Testosterone Decreases the Number of Implanting Embryos, Expression of Pinopode and L-selectin Ligand (MECA-79) in the Endometrium of Early Pregnant Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals & Hormone Treatment
2.2. Measurement of Serum Hormone Levels
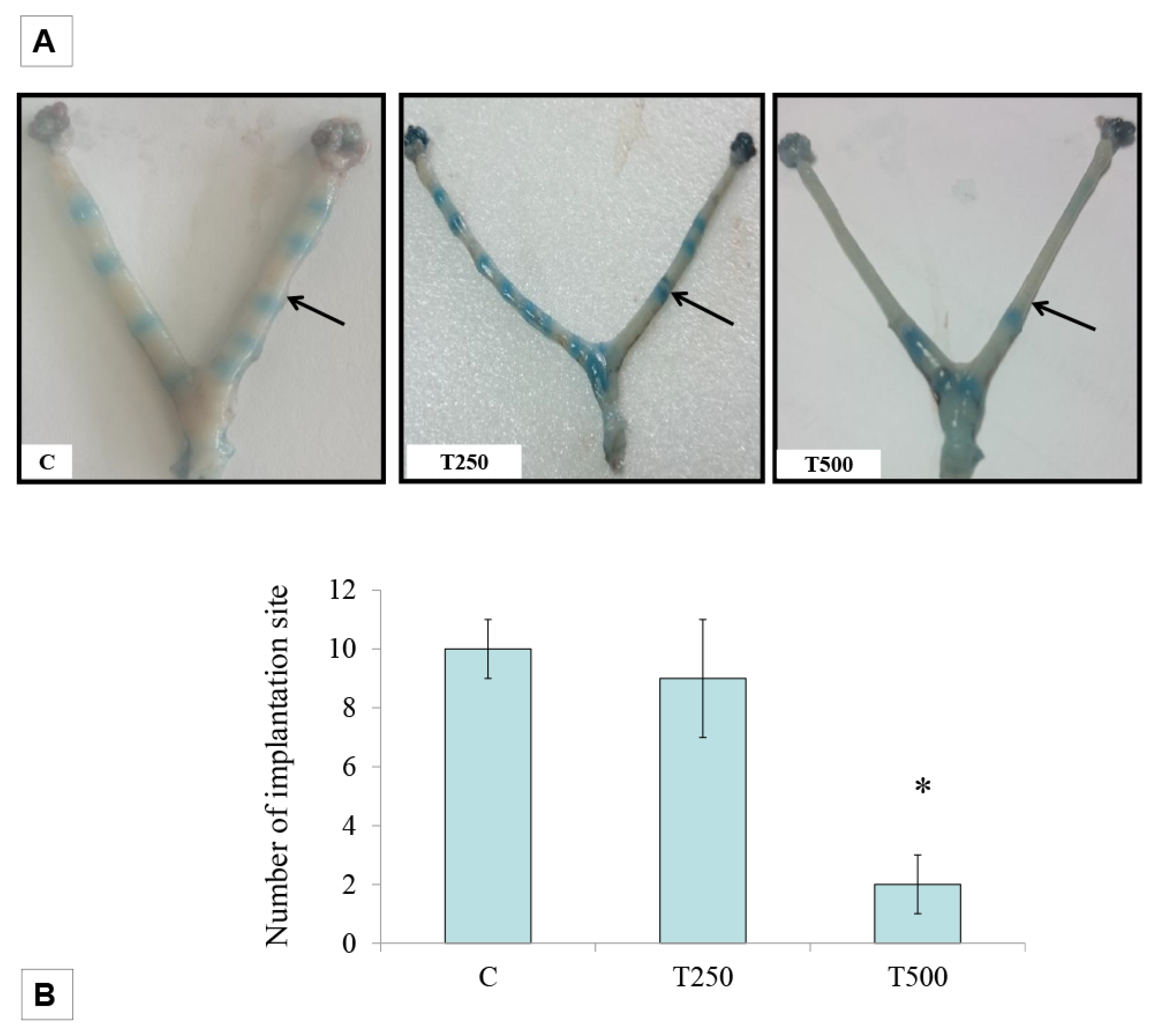
2.3. Determination of the Number of Implantation Sites
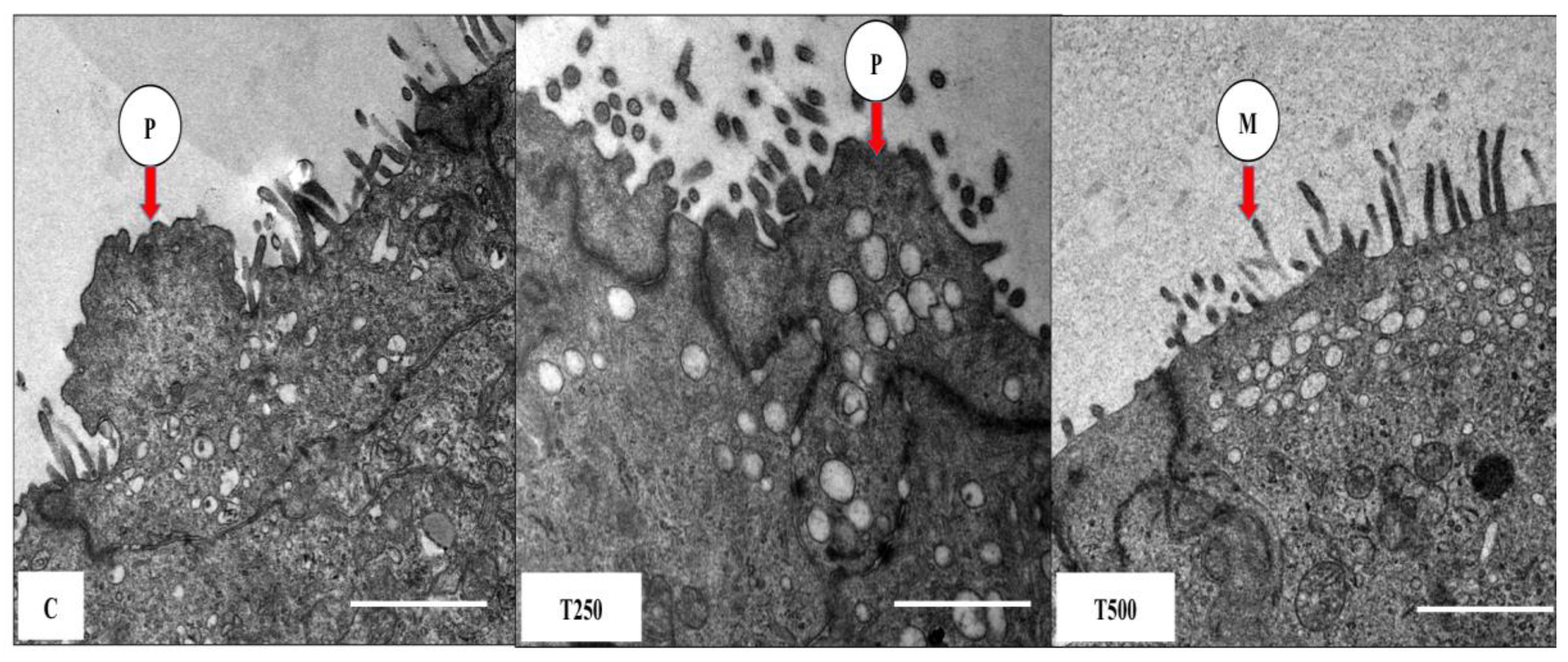
2.4. Transmission Electron Microscopy (TEM)
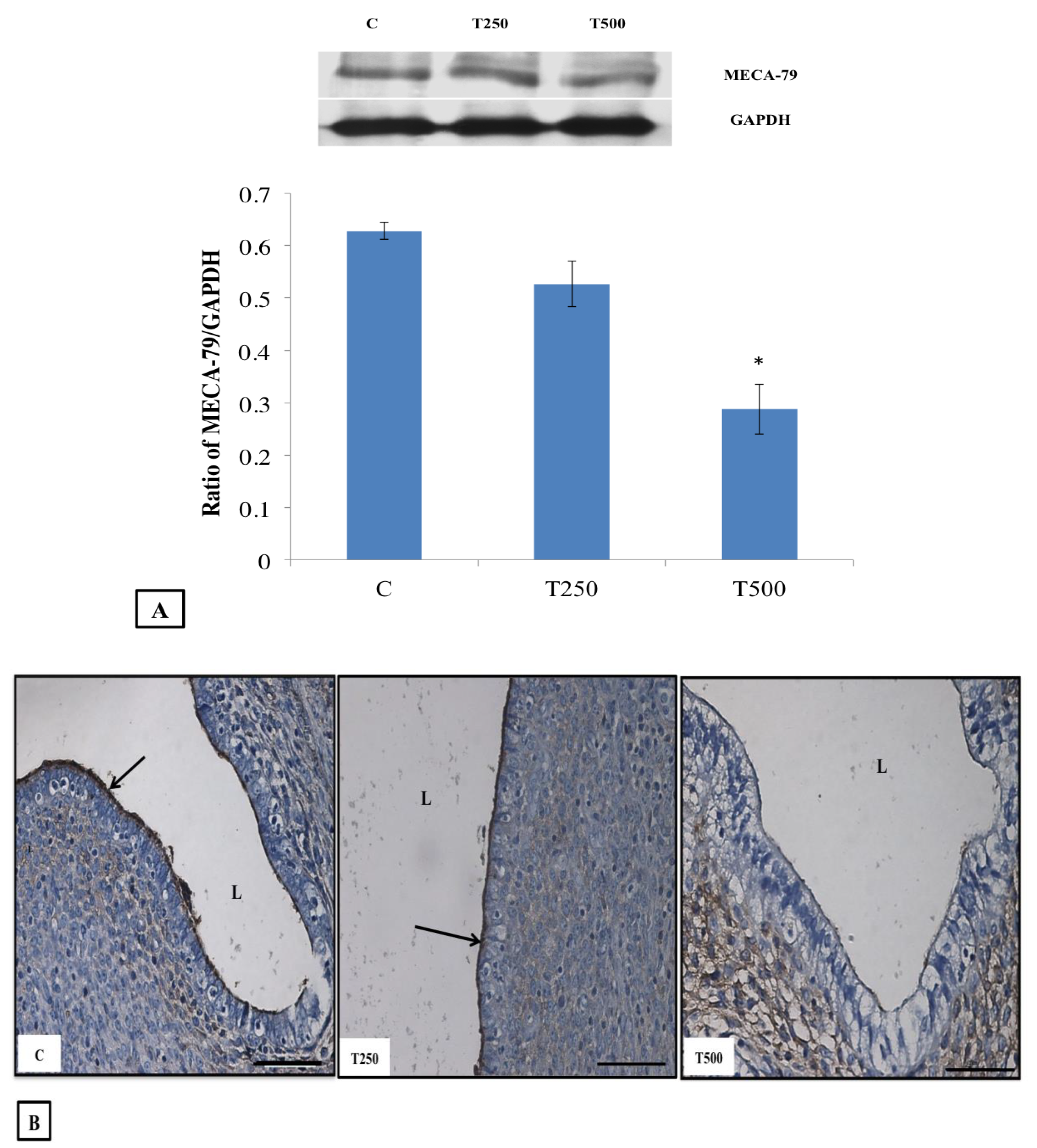
2.5. Protein Distribution Analysis by Immunohistochemistry (IHC)
2.6. Protein Quantification by Western Blotting
2.7. Statistical Analysis
3. Results
3.1. Plasma Levels of Estrogen, Progesterone and Testosterone in Pregnant Rats
3.2. Number of embryo implantation sites
3.3. TEM Images of Pinopodes
3.4. MECA-79 Protein Expression and Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lim, H.J.; Dey, S.K. HB-EGF: A unique mediator of embryo-uterine interactions during implantation. Exp. Cell Res. 2009, 315, 619–626. [Google Scholar] [CrossRef]
- Aghajanova, L.; Hamilton, A.E.; Giudice, L.C. Uterine receptivity to human embryonic implantation: Histology, biomarkers, and transcriptomics. Semin. Cell Dev. Biol. 2008, 19, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Paulson, R.J. Hormonal induction of endometrial receptivity. Fertil. Steril. 2011, 96, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Ruijter-Villani, M.; Stout, T.A.E. The Role of Conceptus–maternal Signalling in the Acquisition of Uterine Receptivity to Implantation in Mammals. Reprod. Domest. Anim. 2015, 50 (Suppl. 3), 7–14. [Google Scholar] [CrossRef]
- Nikas, G. Cell-surface morphological events relevant to human implantation. Hum. Reprod. 1999, 14 (Suppl. 2), 37–44. [Google Scholar] [CrossRef][Green Version]
- Nejatbakhsh, R.; Kabir-Salmani, M.; Dimitriadis, E.; Hosseini, A.; Taheripanah, R.; Sadeghi, Y.; Akimoto, Y.; Iwashita, M. Subcellular localization of L-selectin ligand in the endometrium implies a novel function for pinopodes in endometrial receptivity. Reprod. Biol. Endocrinol. RbE 2012, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Shamonki, M.I.; Kligman, I.; Shamonki, J.M.; Schattman, G.L.; Hyjek, E.; Spandorfer, S.D.; Zaninovic, N.; Rosenwaks, Z. Immunohistochemical expression of endometrial L-selectin ligand is higher in donor egg recipients with embryonic implantation. Fertil. Steril. 2006, 86, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.M.; Chauhan, S.C.; Trivedi, R.N.; Maitra, S.C.; Kamboj, V.P. Correlation of pinopod development on uterine luminal epithelial surface with hormonal events and endometrial sensitivity in rat. Eur. J. Endocrinol. 1996, 135, 107–117. [Google Scholar] [CrossRef]
- Salleh, N.; Mokhtar, H.M.; Kassim, N.M.; Giribabu, N. Testosterone Induces Increase in Aquaporin (AQP)-1, 5, and 7 Expressions in the Uteri of Ovariectomized Rats. J. Membr. Biol. 2015, 248, 1097–1105. [Google Scholar] [CrossRef]
- Mohd Mokhtar, H.; Giribabu, N.; Kassim, N.; Muniandy, S.; Salleh, N. Testosterone decreases fluid and chloride secretions in the uterus of adult female rats via down-regulating cystic fibrosis transmembrane regulator (CFTR) expression and functional activity. J. Steroid Biochem. Mol. Biol. 2014, 144, 361–372. [Google Scholar] [CrossRef]
- Diao, H.-L.; Su, R.-W.; Tan, H.-N.; Li, S.-J.; Lei, W.; Deng, W.-B.; Yang, Z.-M. Effects of androgen on embryo implantation in the mouse delayed-implantation model. Fertil. Steril. 2008, 90, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Cermik, D.; Selam, B.; Taylor, H.S. Regulation of HOXA-10 Expression by Testosterone in Vitro and in the Endometrium of Patients with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.K.; Lim, H.; Das, S.K.; Reese, J.; Paria, B.C.; Daikoku, T.; Wang, H. Molecular cues to implantation. Endocr. Rev. 2004, 25, 341–373. [Google Scholar] [CrossRef] [PubMed]
- Strowitzki, T.; Germeyer, A.; Popovici, R.; von Wolff, M. The human endometrium as a fertility-determining factor. Hum. Reprod. Update 2006, 12, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Foulk, R.A.; Zdravkovic, T.; Genbacev, O.; Prakobphol, A. Expression of L-selectin ligand MECA-79 as a predictive marker of human uterine receptivity. J. Assist. Reprod. Genet. 2007, 24, 316–321. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pantos, K.; Nikas, G.; Makrakis, E.; Stavrou, D.; Karantzis, P.; Grammatis, M. Clinical value of endometrial pinopodes detection in artificial donation cycles. Reprod. Biomed. Online 2004, 9, 86–90. [Google Scholar] [CrossRef]
- Nelson, L.R.; Bulun, S.E. Estrogen production and action. J. Am. Acad. Dermatol. 2001, 45 (Suppl. 3), S116–S124. [Google Scholar] [CrossRef]
- Simpson, E.R. Aromatization of androgens in women: Current concepts and findings. Fertil. Steril. 2002, 77, 6–10. [Google Scholar] [CrossRef]
- Diedrich, K.; Fauser, B.C.J.M.; Devroey, P.; Griesinger, G. The role of the endometrium and embryo in human implantation. Hum. Reprod. Update 2007, 13, 365–377. [Google Scholar] [CrossRef]
- Salleh, N.; Baines, D.L.; Naftalin, R.J.; Milligan, S.R. The Hormonal Control of Uterine Luminal Fluid Secretion and Absorption. J. Membr. Biol. 2005, 206, 17–28. [Google Scholar] [CrossRef]
- Naftalin, R.J.; Thiagarajah, J.R.; Pedley, K.C.; Pocock, V.J.; Milligan, S.R. Progesterone stimulation of fluid absorption by the rat uterine gland. Reproduction (Cambridge, UK) 2002, 123, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Stavreus-Evers, A.; Nikas, G.; Sahlin, L.; Eriksson, H.; Landgren, B.M. Formation of pinopodes in human endometrium is associated with the concentrations of progesterone and progesterone receptors. Fertil. Steril. 2001, 76, 782–791. [Google Scholar] [CrossRef]
- Parr, M.B.; Parr, E.L. Relationship of apical domes in the rabbit uterine epithelium during the peri-implantation period to endocytosis, apocrine secretion and fixation. J. Reprod. Fertil. 1982, 66, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, H.M.; Giribabu, N.; Muniandy, S.; Salleh, N. Testosterone decreases the expression of endometrial pinopode and L-selectin ligand (MECA-79) in adult female rats during uterine receptivity period. Int. J. Clin. Exp. Pathol. 2014, 7, 1967–1976. [Google Scholar]
- Genbacev, O.D.; PAFoulk, R.A.; Krtolica, A.R.; Ilic, D.; Singer, M.S.; Yang, Z.Q.; Kiessling, L.L.; Rosen, S.D.; Fisher, S.J. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science 2003, 299, 405–408. [Google Scholar] [CrossRef]
- Ashary, N.; Tiwari, A.; Modi, D. Embryo Implantation: War in Times of Love. Endocrinology 2018, 159, 1188–1198. [Google Scholar] [CrossRef]
| HORMONES | GROUPS | PLASMA LEVELS |
|---|---|---|
| ESTROGEN | C | 49.65 ± 3.66 pmol/L |
| T250 | *83.35 ± 2.91 pmol/L | |
| T500 | *102.67 ± 1.76 pmol/L | |
| PROGESTERONE | C | 166.93 ± 8.71 nmol/L |
| T250 | 134.8 ± 12.20 nmol/L | |
| T500 | 161.73 ± 3.98 nmol/L | |
| TESTOSTERONE | C | 0.60 ± 0.2 nmol/L |
| T250 | *5.90 ± 1.44 nmol/L | |
| T500 | *30.07 ± 1.47 nmol/L |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokhtar, M.H.; Giribabu, N.; Salleh, N. Testosterone Decreases the Number of Implanting Embryos, Expression of Pinopode and L-selectin Ligand (MECA-79) in the Endometrium of Early Pregnant Rats. Int. J. Environ. Res. Public Health 2020, 17, 2293. https://doi.org/10.3390/ijerph17072293
Mokhtar MH, Giribabu N, Salleh N. Testosterone Decreases the Number of Implanting Embryos, Expression of Pinopode and L-selectin Ligand (MECA-79) in the Endometrium of Early Pregnant Rats. International Journal of Environmental Research and Public Health. 2020; 17(7):2293. https://doi.org/10.3390/ijerph17072293
Chicago/Turabian StyleMokhtar, Mohd Helmy, Nelli Giribabu, and Naguib Salleh. 2020. "Testosterone Decreases the Number of Implanting Embryos, Expression of Pinopode and L-selectin Ligand (MECA-79) in the Endometrium of Early Pregnant Rats" International Journal of Environmental Research and Public Health 17, no. 7: 2293. https://doi.org/10.3390/ijerph17072293
APA StyleMokhtar, M. H., Giribabu, N., & Salleh, N. (2020). Testosterone Decreases the Number of Implanting Embryos, Expression of Pinopode and L-selectin Ligand (MECA-79) in the Endometrium of Early Pregnant Rats. International Journal of Environmental Research and Public Health, 17(7), 2293. https://doi.org/10.3390/ijerph17072293





