Maternal Resveratrol Treatment Re-Programs and Maternal High-Fat Diet-Induced Retroperitoneal Adiposity in Male Offspring
Abstract
1. Introduction
2. Materials and Methods
2.1. Rats and Experimental Design
2.2. Test for Intraperitoneal Glucose Tolerance
2.3. Tissue Collection and Blood Sampling
2.4. Histological Examination
2.5. Western Blot Analysis
2.6. For Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
2.7. Statistical Analysis
3. Results
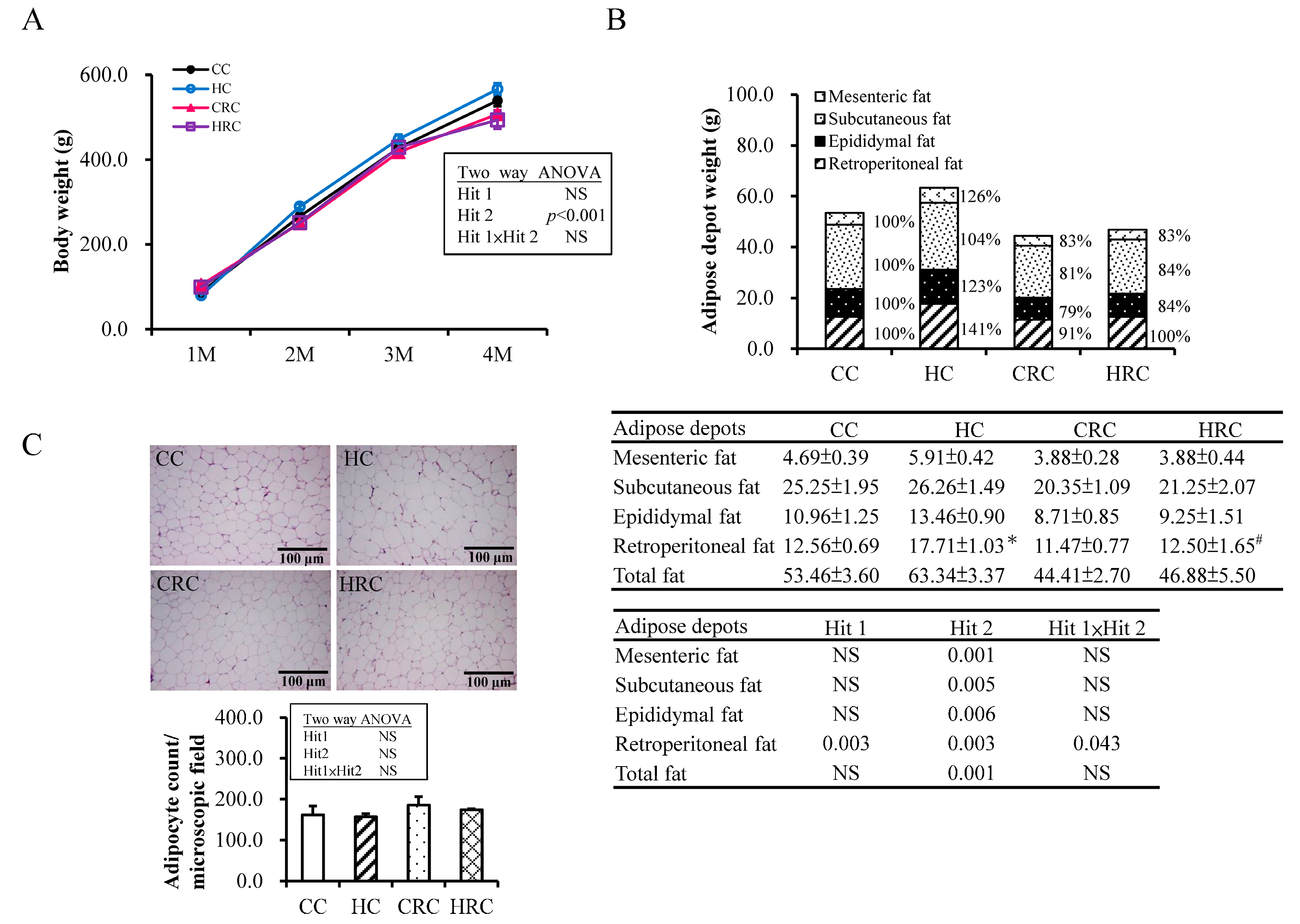
3.1. Maternal Resveratrol Treatment Re-Programmed the Maternal HF Diet Exposure-Induced Visceral Adiposity in the Offspring
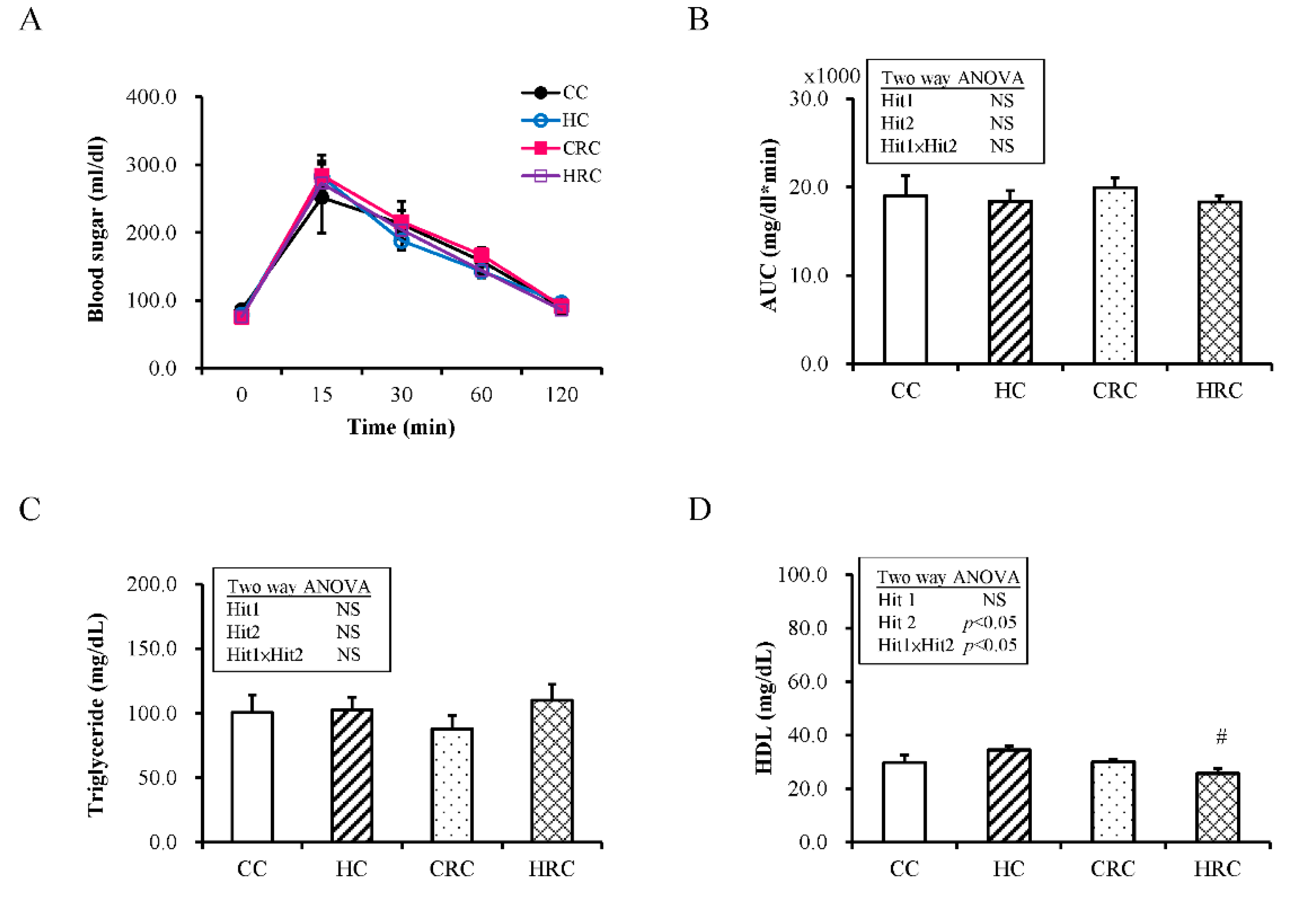
3.2. Maternal Resveratrol Treatment Reduced the Maternal HF Diet Exposure-Induced Alterations in Liver Enzyme Concentrations
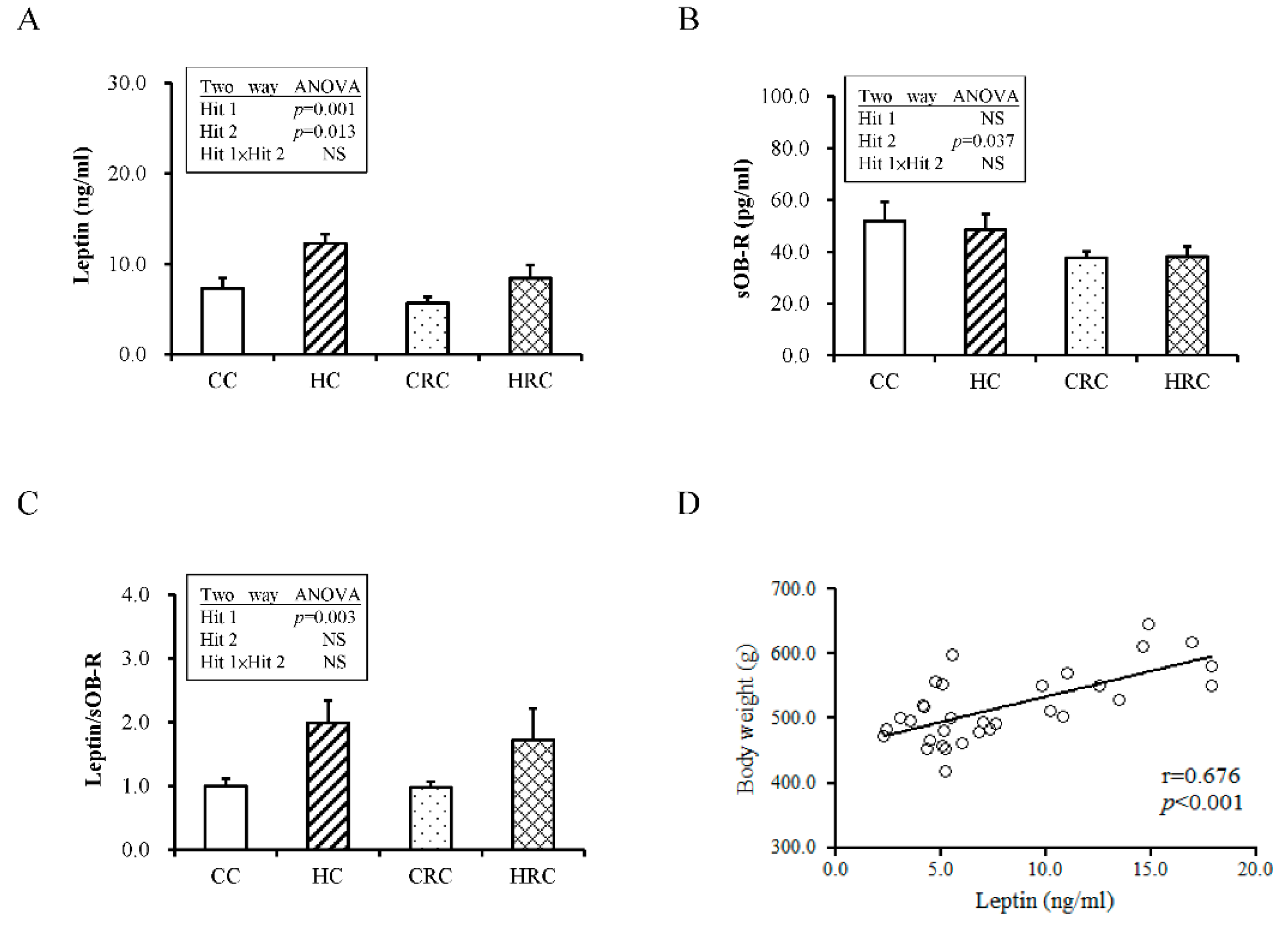
3.3. Maternal Resveratrol Treatment Ameliorated Leptin Dysregulation Induced by Maternal HF Diet Exposure
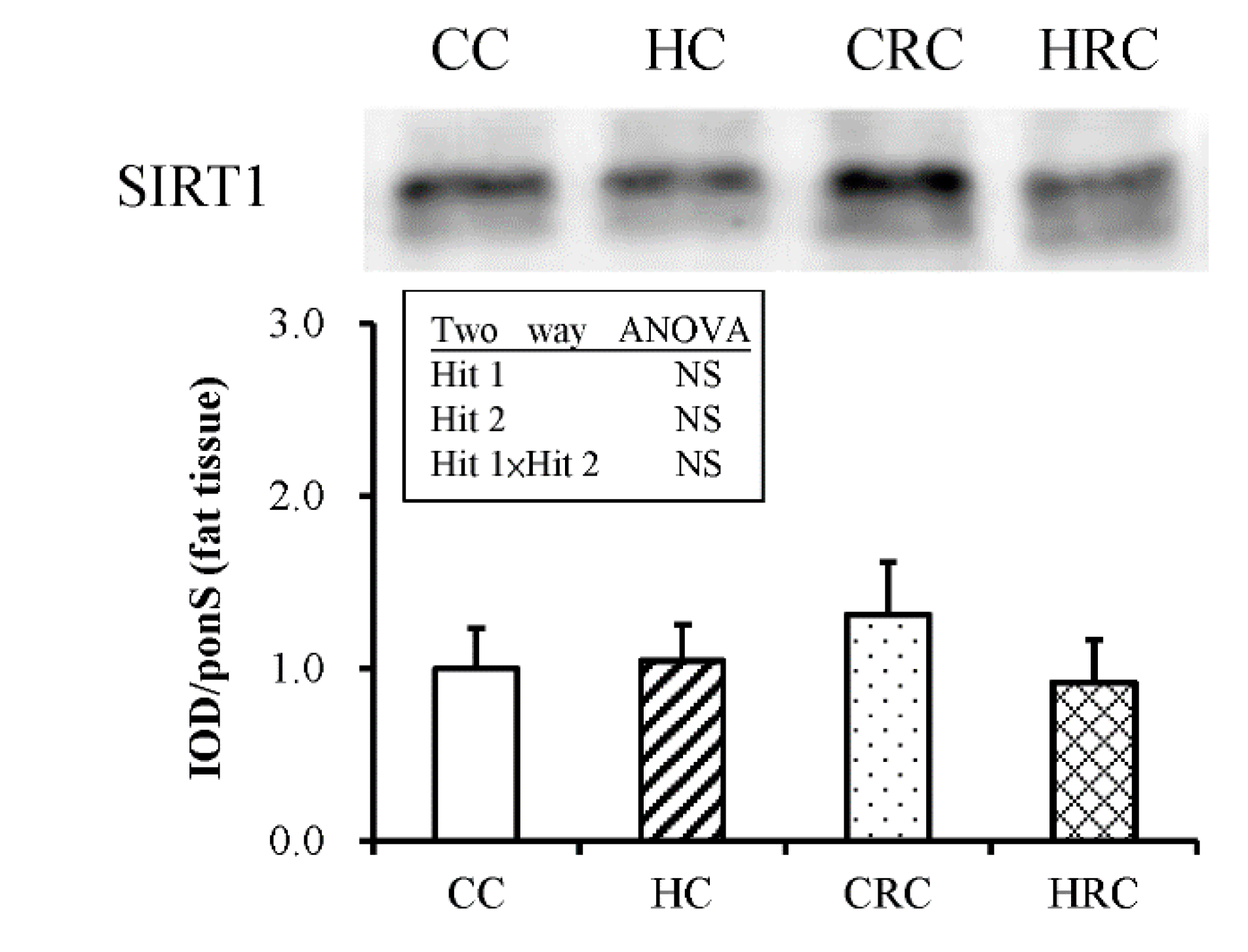
3.4. Maternal Resveratrol Treatment Reduced Maternal HF Diet Exposure-Induced Adiposity in the Offspring via a SIRT1-Independent Mechanism
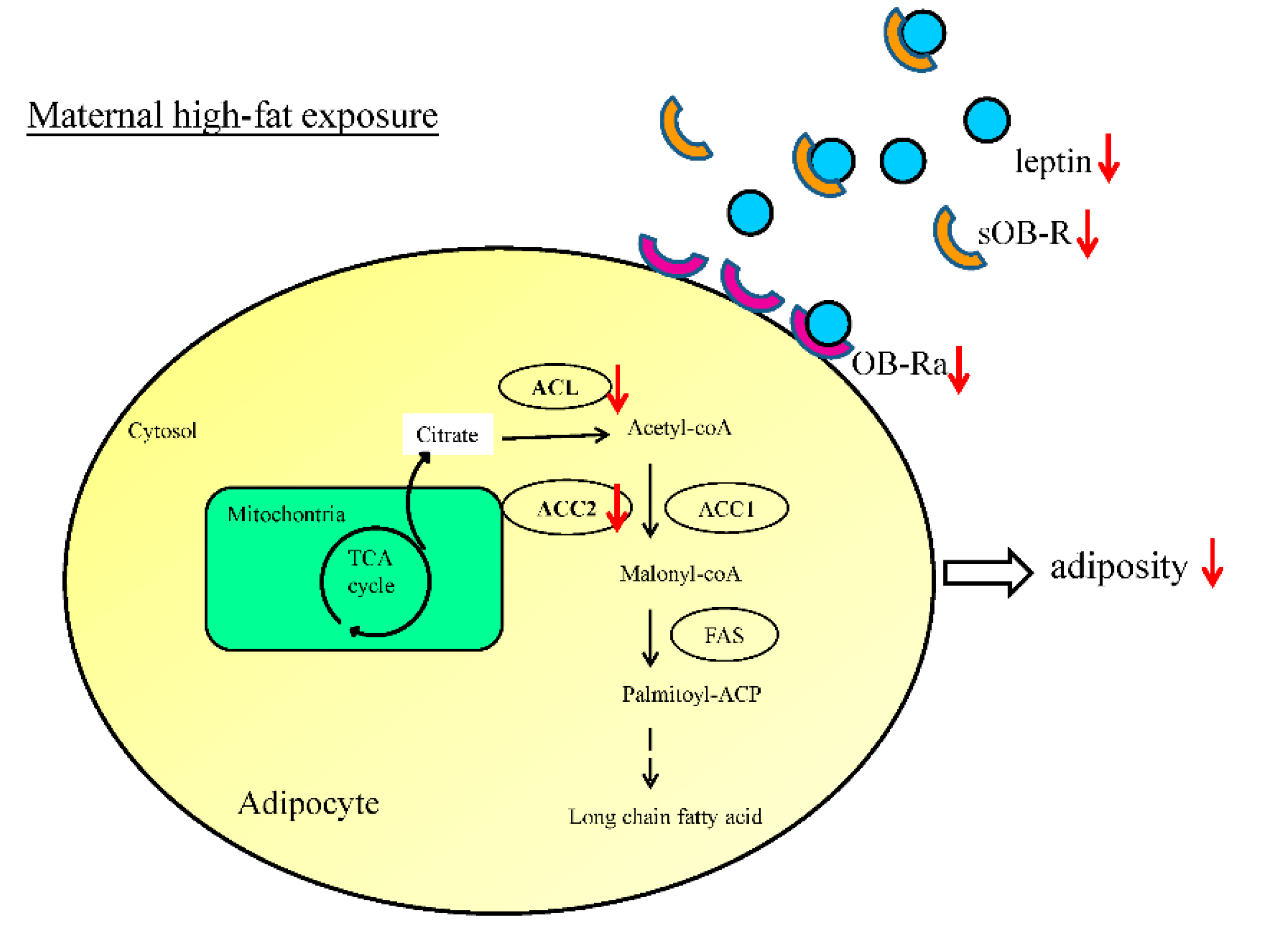
3.5. Administration of Resveratrol during Pregnancy Altered the Expression of Genes Crucial for Fatty Acid Synthesis in the Offspring
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barker, D.J. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Breton, C. The hypothalamus-adipose axis is a key target of developmental programming by maternal nutritional manipulation. J. Endocrinol. 2013, 216, R19–R31. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M. Metabolically healthy overweight and obesity. Ann. Intern. Med. 2014, 160, 515–516. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yu, X.; Keim, S.; Li, L.; Zhang, L.; Zhang, J. Maternal prepregnancy obesity and child neurodevelopment in the Collaborative Perinatal Project. Int. J. Epidemiol. 2014, 43, 783–792. [Google Scholar] [CrossRef]
- Rivera, H.M.; Christiansen, K.J.; Sullivan, E.L. The role of maternal obesity in the risk of neuropsychiatric disorders. Front. Neurosci. 2015, 9, 194. [Google Scholar] [CrossRef]
- Rodriguez, A.; Miettunen, J.; Henriksen, T.B.; Olsen, J.; Obel, C.; Taanila, A.; Ebeling, H.; Linnet, K.M.; Moilanen, I.; Jarvelin, M.R. Maternal adiposity prior to pregnancy is associated with ADHD symptoms in offspring: Evidence from three prospective pregnancy cohorts. Int. J. Obes. 2008, 32, 550–557. [Google Scholar] [CrossRef]
- Krakowiak, P.; Walker, C.K.; Bremer, A.A.; Baker, A.S.; Ozonoff, S.; Hansen, R.L.; Hertz-Picciotto, I. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 2012, 129, e1121–e1128. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.; Eriksson, J.G.; Broekman, B.F. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef]
- Parlee, S.D.; MacDougald, O.A. Maternal nutrition and risk of obesity in offspring: The Trojan horse of developmental plasticity. Biochim. Biophys. Acta 2014, 1842, 495–506. [Google Scholar] [CrossRef]
- Reynolds, R.M.; Allan, K.M.; Raja, E.A.; Bhattacharya, S.; McNeill, G.; Hannaford, P.C.; Sarwar, N.; Lee, A.J.; Bhattacharya, S.; Norman, J.E. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: Follow-up of 1,323,275 person years. BMJ 2013, 347, f4539. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Developmental Programming of the Metabolic Syndrome: Can We Reprogram with Resveratrol? Int. J. Mol. Sci. 2018, 19, 2584. [Google Scholar] [CrossRef]
- Chang, G.Q.; Gaysinskaya, V.; Karatayev, O.; Leibowitz, S.F. Maternal high-fat diet and fetal programming: Increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 12107–12119. [Google Scholar] [CrossRef]
- Rajia, S.; Chen, H.; Morris, M.J. Maternal overnutrition impacts offspring adiposity and brain appetite markers-modulation by post-weaning diet. J. Neuroendocrinol. 2010, 22, 905–914. [Google Scholar]
- Miles, E.A.; Calder, P.C. Maternal diet and its influence on the development of allergic disease. Clin. Exp. Allergy 2015, 45, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.Y.; Tain, Y.L.; Yu, H.R.; Huang, L.T. The Effects of Resveratrol in the Treatment of Metabolic Syndrome. Int. J. Mol. Sci. 2019, 20, 535. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.S.; Canto, C. The molecular targets of resveratrol. Biochim. Et Biophys. Acta 2015, 1852, 1114–1123. [Google Scholar] [CrossRef]
- Huang, Y.C.; Huang, L.T.; Sheen, J.M.; Hou, C.Y.; Yeh, Y.T.; Chiang, C.P.; Lin, I.C.; Tiao, M.M.; Tsai, C.C.; Lin, Y.J.; et al. Resveratrol treatment improves the altered metabolism and related dysbiosis of gut programed by prenatal high-fat diet and postnatal high-fat diet exposure. J. Nutr. Biochem. 2020, 75, 108260. [Google Scholar] [CrossRef] [PubMed]
- Ros, P.; Diaz, F.; Freire-Regatillo, A.; Argente-Arizon, P.; Barrios, V.; Argente, J.; Controlen, J.A. Resveratrol Intake During Pregnancy and Lactation Modulates the Early Metabolic Effects of Maternal Nutrition Differently in Male and Female Offspring. Endocrinology 2018, 159, 810–825. [Google Scholar] [CrossRef]
- Yu, H.R.; Sheen, J.M.; Tiao, M.M.; Tain, Y.L.; Chen, C.C.; Lin, I.C.; Lai, Y.J.; Tsai, C.C.; Lin, Y.J.; Tsai, C.C.; et al. Resveratrol Treatment Ameliorates Leptin Resistance and Adiposity Programed by the Combined Effect of Maternal and Post-Weaning High-Fat Diet. Mol. Nutr. Food Res. 2019, e1801385. [Google Scholar] [CrossRef]
- Chen, H.-E.; Lin, Y.-J.; Lin, I.-C.; Yu, H.-R.; Sheen, J.-M.; Tsai, C.-C.; Huang, L.-T.; Tain, Y.-L. Resveratrol prevents combined prenatal NG-nitro-L-arginine-methyl ester (L-NAME) treatment plus postnatal high-fat diet induced programmed hypertension in adult rat offspring: Interplay between nutrient-sensing signals, oxidative stress and gut microbiota. J. Nutr. Biochem. 2019, 70, 28–37. [Google Scholar] [CrossRef]
- Yu, H.R.; Tain, Y.L.; Tiao, M.M.; Chen, C.C.; Sheen, J.M.; Lin, I.C.; Li, S.W.; Tsai, C.C.; Lin, Y.J.; Hsieh, K.S.; et al. Prenatal dexamethasone and postnatal high-fat diet have a synergistic effect of elevating blood pressure through a distinct programming mechanism of systemic and adipose renin-angiotensin systems. Lipids Health Dis. 2018, 17, 50. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.R.; Tain, Y.L.; Sheen, J.M.; Tiao, M.M.; Chen, C.C.; Kuo, H.C.; Hung, P.L.; Hsieh, K.S.; Huang, L.T. Prenatal Dexamethasone and Postnatal High-Fat Diet Decrease Interferon Gamma Production through an Age-Dependent Histone Modification in Male Sprague-Dawley Rats. Int. J. Mol. Sci. 2016, 17, 1610. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Su, Y.T.; Liu, T.Y.; Tsai, C.M.; Chang, C.H.; Yu, H.R. L-Arginine and L-Citrulline Supplementation Have Different Programming Effect on Regulatory T-Cells Function of Infantile Rats. Front. Immunol. 2018, 9, 2911. [Google Scholar] [CrossRef]
- Herrick, J.E.; Panza, G.S.; Gollie, J.M. Leptin, Leptin Soluble Receptor, and the Free Leptin Index following a Diet and Physical Activity Lifestyle Intervention in Obese Males and Females. J. Obes. 2016, 2016, 8375828. [Google Scholar] [CrossRef] [PubMed]
- Sandhofer, A.; Laimer, M.; Ebenbichler, C.F.; Kaser, S.; Paulweber, B.; Patsch, J.R. Soluble leptin receptor and soluble receptor-bound fraction of leptin in the metabolic syndrome. Obes. Res. 2003, 11, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Owecki, M.; Nikisch, E.; Miczke, A.; Pupek-Musialik, D.; Sowinski, J. Leptin, soluble leptin receptors, free leptin index, and their relationship with insulin resistance and BMI: High normal BMI is the threshold for serum leptin increase in humans. Horm. Metab. Res. 2010, 42, 585–589. [Google Scholar] [CrossRef]
- Busserolles, J.; Mazur, A.; Gueux, E.; Rock, E.; Rayssiguier, Y. Metabolic syndrome in the rat: Females are protected against the pro-oxidant effect of a high sucrose diet. Exp. Biol. Med. 2002, 227, 837–842. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Fairus, A.; Ima-Nirwana, S. Animal models of metabolic syndrome: A review. Nutr. Metab. 2016, 13, 65. [Google Scholar] [CrossRef]
- Hung, C.S.; Lee, J.K.; Yang, C.Y.; Hsieh, H.R.; Ma, W.Y.; Lin, M.S.; Liu, P.H.; Shih, S.R.; Liou, J.M.; Chuang, L.M.; et al. Measurement of visceral fat: Should we include retroperitoneal fat? PLoS ONE 2014, 9, e112355. [Google Scholar] [CrossRef]
- Brennan, A.M.; Mantzoros, C.S. Drug Insight: The role of leptin in human physiology and pathophysiology—Emerging clinical applications. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 318–327. [Google Scholar] [CrossRef]
- Huan, J.N.; Li, J.; Han, Y.; Chen, K.; Wu, N.; Zhao, A.Z. Adipocyte-selective reduction of the leptin receptors induced by antisense RNA leads to increased adiposity, dyslipidemia, and insulin resistance. J. Biol. Chem. 2003, 278, 45638–45650. [Google Scholar] [CrossRef] [PubMed]
- Lukaszewski, M.A.; Eberle, D.; Vieau, D.; Breton, C. Nutritional manipulations in the perinatal period program adipose tissue in offspring. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1195–E1207. [Google Scholar] [CrossRef]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L.; et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. New Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.; Caron, A.; Richard, D.; Picard, F. Role of leptin resistance in the development of obesity in older patients. Clin. Interv. Aging 2013, 8, 829–844. [Google Scholar] [PubMed]
- Seron, K.; Corset, L.; Vasseur, F.; Boutin, P.; Gomez-Ambrosi, J.; Salvador, J.; Fruhbeck, G.; Froguel, P. Distinct impaired regulation of SOCS3 and long and short isoforms of the leptin receptor in visceral and subcutaneous fat of lean and obese women. Biochem. Biophys. Res. Commun. 2006, 348, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.K.; Opentanova, I.; Ohannesian, J.P.; Kolaczynski, J.W.; Heiman, M.L.; Hale, J.; Becker, G.W.; Bowsher, R.R.; Stephens, T.W.; Caro, J.F. Evidence of free and bound leptin in human circulation. Studies in lean and obese subjects and during short-term fasting. J. Clin. Investig. 1996, 98, 1277–1282. [Google Scholar] [CrossRef]
- Schaab, M.; Kausch, H.; Klammt, J.; Nowicki, M.; Anderegg, U.; Gebhardt, R.; Rose-John, S.; Scheller, J.; Thiery, J.; Kratzsch, J. Novel regulatory mechanisms for generation of the soluble leptin receptor: Implications for leptin action. PLoS ONE 2012, 7, e34787. [Google Scholar] [CrossRef]
- Sun, Q.; van Dam, R.M.; Meigs, J.B.; Franco, O.H.; Mantzoros, C.S.; Hu, F.B. Leptin and soluble leptin receptor levels in plasma and risk of type 2 diabetes in U.S. women: A prospective study. Diabetes 2010, 59, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Medici, V.; Ali, M.R.; Seo, S.; Aoki, C.A.; Rossaro, L.; Kim, K.; Fuller, W.D.; Vidovszky, T.J.; Smith, W.; Jiang, J.X.; et al. Increased soluble leptin receptor levels in morbidly obese patients with insulin resistance and nonalcoholic fatty liver disease. Obesity 2010, 18, 2268–2273. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Emoto, M.; Morioka, T.; Kawano, N.; Lee, E.; Urata, H.; Tsuchikura, S.; Motoyama, K.; Mori, K.; Fukumoto, S. Clinical impact of the leptin to soluble leptin receptor ratio on subclinical carotid atherosclerosis in patients with type 2 diabetes. J. Atheroscler. Thromb. 2013, 20, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Catli, G.; Anik, A.; Tuhan, H.U.; Kume, T.; Bober, E.; Abaci, A. The relation of leptin and soluble leptin receptor levels with metabolic and clinical parameters in obese and healthy children. Peptides 2014, 56, 72–76. [Google Scholar] [CrossRef]
- Mohammadzadeh, G.; Ghaffari, M.A.; Bafandeh, A.; Hosseini, S.M. Association of serum soluble leptin receptor and leptin levels with breast cancer. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 433–438. [Google Scholar]
- Liu, R.B.; Liu, Y.; Lv, L.Q.; Xiao, W.; Gong, C.; Yue, J.X. Effects of Metformin Treatment on Soluble Leptin Receptor Levels in Women with Polycystic Ovary Syndrome. Curr. Med. Sci. 2019, 39, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Van Dielen, F.M.; van ’t Veer, C.; Buurman, W.A.; Greve, J.W. Leptin and soluble leptin receptor levels in obese and weight-losing individuals. J. Clin. Endocrinol. Metab. 2002, 87, 1708–1716. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, Q.; Yu, Y.; Zhao, F.; Huang, P.; Zeng, R.; Qi, R.Z.; Li, W.; Liu, Y. Leptin contributes to the adaptive responses of mice to high-fat diet intake through suppressing the lipogenic pathway. PLoS ONE 2009, 4, e6884. [Google Scholar] [CrossRef] [PubMed]
- Ferre, P.; Foufelle, F. SREBP-1c transcription factor and lipid homeostasis: Clinical perspective. Horm. Res. 2007, 68, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Guay, C.; Madiraju, S.R.; Aumais, A.; Joly, E.; Prentki, M. A role for ATP-citrate lyase, malic enzyme, and pyruvate/citrate cycling in glucose-induced insulin secretion. J. Biol. Chem. 2007, 282, 35657–35665. [Google Scholar] [CrossRef]
- Munday, M.R. Regulation of mammalian acetyl-CoA carboxylase. Biochem. Soc. Trans. 2002, 30, 1059–1064. [Google Scholar] [CrossRef]
- Abu-Elheiga, L.; Brinkley, W.R.; Zhong, L.; Chirala, S.S.; Woldegiorgis, G.; Wakil, S.J. The subcellular localization of acetyl-CoA carboxylase 2. Proc. Natl. Acad. Sci. USA 2000, 97, 1444–1449. [Google Scholar] [CrossRef]
- Kreuz, S.; Schoelch, C.; Thomas, L.; Rist, W.; Rippmann, J.F.; Neubauer, H. Acetyl-CoA carboxylases 1 and 2 show distinct expression patterns in rats and humans and alterations in obesity and diabetes. Diabetes Metab. Res. Rev. 2009, 25, 577–586. [Google Scholar] [CrossRef]
- González-Rodríguez, Á.; Santamaría, B.; Mas-Gutierrez, J.A.; Rada, P.; Fernández-Millán, E.; Pardo, V.; Álvarez, C.; Cuadrado, A.; Ros, M.; Serrano, M.; et al. Resveratrol treatment restores peripheral insulin sensitivity in diabetic mice in a sirt1-independent manner. Mol. Nutr. Food Res. 2015, 59, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Kume, S.; Imaizumi, N.; Koya, D. Resveratrol improves oxidative stress and protects against diabetic nephropathy through normalization of Mn-SOD dysfunction in AMPK/SIRT1-independent pathway. Diabetes 2011, 60, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Resveratrol inhibits insulin responses in a SirT1-independent pathway. Biochem. J. 2006, 397, 519–527. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, T.-A.; Tsai, C.-K.; Huang, L.-T.; Sheen, J.-M.; Tiao, M.-M.; Tain, Y.-L.; Chen, C.-C.; Lin, I.-C.; Lai, Y.-J.; Tsai, C.-C.; et al. Maternal Resveratrol Treatment Re-Programs and Maternal High-Fat Diet-Induced Retroperitoneal Adiposity in Male Offspring. Int. J. Environ. Res. Public Health 2020, 17, 2780. https://doi.org/10.3390/ijerph17082780
Tsai T-A, Tsai C-K, Huang L-T, Sheen J-M, Tiao M-M, Tain Y-L, Chen C-C, Lin I-C, Lai Y-J, Tsai C-C, et al. Maternal Resveratrol Treatment Re-Programs and Maternal High-Fat Diet-Induced Retroperitoneal Adiposity in Male Offspring. International Journal of Environmental Research and Public Health. 2020; 17(8):2780. https://doi.org/10.3390/ijerph17082780
Chicago/Turabian StyleTsai, Ti-An, Chang-Ku Tsai, Li-Tung Huang, Jiunn-Ming Sheen, Mao-Meng Tiao, You-Lin Tain, Chih-Cheng Chen, I-Chun Lin, Yun-Ju Lai, Ching-Chou Tsai, and et al. 2020. "Maternal Resveratrol Treatment Re-Programs and Maternal High-Fat Diet-Induced Retroperitoneal Adiposity in Male Offspring" International Journal of Environmental Research and Public Health 17, no. 8: 2780. https://doi.org/10.3390/ijerph17082780
APA StyleTsai, T.-A., Tsai, C.-K., Huang, L.-T., Sheen, J.-M., Tiao, M.-M., Tain, Y.-L., Chen, C.-C., Lin, I.-C., Lai, Y.-J., Tsai, C.-C., Lin, Y.-J., & Yu, H.-R. (2020). Maternal Resveratrol Treatment Re-Programs and Maternal High-Fat Diet-Induced Retroperitoneal Adiposity in Male Offspring. International Journal of Environmental Research and Public Health, 17(8), 2780. https://doi.org/10.3390/ijerph17082780








