Carbonyls and Carbon Monoxide Emissions from Electronic Cigarettes Affected by Device Type and Use Patterns
Abstract
1. Introduction
2. Materials and Methods
2.1. E-Cigarette Devices and E-Liquids
2.2. E-Cigarette Aerosol Generation
2.3. CO Measurements
2.4. Carbonyl Analysis
2.5. Nicotine Analysis
2.6. Statistical Analyses
3. Results and Discussion
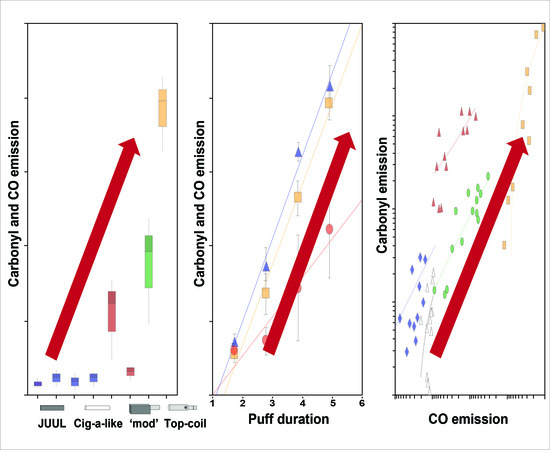
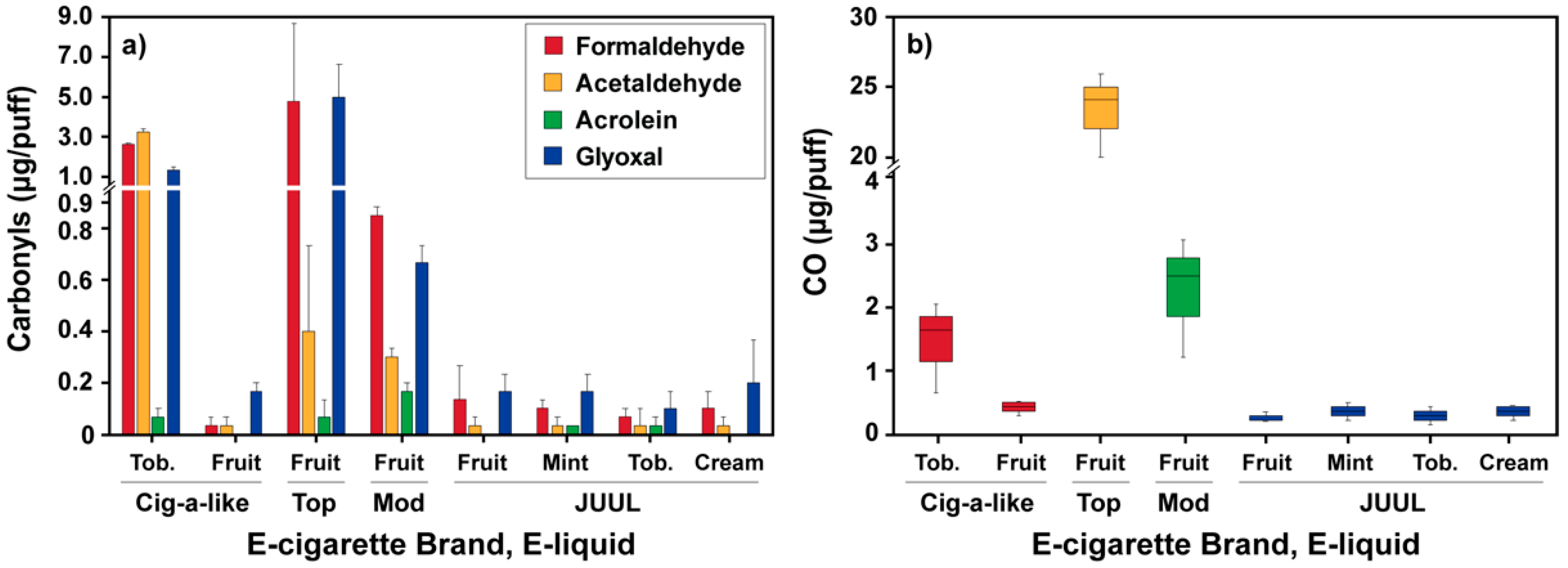
3.1. Carbonyls and CO Emissions from the Different E-Cigarettes
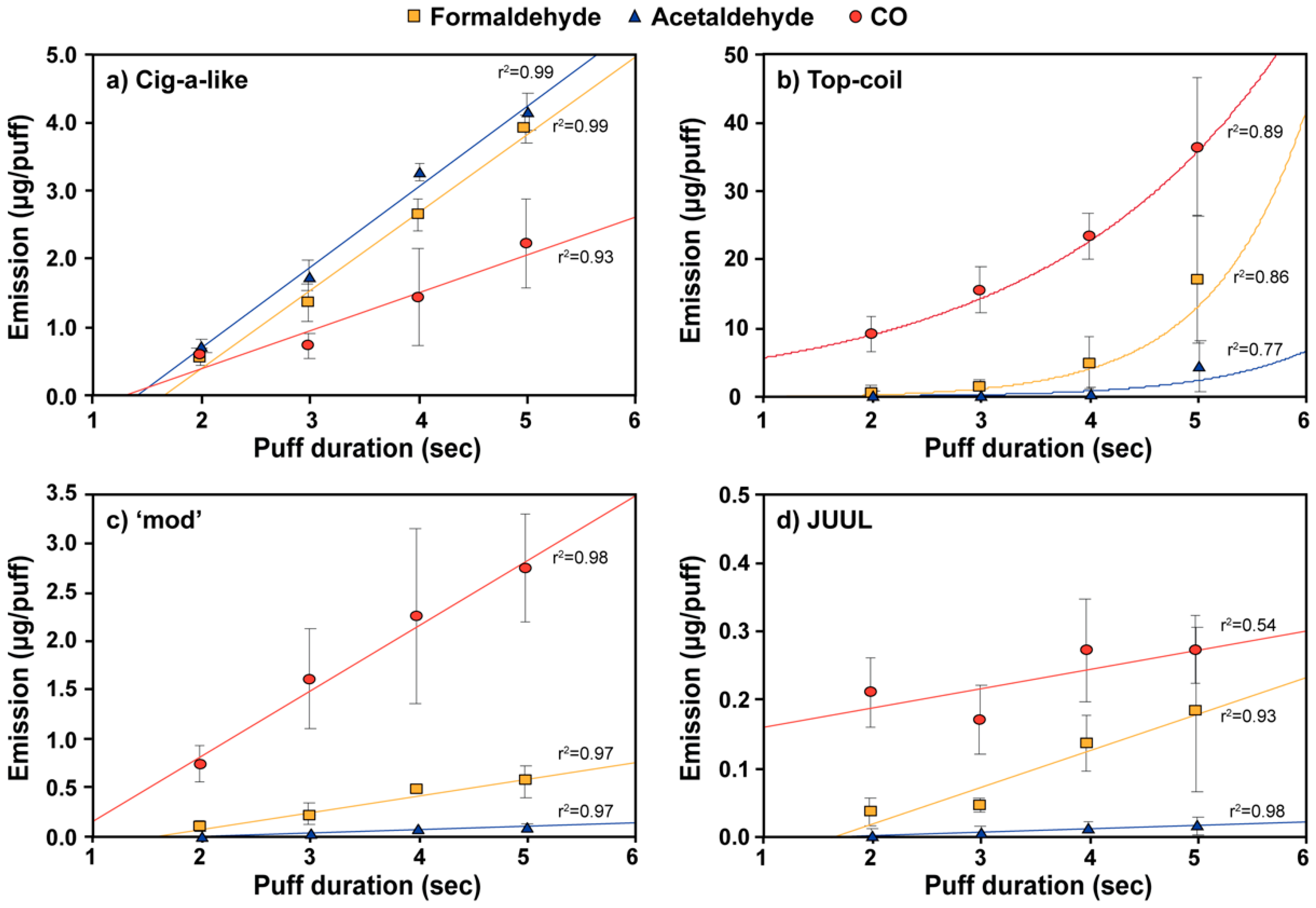
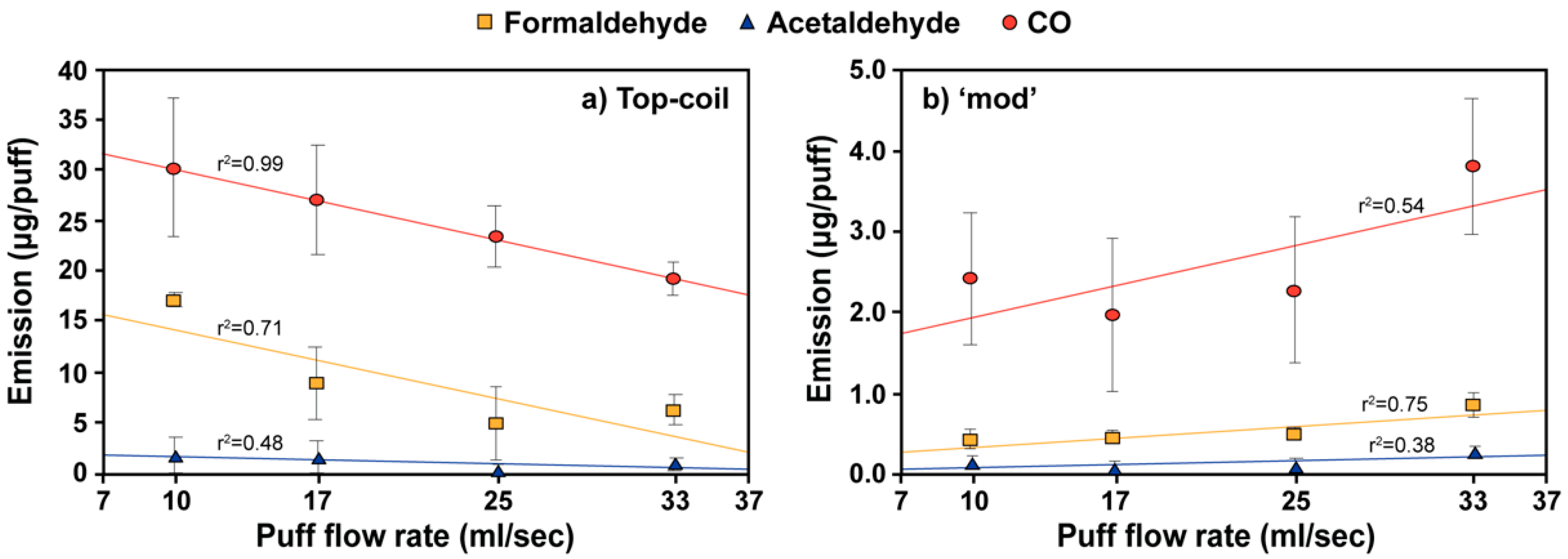
3.2. Effect of Vaping Topography on E-Cigarette Emissions
3.3. Effect of Power Output and Flavor on CO Emission
3.4. Correlation between Carbonyls and CO Emissions
3.5. E-Cigarette vs. Conventional Cigarette Emissions
3.6. Public Health Implications
3.7. Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Electronic nicotine delivery systems, Report by WHO. In Proceedings of the Conference of the Parties to the World Health Organization Framework Convention on Tobacco Control, Moscow, Russia, 13–18 October 2014. [Google Scholar]
- Allen, J.G.; Flanigan, S.S.; LeBlanc, M.; Vallarino, J.; MacNaughton, P.; Stewart, J.H.; Christiani, D.C. Flavoring Chemicals in E-Cigarettes: Diacetyl, 2, 3-Pentanedione, and Acetoin in a Sample of 51 Products, Including Fruit-, Candy-, and Cocktail-Flavored E-Cigarettes. Environ. Health Perspect. 2015, 124, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Khlystov, A.; Samburova, V. Flavoring compounds dominate toxic aldehyde production during e-cigarette vaping. Environ. Sci. Technol. 2016, 50, 13080–13085. [Google Scholar] [CrossRef] [PubMed]
- Geiss, O.; Bianchi, I.; Barrero-Moreno, J. Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. Int. J. Hyg. Envirn. Health 2016, 219, 268–277. [Google Scholar] [CrossRef]
- Gillman, I.; Kistler, K.; Stewart, E.; Paolantonio, A. Effect of variable power levels on the yield of total aerosol mass and formation of aldehydes in e-cigarette aerosols. Regul. Toxicol. Pharmacol. 2016, 75, 58–65. [Google Scholar] [CrossRef]
- Kosmider, L.; Sobczak, A.; Fik, M.; Knysak, J.; Zaciera, M.; Kurek, J.; Goniewicz, M.L. Carbonyl compounds in electronic cigarette vapors—Effects of nicotine solvent and battery output voltage. Nicotine Tob. Res. 2014, 16, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Goniewicz, M.L.; Gawron, M.; Smith, D.M.; Peng, M.; Jacob, P.; Benowitz, N.L. Exposure to nicotine and selected toxicants in cigarette smokers who switched to electronic cigarettes: A longitudinal within-subjects observational study. Nicotine Tob. Res. 2017, 19, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, M.; Logue, J.M.; Montesinos, V.N.; Russell, M.L.; Litter, M.I.; Gundel, L.A.; Destaillats, H. Emissions from electronic cigarettes: Key parameters affecting the release of harmful chemicals. Environ. Sci. Technol. 2016, 50, 9644–9651. [Google Scholar] [CrossRef]
- Pankow, J.F.; Kim, K.; McWhirter, K.J.; Luo, W.; Escobedo, J.O.; Strongin, R.M.; Duell, A.K.; Peyton, D.H. Benzene formation in electronic cigarettes. PLoS ONE 2017, 12, e0173055. [Google Scholar] [CrossRef]
- Schripp, T.; Markewitz, D.; Uhde, E.; Salthammer, T. Does e-cigarette consumption cause passive vaping? Indoor Air 2013, 23, 25–31. [Google Scholar] [CrossRef]
- Williams, M.; Villarreal, A.; Bozhilov, K.; Lin, S.; Talbot, P. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS ONE 2013, 8, e57987. [Google Scholar] [CrossRef]
- Olmedo, P.; Goessler, W.; Tanda, S.; Grau-Perez, M.; Jarmul, S.; Aherrera, A.; Chen, R.; Hilpert, M.; Cohen, J.E.; Navas-Acien, A. Metal concentrations in e-cigarette liquid and aerosol samples: The contribution of metallic coils. Environ. Health Perspect. 2018, 126, 027010. [Google Scholar] [CrossRef]
- Talih, S.; Balhas, Z.; Salman, R.; El-Hage, R.; Karaoghlanian, N.; El-Hellani, A.; Baassiri, M.; Jaroudi, E.; Eissenberg, T.; Saliba, N. Transport phenomena governing nicotine emissions from electronic cigarettes: Model formulation and experimental investigation. Aerosol Sci. Technol. 2017, 51, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Talih, S.; Balhas, Z.; Eissenberg, T.; Salman, R.; Karaoghlanian, N.; El Hellani, A.; Baalbaki, R.; Saliba, N.; Shihadeh, A. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: Measurements and model predictions. Nicotine Tob. Res. 2015, 17, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Kim, K.-H.; Szulejko, J.E. The effect of flavor content in e-liquids on e-cigarette emissions of carbonyl compounds. Environ. Res. 2018, 166, 324–333. [Google Scholar] [CrossRef]
- Zhao, T.; Shu, S.; Guo, Q.; Zhu, Y. Effects of design parameters and puff topography on heating coil temperature and mainstream aerosols in electronic cigarettes. Atmos. Environ. 2016, 134, 61–69. [Google Scholar] [CrossRef]
- Korzun, T.; Lazurko, M.; Munhenzva, I.; Barsanti, K.C.; Huang, Y.; Jensen, R.P.; Escobedo, J.O.; Luo, W.; Peyton, D.H.; Strongin, R.M. E-Cigarette Airflow Rate Modulates Toxicant Profiles and Can Lead to Concerning Levels of Solvent Consumption. ACS Omega 2018, 3, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Dautzenberg, B.; Bricard, D. Real-Time Characterization of E-Cigarettes Use: The 1 Million Puffs Study. J. Addict. Res. Ther. 2015, 6, 229. [Google Scholar] [CrossRef]
- Son, Y.; Weisel, C.; Wackowski, O.; Mainelis, G.; Schwander, S.; Delnevo, C.; Kipen, H.; Meng, Q. Evaluation of e-vapor nicotine and nicotyrine concentrations emitted under various e-liquid compositions, device settings and vaping topographies. Chem. Res. Toxicol. 2018, 31, 861–868. [Google Scholar] [CrossRef]
- Casebolt, R.; Cook, S.J.; Islas, A.; Brown, A.; Castle, K.; Dutcher, D.D. Carbon monoxide concentration in mainstream E-cigarette emissions measured with diode laser spectroscopy. Tob. Control 2019, 1–4. [Google Scholar] [CrossRef]
- El-Hellani, A.; Al-Moussawi, S.; El-Hage, R.; Talih, S.; Salman, R.; Shihadeh, A.; Saliba, N.A. Carbon monoxide and small hydrocarbon emissions from sub-ohm electronic cigarettes. Chem. Res. Toxicol. 2019, 32, 312–317. [Google Scholar] [CrossRef]
- D’ruiz, C.D.; O’connell, G.; Graff, D.W.; Yan, X.S. Measurement of cardiovascular and pulmonary function endpoints and other physiological effects following partial or complete substitution of cigarettes with electronic cigarettes in adult smokers. Regul. Toxicol. Pharmacol. 2017, 87, 36–53. [Google Scholar] [CrossRef]
- McRobbie, H.; Phillips, A.; Goniewicz, M.L.; Smith, K.M.; Knight-West, O.; Przulj, D.; Hajek, P. Effects of switching to electronic cigarettes with and without concurrent smoking on exposure to nicotine, carbon monoxide, and acrolein. Cancer Prev. Res. 2015, 8, 873–878. [Google Scholar] [CrossRef]
- Walele, T.; Bush, J.; Koch, A.; Savioz, R.; Martin, C.; O’Connell, G. Evaluation of the safety profile of an electronic vapour product used for two years by smokers in a real-life setting. Regul. Toxicol. Pharmacol. 2018, 92, 226–238. [Google Scholar] [CrossRef]
- Lin, Y.-C. Catalytic valorization of glycerol to hydrogen and syngas. Int. J. Hydrog. Energy 2013, 38, 2678–2700. [Google Scholar] [CrossRef]
- Rosado-Reyes, C.M.; Tsang, W. Thermal Stability of Larger Carbonyl Compounds: 2-Methylbutyraldehyde. Int. J. Chem. Kinet. 2014, 46, 285–293. [Google Scholar] [CrossRef]
- Kavuluru, R.; Han, S.; Hahn, E.J. On the popularity of the USB flash drive-shaped electronic cigarette Juul. Tob. Control 2018, 28, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Herzog, B.; Kanada, P. Nielsen: Tobacco All Channel Data Thru 8/11-Cig Vol Decelerates; Wells Fargo Securities, LLC: San Francisco, CA, USA, 2018. [Google Scholar]
- Goniewicz, M.L.; Boykan, R.; Messina, C.R.; Eliscu, A.; Tolentino, J. High exposure to nicotine among adolescents who use Juul and other vape pod systems (‘pods’). Tob. Control 2019, 28, 676–677. [Google Scholar] [CrossRef]
- Tierney, P.A.; Karpinski, C.D.; Brown, J.E.; Luo, W.; Pankow, J.F. Flavour chemicals in electronic cigarette fluids. Tob. Control 2015, 25, e10–e15. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Schwander, S.; Son, Y.; Rivas, C.; Delnevo, C.; Graber, J.; Giovenco, D.; Bruen, U.; Mathew, R.; Robson, M. Has the mist been peered through? Revisiting the building blocks of human health risk assessment for electronic cigarette use. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 558–579. [Google Scholar] [CrossRef]
- Robinson, R.; Hensel, E.; Morabito, P.; Roundtree, K. Electronic cigarette topography in the natural environment. PLoS ONE 2015, 10, e0129296. [Google Scholar] [CrossRef]
- Spindle, T.R.; Breland, A.B.; Karaoghlanian, N.V.; Shihadeh, A.L.; Eissenberg, T. Preliminary results of an examination of electronic cigarette user puff topography: The effect of a mouthpiece-based topography measurement device on plasma nicotine and subjective effects. Nicotine Tob. Res. 2014, 17, 142–149. [Google Scholar] [CrossRef]
- CORESTA. Routine Analytical Machine for E-Cigarette Aerosol Generation and Collection-Definitions and Standard Conditions; Cooperation Centre for Scientific Research Relative to Tobacco: Paris, France, 2015. [Google Scholar]
- Perfetti, T.A. Structural study of nicotine salts. Beitr. Table Contrib. Tob. Res. 1983, 12, 43–54. [Google Scholar] [CrossRef]
- Robinson, R.J.; Eddingsaas, N.C.; DiFrancesco, A.G.; Jayasekera, S.; Hensel, E.C., Jr. A framework to investigate the impact of topography and product characteristics on electronic cigarette emissions. PLoS ONE 2018, 13, e0206341. [Google Scholar] [CrossRef] [PubMed]
- Goniewicz, M.L.; Kuma, T.; Gawron, M.; Knysak, J.; Kosmider, L. Nicotine levels in electronic cigarettes. Nicotine Tob. Res. 2012, 15, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.E.; Dhawan, S.; Sumner, W.; Williams, B.J. On-line chemical composition analysis of refillable electronic cigarette aerosol—Measurement of nicotine and nicotyrine. Nicotine Tob. Res. 2015, 17, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Tayyarah, R.; Long, G.A. Comparison of select analytes in aerosol from e-cigarettes with smoke from conventional cigarettes and with ambient air. Regul. Toxicol. Pharmacol. 2014, 70, 704–710. [Google Scholar] [CrossRef]
- Uchiyama, S.; Senoo, Y.; Hayashida, H.; Inaba, Y.; Nakagome, H.; Kunugita, N. Determination of Chemical compounds generated from Second-generation E-cigarettes using a Sorbent Cartridge followed by a Two-step Elution Method. Anal. Sci. 2016, 32, 549–555. [Google Scholar] [CrossRef]
- Farsalinos, K.E.; Kistler, K.A.; Gillman, G.; Voudris, V. Evaluation of electronic cigarette liquids and aerosol for the presence of selected inhalation toxins. Nicotine Tob. Res. 2014, 17, 168–174. [Google Scholar] [CrossRef]
- Flora, J.W.; Wilkinson, C.T.; Wilkinson, J.W.; Lipowicz, P.J.; Skapars, J.A.; Anderson, A.; Miller, J.H. Method for the Determination of Carbonyl Compounds in E-Cigarette Aerosols. J. Chromatogr. Sci. 2017, 55, 142–148. [Google Scholar] [CrossRef]
- Seeman, J.I.; Laffoon, S.W.; Kassman, A.J. Evaluation of relationships between mainstream smoke acetaldehyde and “tar” and carbon monoxide yields in tobacco smoke and reducing sugars in tobacco blends of US commercial cigarettes. Inhal. Toxicol. 2003, 15, 373–395. [Google Scholar] [CrossRef]
- Baker, R.R.; Bishop, L.J. The pyrolysis of tobacco ingredients. J. Anal. Appl. Pyrolysis 2004, 71, 223–311. [Google Scholar] [CrossRef]
- Wang, P.; Chen, W.; Liao, J.; Matsuo, T.; Ito, K.; Fowles, J.; Shusterman, D.; Mendell, M.; Kumagai, K. A Device-Independent Evaluation of Carbonyl Emissions from Heated Electronic Cigarette Solvents. PLoS ONE 2017, 12, e0169811. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, M.V.; Stellman, S.D.; Zang, E. Doses of nicotine and lung carcinogens delivered to cigarette smokers. J. Natl. Cancer Inst. 2000, 92, 106–111. [Google Scholar] [CrossRef] [PubMed]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 83: Tobacco Smoke and Involuntary Smoking; International Agency for Research on Cancer: Lyon, France, 2004. [Google Scholar]
- Moir, D.; Rickert, W.S.; Levasseur, G.; Larose, Y.; Maertens, R.; White, P.; Desjardins, S. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem. Res. Toxicol. 2007, 21, 494–502. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. Integrated Risk Information System (IRIS). Available online: https://www.epa.gov/iris (accessed on 13 April 2020).
- Rodu, B.; Plurphanswat, N. E-cigarette Use Among US Adults: Population Assessment of Tobacco and Health (PATH) Study. Nicotine Tob. Res. 2018, 20, 940–948. [Google Scholar] [CrossRef]
- Dawkins, L.E.; Kimber, C.F.; Doig, M.; Feyerabend, C.; Corcoran, O. Self-titration by experienced e-cigarette users: Blood nicotine delivery and subjective effects. Psychopharmacology 2016, 233, 2933–2941. [Google Scholar] [CrossRef]
- Fujioka, K.; Shibamoto, T. Determination of toxic carbonyl compounds in cigarette smoke. Environ. Toxicol. 2006, 21, 47–54. [Google Scholar] [CrossRef]
- Talih, S.; Salman, R.; Karaoghlanian, N.; El-Hellani, A.; Saliba, N.; Eissenberg, T.; Shihadeh, A. “Juice monsters”: Sub-ohm vaping and toxic volatile aldehyde emissions. Chem. Res. Toxicol. 2017, 30, 1791–1793. [Google Scholar] [CrossRef]
- Aalto-Korte, K.; Mäkelä, E.A.; Huttunen, M.; Suuronen, K.; Jolanki, R. Occupational contact allergy to glyoxal. Contact Dermat. 2005, 52, 276–281. [Google Scholar] [CrossRef]
- U.S. FDA. Tobacco Product Standard for Nicotine Level of Combusted Cigarettes, Proposed Rule (21 CFR 1130); Food and Drug Administration: Silver Spring, MD, USA, 2018.
- Institute of Medicine. Ending the Tobacco Problem: A Blueprint for the Nation; The National Academies Press: Washington, DC, USA, 2007; p. 732. [Google Scholar]
- Primack, B.A.; Shensa, A.; Sidani, J.E.; Hoffman, B.L.; Soneji, S.; Sargent, J.D.; Hoffman, R.M.; Fine, M.J. Initiation of traditional cigarette smoking after electronic cigarette use among tobacco-naïve US young adults. Am. J. Med. 2018, 131, 443. [Google Scholar] [CrossRef]
- McKelvey, K.; Baiocchi, M.; Halpern-Felsher, B. Adolescents’ and young adults’ use and perceptions of pod-based electronic cigarettes. JAMA Netw. Open 2018, 1, e183535. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. Exposure Factors Handbook 2011 Edition (Final); U.S. Environmental Protection Agency: Washington, DC, USA, 2011.
- ATSDR. Minimal Risk Levels (MRLs) List. Available online: https://www.atsdr.cdc.gov/mrls/mrllist.asp (accessed on 13 April 2020).
- U.S. EPA. Compendium Method TO-11A: Determination of Formaldehyde in Ambient Air Using Adsorbent Cartridge Followed by High. Performance Liquid Chromatography (HPLC) [Active Sampling Methodology], 2nd ed.; U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1999.
- ISO. Vapour Products-Routine Analytical Vaping Machine—Definitions and Standard Conditions; ISO 20768:2018(en); International Organization for Standardization: Geneva, Switzerland, 2018. [Google Scholar]


| Type | E-liquid (Flavor, Nicotine) | Puff Duration (s) | Puff Volume (mL) | Power Output (watt) |
|---|---|---|---|---|
| Cig-a-like | Tobacco, 18 mg/mL | 2, 3, 4, 5 | 100, 133 | 5.2 |
| Grape, 18 mg/mL | 4 | 100 | 5.2 | |
| Top-coil | Strawberry-watermelon, 3 mg/mL | 2, 3, 4, 5 | 40, 67, 100, 133 | 4.9 |
| ‘mod’ | Strawberry-watermelon, 3 mg/mL | 2, 3, 4, 5 | 40, 67, 100, 133 | 40, 50, 60 |
| Peach-lemonade, 3 mg/mL | 4 | 40 | 50 | |
| Vanilla-almond-milk, 6 mg/mL | 4 | 40 | 50 | |
| Unflavored, no-nicotine | 4 | 40 | 40, 50, 60 | |
| ‘pod’ (JUUL) | Fruit melody, 59 mg/mL | 2, 3, 4, 5 | 100, 133 | 6–9 |
| Cool mint, 59 mg/mL | 4 | 100 | 6–9 | |
| Virginia tobacco, 59 mg/mL | 4 | 100 | 6–9 | |
| Creme brulee, 59 mg/mL | 4 | 100 | 6–9 |
| E-Cigarette Type | Formaldehyde | Acetaldehyde | Acrolein | Glyoxal |
|---|---|---|---|---|
| Cig-a-like (tobacco) | 0.820 * | 0.821 * | 0.837 * | 0.823 * |
| Cig-a-like (grape) | 0.476 | 0.380 | – | 0.478 |
| Top-coil | 0.909 * | 0.857 * | 0.901 * | 0.620 * |
| ‘mod’ | 0.847 * | 0.855 * | 0.776 * | 0.609 * |
| JUUL | 0.335 | 0.429 | – | 0.296 |
| Device Type | Nicotine (μg/puff) | Nicotine Normalized Concentration (ng/puff/μg Nicotine) | ||||
|---|---|---|---|---|---|---|
| COn | Formaldehyden | Acetaldehyden | Acroleinn | Glyoxaln | ||
| Cig-a-like | 104 ± 10.4 | 13.8 ± 68.8 | 25.6 ± 6.13 | 31.6 ± 16.4 | 0.47 ± 1.7 | 13.8 ± 68.8 |
| Top-coil 2 | 6.0 ± 1.3 | 556 ± 337 | 114 ± 427 | 8.92 ± 38.5 | 1.52 ± 6.99 | 556 ± 337 |
| ‘mod’ | 106 ± 11.8 | 21.1 ± 76.3 | 7.77 ± 1.12 | 2.61 ± 0.54 | 1.64 ± 2.89 | 21.1 ± 76.3 |
| JUUL | 390 ± 30.5 | 1.01 ± 7.14 | 0.34 ± 4.34 | 0.06 ± 2.06 | 0.02 ± 0.66 | 1.01 ± 7.14 |
| Cigarette 3 | 134 ± 75.1 | 10893 ± 7313 | 72.4 ± 84.8 | 551 ± 669 | 127 ± 78.4 | 10893 ± 7313 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, Y.; Bhattarai, C.; Samburova, V.; Khlystov, A. Carbonyls and Carbon Monoxide Emissions from Electronic Cigarettes Affected by Device Type and Use Patterns. Int. J. Environ. Res. Public Health 2020, 17, 2767. https://doi.org/10.3390/ijerph17082767
Son Y, Bhattarai C, Samburova V, Khlystov A. Carbonyls and Carbon Monoxide Emissions from Electronic Cigarettes Affected by Device Type and Use Patterns. International Journal of Environmental Research and Public Health. 2020; 17(8):2767. https://doi.org/10.3390/ijerph17082767
Chicago/Turabian StyleSon, Yeongkwon, Chiranjivi Bhattarai, Vera Samburova, and Andrey Khlystov. 2020. "Carbonyls and Carbon Monoxide Emissions from Electronic Cigarettes Affected by Device Type and Use Patterns" International Journal of Environmental Research and Public Health 17, no. 8: 2767. https://doi.org/10.3390/ijerph17082767
APA StyleSon, Y., Bhattarai, C., Samburova, V., & Khlystov, A. (2020). Carbonyls and Carbon Monoxide Emissions from Electronic Cigarettes Affected by Device Type and Use Patterns. International Journal of Environmental Research and Public Health, 17(8), 2767. https://doi.org/10.3390/ijerph17082767