker, M.; von Döhren, H. Cyanobacterial peptides nature’s own combinatorial biosynthesis. FEMS Microbiol. Rev. 2006, 30, 530–563. [Google Scholar] [CrossRef]
- Sivonen, K.; Börner, T. Bioactive compounds produced by cyanobacteria. In The Cyanobacteria; Herrero, A., Flores, E., Eds.; Caister Academic Press: Norfolk, UK, 2008; pp. 159–197. [Google Scholar]
- Mantzouki, E.; Lürling, M.; Fastner, J.; de Senerpont Domis, L.; Wilk-Woźniak, E.; Koreivienė, J.; Seelen, L.; Teurlincx, S.; Verstijnen, Y.; Krztoń, W.; et al. Temperature effects explain continental scale distribution of cyanobacterial toxins. Toxins 2018, 10, 156. [Google Scholar] [CrossRef]
- Kurmayer, R.; Schober, E.; Tonk, L.; Visser, P.M.; Christiansen, G. Spatial divergence in the proportions of the genes encoding toxic peptides synthesis among populations of the cyanobacterium Planktothrix in European lakes. FEMS Microbiol. Lett. 2011, 317, 127–137. [Google Scholar] [CrossRef]
- Toporowska, M.; Pawlik-Skowrońska, B.; Kalinowska, R. Mass development of diazotrophic cyanobacteria (Nostocales) and production of neurotoxic anatoxin-a in a Planktothrix (Oscillatoriales) dominated temperate lake. Water Air Soil Pollut. 2016, 227, 321. [Google Scholar] [CrossRef]
- Kurmayer, R.; Deng, L.; Entfellner, E. Role of toxic and bioactive secondary metabolites in colonization and bloom formation by filamentous cyanobacteria Planktothrix. Harmful Algae 2016, 54, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Schindler, D.W. The dilemma of controlling cultural eutrophication of lakes. Proc. R. Soc. B-Biol. Sci. 2012, 279, 4322–4333. [Google Scholar] [CrossRef] [PubMed]
- Dokulil, M.T.; Teubner, K. Cyanobacterial dominance in lakes. Hydrobiologia 2000, 438, 1–12. [Google Scholar] [CrossRef]
- Rohrlack, T.; Skulberg, R.; Skulberg, O.M. Distribution of oligopeptide chemotypes of the cyanobacterium Planktothrix and their persistence in selected lakes in Fennoscandia. J. Phycol. 2009, 45, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Rohrlack, T.; Christiansen, G.; Kurmayer, R. Putative antiparasite defensive system involving ribosomal and non-ribosomal oligopeptides in cyanobacteria of the genus Planktothrix. Appl. Environ. Microbiol. 2013, 79, 2642–2647. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, M.; Kobos, J.; Toruńska-Sitarz, A.; Mazur-Marzec, H. Non-ribosomal peptides produced by Planktothrix agardhii from Siemianówka Dam Reservoir SDR (northeast Poland). Arch. Microbiol. 2014, 196, 697–707. [Google Scholar] [CrossRef]
- Pawlik-Skowrońska, B.; Toporowska, M.; Mazur-Marzec, H. Toxic oligopeptides in the cyanobacterium Planktothrix agardhii-dominated blooms and their effects on duckweed (Lemnaceae) development. Knowl. Manag. Aquat. Ecosyst. 2018, 419, 1–9. [Google Scholar] [CrossRef]
- Briand, E.; Yéprémian, C.; Humbert, J.F.; Quiblier, C. Competition between microcystin-and non-microcystin-producing Planktothrix agardhii (cyanobacteria) strains under different environmental conditions. Environ. Microbiol. 2008, 10, 3337–3348. [Google Scholar] [CrossRef]
- Agha, R.; Quesada, A. Oligopeptides as biomarkers of cyanobacterial subpopulations. Toward an understanding of their biological role. Toxins 2014, 6, 1929–1950. [Google Scholar] [CrossRef]
- Nasri, A.B.; Bouaïcha, N.; Fastner, J. First report of a microcystin-containing bloom of the cyanobacteria Microcystis spp. in Lake Oubeira, Eastern Algeria. Arch. Environ. Contam. Toxicol. 2004, 46, 197–202. [Google Scholar] [CrossRef]
- Jones, G.J.; Orr, P.T. Release and degradation of microcystin following algicide treatment of a Microcystis aeruginosa bloom in a recreational lake, as determined by HPLC and protein phosphatase inhibition assay. Water Res. 1994, 28, 871–876. [Google Scholar] [CrossRef]
- Chlipala, G.E.; Mo, S.; Orjala, J. Chemodiversity in freshwater and terrestrial cyanobacteria—A source for drug discovery. Curr. Drug Targets 2011, 12, 1654–1673. [Google Scholar] [CrossRef] [PubMed]
- Janssen, E.M.-L. Cyanobacterial peptides beyond microcystins—A review on co-occurrence, toxicity, and challenges for risk assessment. Water Res. 2019, 151, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Rohrlack, T.; Christoffersen, K.; Kaebernick, M.; Neilan, B.A. Cyanobacterial protease inhibitor microviridin J causes a lethal molting disruption in Daphnia pulicaria. Appl. Environ. Microbiol. 2004, 70, 5047–5050. [Google Scholar] [CrossRef] [PubMed]
- Suikkanen, S.; Fistarol, G.O.; Granéli, E. Allelopathic effects of the Baltic cyanobacteria Nodularia spumigena, Aphanizomenon flos-aquae and Anabaena lemmermannii on algal monocultures. J. Exp. Mar. Biol. Ecol. 2004, 308, 85–101. [Google Scholar] [CrossRef]
- Pflugmacher, S. Promotion of oxidative stress in the aquatic macrophyte Ceratophyllum demersum during biotransformation of the cyanobacterial toxin microcystin-LR. Aquat. Toxicol. 2004, 70, 169–178. [Google Scholar] [CrossRef]
- Kehr, J.C.; Zilliges, Y.; Springer, A.; Disney, M.D.; Ratner, D.D.; Bouchier, C.; Seeberger, P.H.; de Marsac, N.T.; Dittmann, E. A mannan binding lectin is involved in cell-cell attachment in a toxic strain of Microcystis aeruginosa. Mol. Microbiol. 2006, 59, 893–906. [Google Scholar] [CrossRef]
- Schatz, D.; Keren, Y.; Vardi, A.; Sukenik, A.; Carmeli, S.; Boerner, T.; Dittmann, E.; Kaplan, A. Towards clarification of the biological role of microcystins, a family of cyanobacterial toxins. Environ. Microbiol. 2007, 9, 965–970. [Google Scholar] [CrossRef]
- Sedmak, B.; Kosi, G. The role of microcystins in heavy cyanobacterial bloom formation. J. Plankton Res. 1998, 20, 691–708. [Google Scholar] [CrossRef]
- Sedmak, B.; Carmeli, S.; Elersek, T. “Non-toxic” cyclic peptides induce lysis of cyanobacteria-An effective cell population density control mechanism in cyanobacterial blooms. Microb. Ecol. 2008, 56, 201–209. [Google Scholar] [CrossRef]
- Šulčius, S.; Mazur-Marzec, H.; Vitonytė, I.; Kvederavičiūtė, K.; Kuznecova, J.; Šimoliūnas, E.; Holmfeldt, K. Insights into cyanophage-mediated dynamics of nodularin and other non-ribosomal peptides in Nodularia spumigena. Harmful Algae 2018, 78, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Suikkanen, S.; Fistarol, G.O.; Granéli, E. Effects of cyanobacterial allelochemicals on a natural plankton community. Mar. Ecol. Prog. Ser. 2005, 287, 1–9. [Google Scholar] [CrossRef]
- Dziga, D.; Kokociński, M.; Barylski, J.; Nowicki, G.; Maksylewicz, A.; Antosiak, A.; Banaś, A.K.; Strzałka, W. Correlation between specific groups of heterotrophic bacteria and microcystin biodegradation in freshwater bodies of central Europe. FEMS Microbiol. Ecol. 2019, 95, fiz162. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, H.; Dürselen, C.D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Goldman, C.R.; Gerletti, M.; Jawornický, P.; Melchiorri-Santolini, U.; Amezaga, E. Primary productivity, bacteria, phyto- and zooplankton in Lake Maggiore: Correlations and relationships with ecological factors. Mem. Inst. Ital. Hydrobiol. 1968, 23, 49–127. [Google Scholar]
- PN-ISO 10260. Water Quality. Measurement of Biochemical Parameters. Spectrophotometric Determination of Chlorophyll-a Concentration; PWN: Warsaw, Poland, 2002; 11p. [Google Scholar]
- PN-EN ISO 6878. Water Quality. Determination of Phosphorus. Ammonium Molybdate Spectrometric Method; PKN: Warsaw, Poland, 2004; 14p. [Google Scholar]
- DIN ISO 13395. Water Quality—Determination of Nitrite Nitrogen and Nitrate Nitrogen and the Sum of Both by Flow Analysis (CFA and FIA) and Spectrometric Detection; International Organization for Standardization: Geneva, Switzerland, 1996; 18p. [Google Scholar]
- DIN EN ISO 29441. Water Quality—Determination of Total Nitrogen after UV Digestion—Method Using Flow Analysis (CFA and FIA) and Spectrometric Detection; International Organization for Standardization: Geneva, Switzerland, 2010; 16p. [Google Scholar]
- Mazur-Marzec, H.; Kaczkowska, M.J.; Błaszczyk, A.; Akcaalan, R.; Spoof, L.; Meriluoto, J. Diversity of peptides produced by Nodularia spumigena from various geographical regions. Mar. Drugs 2013, 11, 1–19. [Google Scholar] [CrossRef]
- Jaccard, P. The distribution of the flora in the alpine zone. New Phytol. 1912, 11, 37–50. [Google Scholar] [CrossRef]
- Sedmak, B.; Eleršek, T. Microcystins induce morphological and physiological changes in selected representative phytoplanktons. Microb. Ecol. 2006, 51, 508–515. [Google Scholar] [CrossRef]
- Eybe, T.; Audinot, J.N.; Bohn, T.; Guignard, C.; Migeon, H.N.; Hoffmann, L. NanoSIMS 50 elucidation of the natural element composition in structures of cyanobacteria and their exposure to halogen compounds. J. Appl. Microb. 2008, 105, 1502–1510. [Google Scholar] [CrossRef]
- Davis, T.W.; Berry, D.L.; Boyer, G.L.; Gobler, C.J. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 2009, 8, 715–725. [Google Scholar] [CrossRef]
- Neilan, B.A.; Pearson, L.A.; Muenchhoff, J.; Moffitt, M.C.; Dittmann, E. Environmental conditions that influence toxin biosynthesis in cyanobacteria. Environ. Microbiol. 2013, 15, 1239–1253. [Google Scholar] [CrossRef] [PubMed]
- Steiner, K.; Wood, S.A.; Puddick, J.; Hawes, I.; Dietrich, D.R.; Hamilton, D.P.A. A comparison of bacterial community structure, activity and microcystins associated with formation and breakdown of a cyanobacterial scum. Aquat. Microb. Ecol. 2017, 80, 243–256. [Google Scholar] [CrossRef]
- Burkholder, J.M.; Glibert, P.M.; Skelton, H.M. Mixotrophy, a major mode of nutrition for harmful algal species in eutrophic waters. Harmful Algae 2008, 8, 77–93. [Google Scholar] [CrossRef]
- Gan, N.; Xiao, Y.; Zhu, L.; Wu, Z.; Liu, J.; Hu, C.; Song, L. The role of microcystins in maintaining colonies of bloom-forming Microcystis spp. Environ. Microbiol. 2012, 14, 730–742. [Google Scholar] [CrossRef]
- Repka, S.; Koivula, M.; Harjunpä, V.; Rouhiainen, L.; Sivonen, K. Effects of phosphate and light on growth of and bioactive peptide production by the cyanobacterium Anabaena strain 90 and its anabaenopeptilide mutant. Appl. Environ. Microbiol. 2004, 70, 4551–4560. [Google Scholar] [CrossRef]
- Briand, E.; Bormans, M.; Quiblier, C.; Salençon, M.J.; Humbert, J.F. Evidence of the cost of the production of microcystins by Microcystis aeruginosa under differing light and nitrate environmental conditions. PLoS ONE 2012, 7, e29981. [Google Scholar] [CrossRef]
- Campbell, D.; Hurry, V.; Clarke, A.K.; Gustafsson, P.; Öquist, G. Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiol. Mol. Biol. Rev. 1998, 62, 667–683. [Google Scholar] [CrossRef]
- Scherer, P.I.; Raeder, U.; Geist, J.; Zwirglmaier, K. Influence of temperature, mixing, and addition of microcystin-LR on microcystin gene expression in Microcystis aeruginosa. Microbiol. Open 2017, 6, e00393. [Google Scholar] [CrossRef]
- Orr, P.T.; Jones, G.J. Relationship between microcystin production and cell division rates in nitrogen-limited Microcystis aeruginosa cultures. Limnol. Oceanogr. 1998, 43, 1604–1614. [Google Scholar] [CrossRef]
- Agha, R.; Cires, S.; Woermer, L.; Antonio Dominguez, J.; Quesada, A. Multi-scale strategies for the monitoring of freshwater cyanobacteria: Reducing the sources of uncertainty. Water Res. 2012, 46, 3043–3053. [Google Scholar] [CrossRef]
- Savadova, K.; Mazur-Marzec, H.; Karosienė, J.; Kasperovičienė, J.; Vitonytė, I.; Toruńska-Sitarz, A.; Koreivienė, J. Effect of increased temperature on native and alien nuisance cyanobacteria from temperate lakes: An experimental approach. Toxins 2018, 10, 445. [Google Scholar] [CrossRef]
- Briand, J.-F.; Jacquet, S.; Flinois, C.; Avois-Jacquet, C.; Maisonnette, C.; Leberre, B.; Humbert, J.-F. Variations in the microcystin production of Planktothrix rubescens (Cyanobacteria) assessed from a four-year survey of Lac du Bourget (France) and from laboratory experiments. Microb. Ecol. 2005, 50, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Tonk, L.; Visser, P.; Christiansen, G.; Dittmann, E.; Sneldfer, E.; Wiedner, C.; Mur, L.; Huisman, J. The microcystin composition of the cyanobacterium Planktothrix agardhii changes toward a more toxic variant with increasing light intensity. Appl. Environ. Microbiol. 2005, 71, 5177–5181. [Google Scholar] [CrossRef] [PubMed]
- Rohrlack, T.; Edvardsen, B.; Skulberg, R.; Halstvedt, C.B.; Utkilen, H.C.; Ptacnik, R.; Skulberg, O.M. Oligopeptide chemotypes of the toxic freshwater cyanobacterium Planktothrix can form subpopulations with dissimilar ecological traits. Limnol. Oceanogr. 2008, 534, 1279–1293. [Google Scholar] [CrossRef]
- Welker, M.; Sejnohova, L.; Nemethova, D.; von Dohren, H.; Jarkovsky, J.; Marsalek, B. Seasonal shifts in chemotype composition of Microcystis sp. communities in the pelagial and the sediment of a shallow reservoir. Limnol. Oceanogr. 2007, 52, 609–619. [Google Scholar] [CrossRef]
- Briand, E.; Bormans, M.; Gugger, M.; Dorrestein, P.C.; Gerwick, W.H. Changes in secondary metabolic profiles of Microcystis aeruginosa strains in response to intraspecific interactions. Environ. Microbiol. 2016, 18, 384–400. [Google Scholar] [CrossRef]
- Fujii, K.; Sivonen, K.; Naganawa, E.; Harada, K. Non-toxic peptides from toxic cyanobacteria, Oscillatoria agardhii. Tetrahedron 2000, 56, 725–733. [Google Scholar] [CrossRef]
| Parameters | Biomasses, Extracts and Samples Used in Experiments (As Controls and Treated) | |||
|---|---|---|---|---|
| Lake, sampling year | Lake Syczyńskie 2010 Pa-A | Lake Czarne S. 2013 Pa-B | Lake Syczyńskie 2015 (conditions correspond to the controls at the beginning of the experiments) | |
| Species contribution to the biomass | Planktothrix agardhii (95%) Microcystis aeruginosa (5%) | P. agardhii (82%) Microcystis spp. (10%) Aphanizomenon gracile (8%) | P. agardhii (96%), Planktolyngbya limnetica (1.4%), other cyanobacteria (0.6%) | |
| Biomass (mg/L) | 2800 ± 80 | 2750 ± 33 | 105 ± 2 | |
| Chl-a (mg/L) | 81.86 ± 2.50 | 80.87 ± 4.1 | 0.357 ± 0.042 | |
| Total MCs (µg/L) | 1030 ± 150 | 13,040 ± 1790 | 38 ± 2 | |
| MCs in P. agardhii biomass (µg/mg) | 368 ± 54 | 4748 ± 651 | 362 ± 19 | |
| MCs’ variants (%) | [Asp3,Dhb7] MC-RR; [Asp3]MC-RR; MC-RR | 47.3 a | 49.0 | 94.4 b |
| [Asp3] MC-HtyrR | 38.1 | n.d. | n.d. | |
| dmMC-LR; MC-LR | 14.6 | 34.1 | 5.6 c | |
| MC-LF | n.d. | 16.9 | n.d. | |
| P-PO4 (mg/L) | 20.07 ± 0.52 | 38.68 ± 0.47 | n.det. | |
| P tot. (mg/L) | 110.70 ± 10.25 | 129.37 ± 14.71 | 0.202 | |
| N-NO3 (mg/L) | 74.63 ± 2.60 | 250.32 ± 3.37 | n.det. | |
| N tot. (mg/L) | 641.48 ± 3.57 | 720.00 ± 3.99 | 6.843 | |
| Extracts | P. agardhii Control | P. agardhii Exposed to the Extract Pa-A | P. agardhii Exposed to the Extract Pa-B | ||
|---|---|---|---|---|---|
| Peptide Classes | Pa-A | Pa-B | |||
| Microcystins (MCs) | 4 | 6 | 3 | 4 | 2 |
| Cyanopeptolins (CPLs) | 11 | 10 | 6 | 7 | 5 |
| Anabaenopeptins (APs) | 10 | 5 | 4 | 4 | 4 |
| Planktopeptin (PLP) | 1 | 1 | 1 | n.d. | n.d. |
| Aeruginosisns (AERs) | 13 | 19 | 8 | 8 | 8 |
| Aeruginosamide (AERD) | n.d. | 1 | n.d. | n.d. | 1 |
| Microginins (MRGs) | n.d. | 4 | n.d. | 1 | n.d. |
| Unidentified peptides | 11 | 9 | 7 | 1 | 3 |
| Total number | 50 | 55 | 29 | 25 | 23 |
| Compared Samples | Extract Pa-B | P. agardhii Control | P. agardhii Exposed to the Extract Pa-A | P. agardhii Exposed to the Extract Pa-B |
|---|---|---|---|---|
| Extract Pa-A | 0.16 | 0.30 | 0.25 | 0.28 |
| Extract Pa-B | - | 0.11 | 0.10 | 0.15 |
| P. agardhii Control | - | - | 0.38 | 0.49 |
| P. agardhii exposed to the extract Pa-A | - | - | - | 0.60 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toporowska, M.; Mazur-Marzec, H.; Pawlik-Skowrońska, B. The Effects of Cyanobacterial Bloom Extracts on the Biomass, Chl-a, MC and Other Oligopeptides Contents in a Natural Planktothrix agardhii Population. Int. J. Environ. Res. Public Health 2020, 17, 2881. https://doi.org/10.3390/ijerph17082881
Toporowska M, Mazur-Marzec H, Pawlik-Skowrońska B. The Effects of Cyanobacterial Bloom Extracts on the Biomass, Chl-a, MC and Other Oligopeptides Contents in a Natural Planktothrix agardhii Population. International Journal of Environmental Research and Public Health. 2020; 17(8):2881. https://doi.org/10.3390/ijerph17082881
Chicago/Turabian StyleToporowska, Magdalena, Hanna Mazur-Marzec, and Barbara Pawlik-Skowrońska. 2020. "The Effects of Cyanobacterial Bloom Extracts on the Biomass, Chl-a, MC and Other Oligopeptides Contents in a Natural Planktothrix agardhii Population" International Journal of Environmental Research and Public Health 17, no. 8: 2881. https://doi.org/10.3390/ijerph17082881
APA StyleToporowska, M., Mazur-Marzec, H., & Pawlik-Skowrońska, B. (2020). The Effects of Cyanobacterial Bloom Extracts on the Biomass, Chl-a, MC and Other Oligopeptides Contents in a Natural Planktothrix agardhii Population. International Journal of Environmental Research and Public Health, 17(8), 2881. https://doi.org/10.3390/ijerph17082881








