Synthesis of Biochar-Supported K-doped g-C3N4 Photocatalyst for Enhancing the Polycyclic Aromatic Hydrocarbon Degradation Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Photocatalysts
2.2. Characterization of Photocatalysts
2.3. Photocatalytic Experiments
2.4. Determination of Intermediates
3. Results and Discussion
3.1. Characterization Results of Samples
3.1.1. XRD Patterns
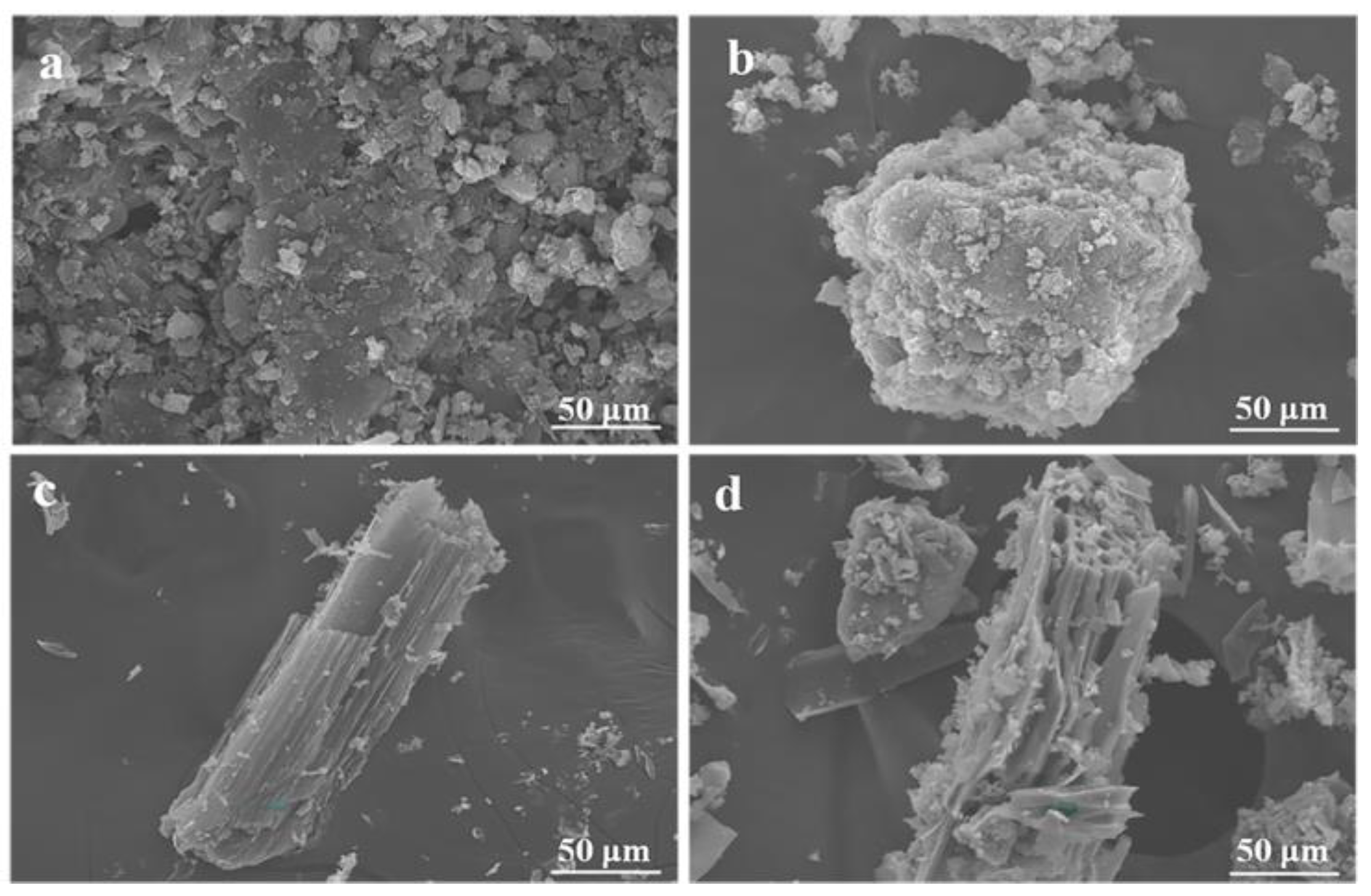
3.1.2. SEM Images
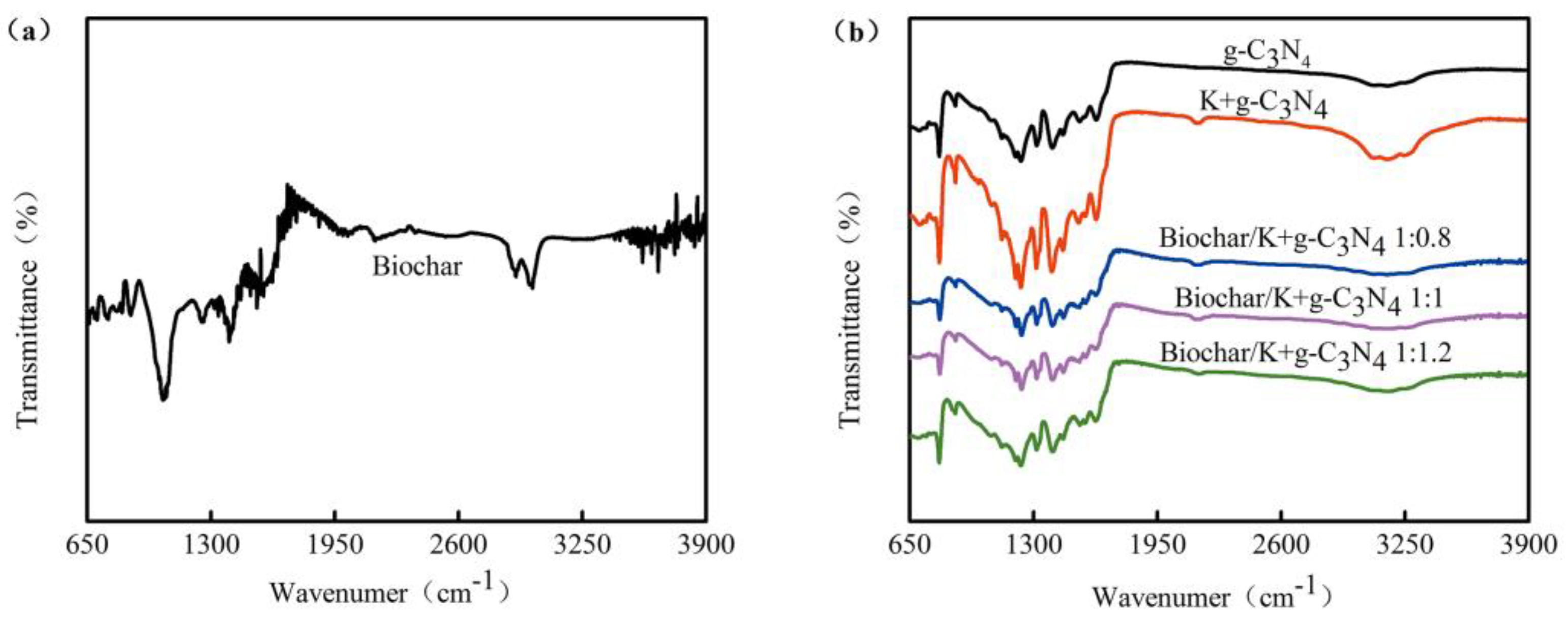
3.1.3. FT-IR Spectra
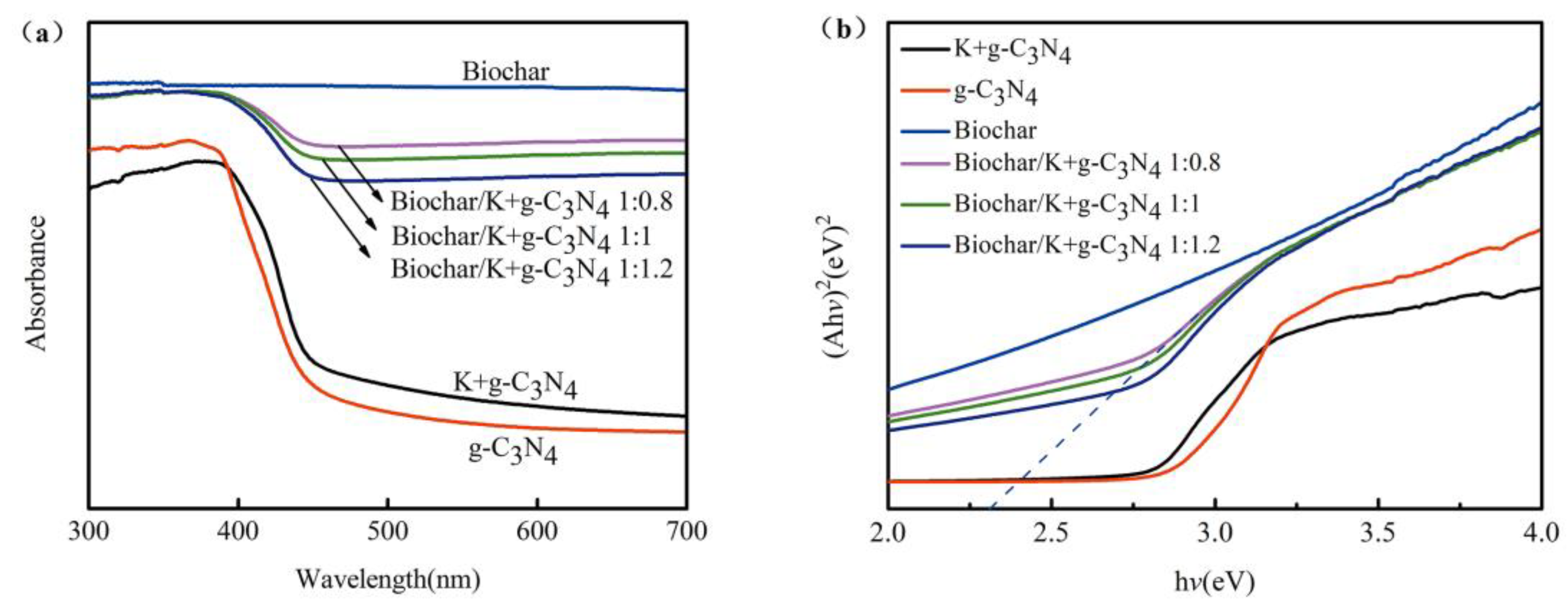
3.1.4. UV-vis Diffuse Reflectance Spectra
3.1.5. PL Spectra
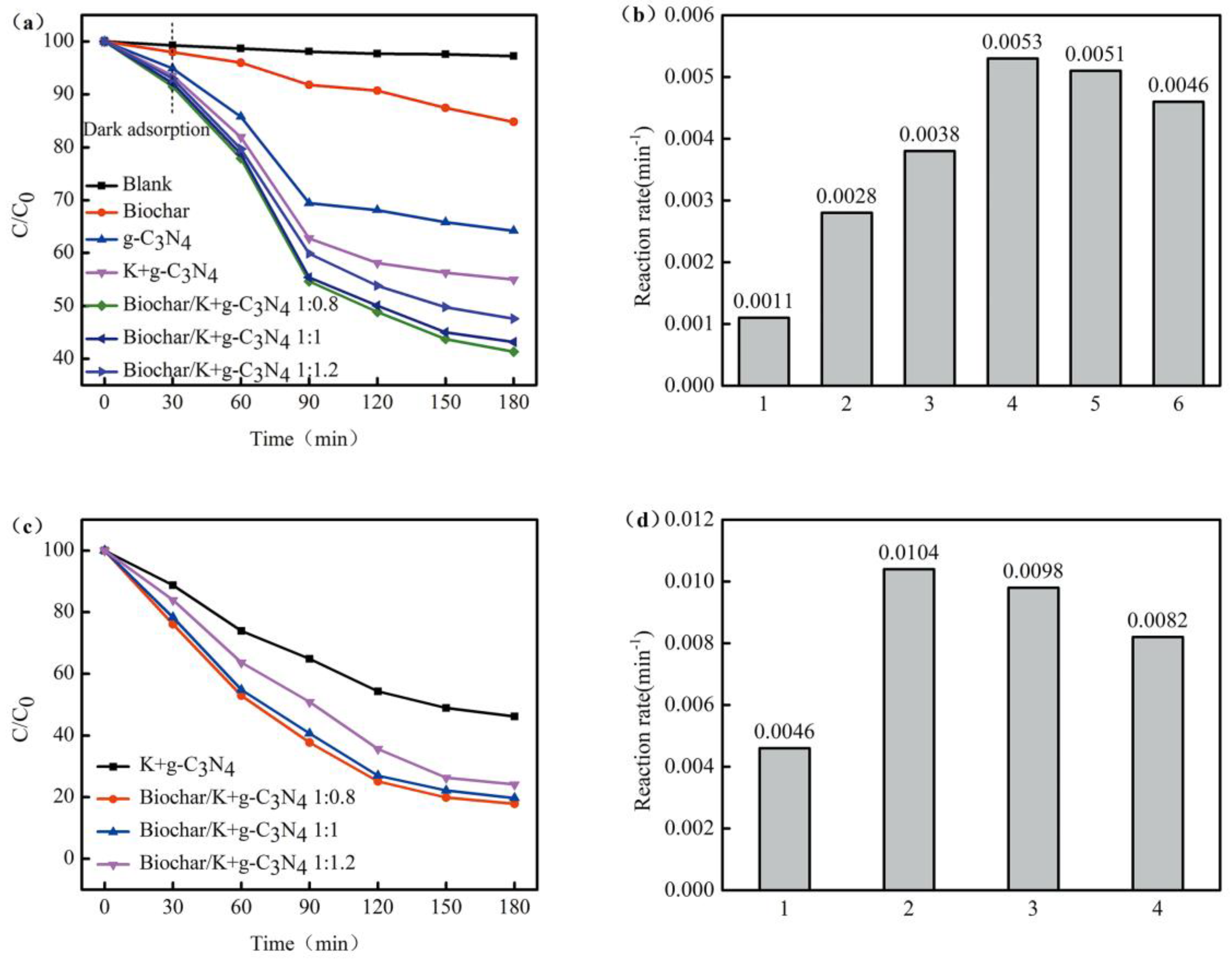
3.2. Photocatalytic Activity
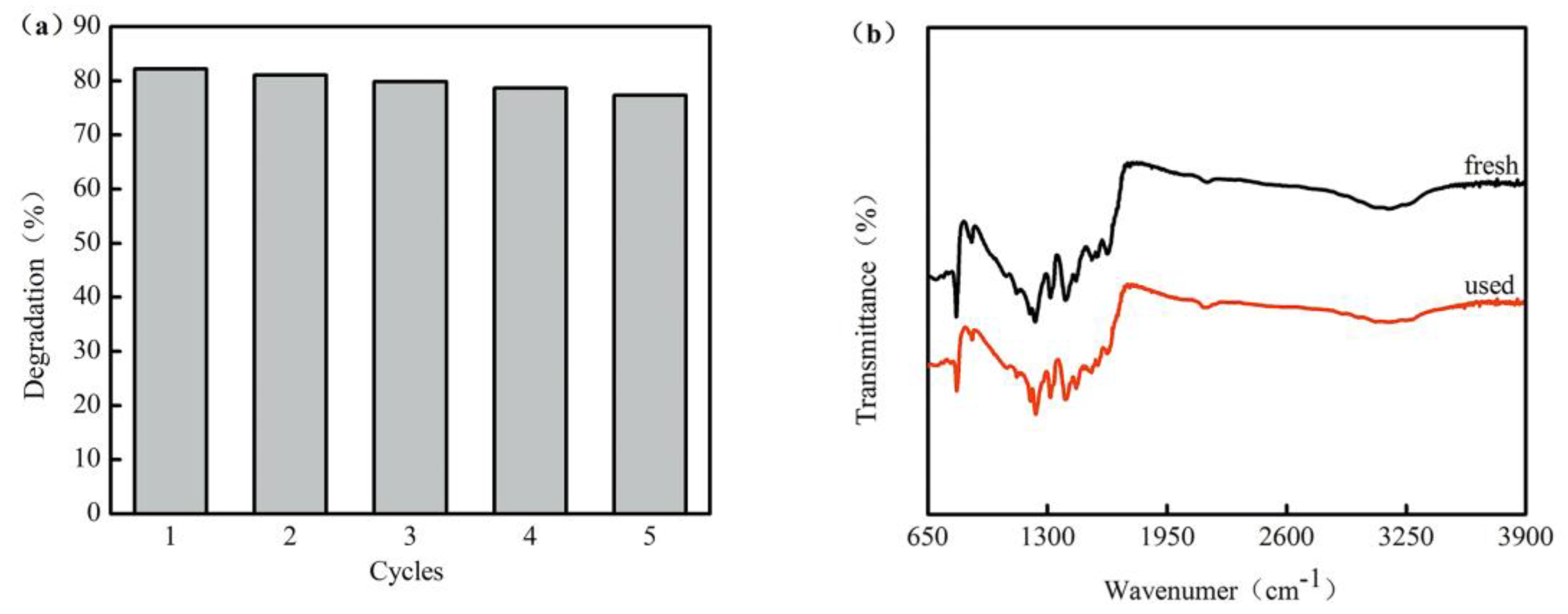
3.3. Photostability
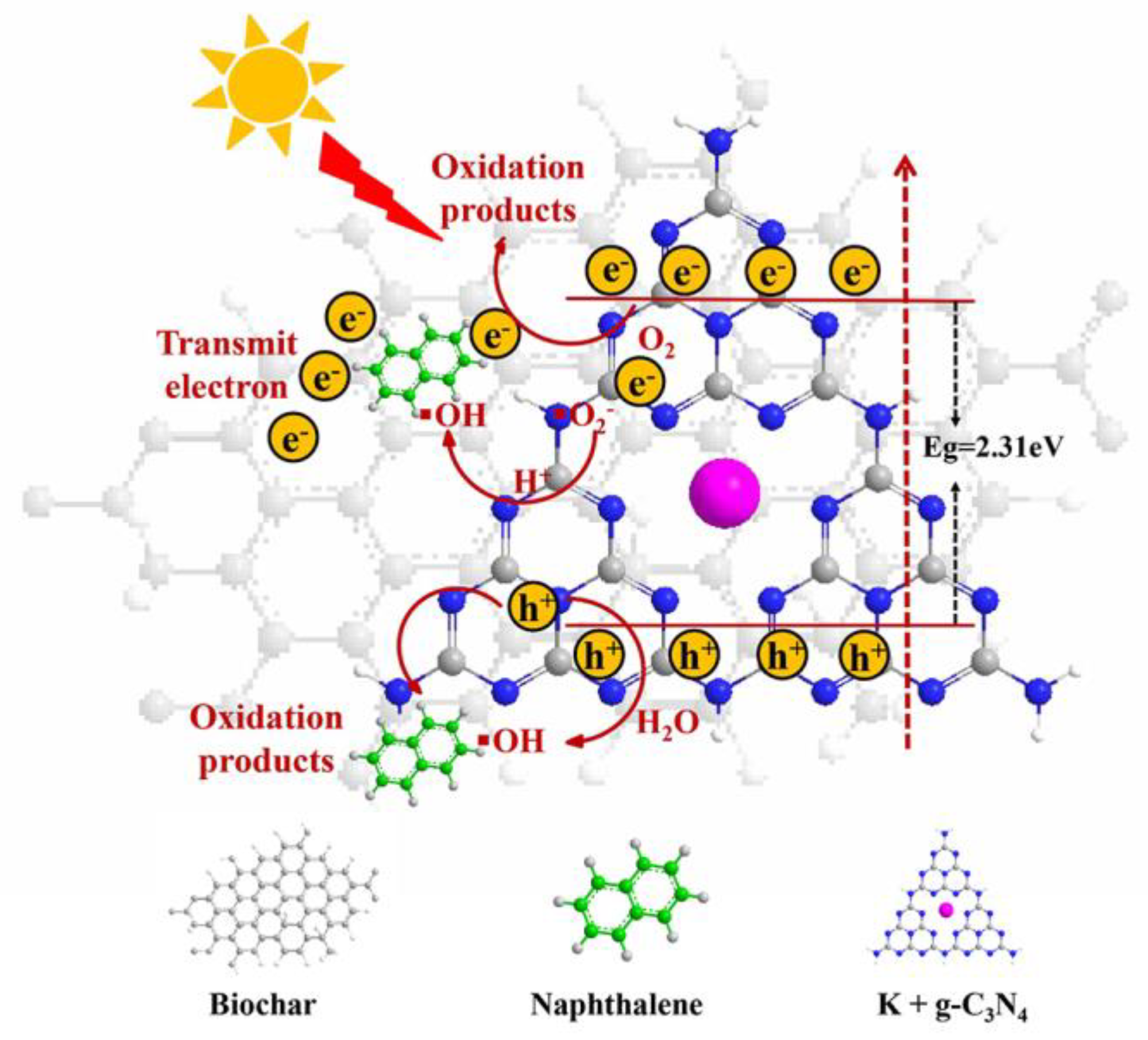
3.4. Photocatalytic Degradation Mechanism of Naphthalene
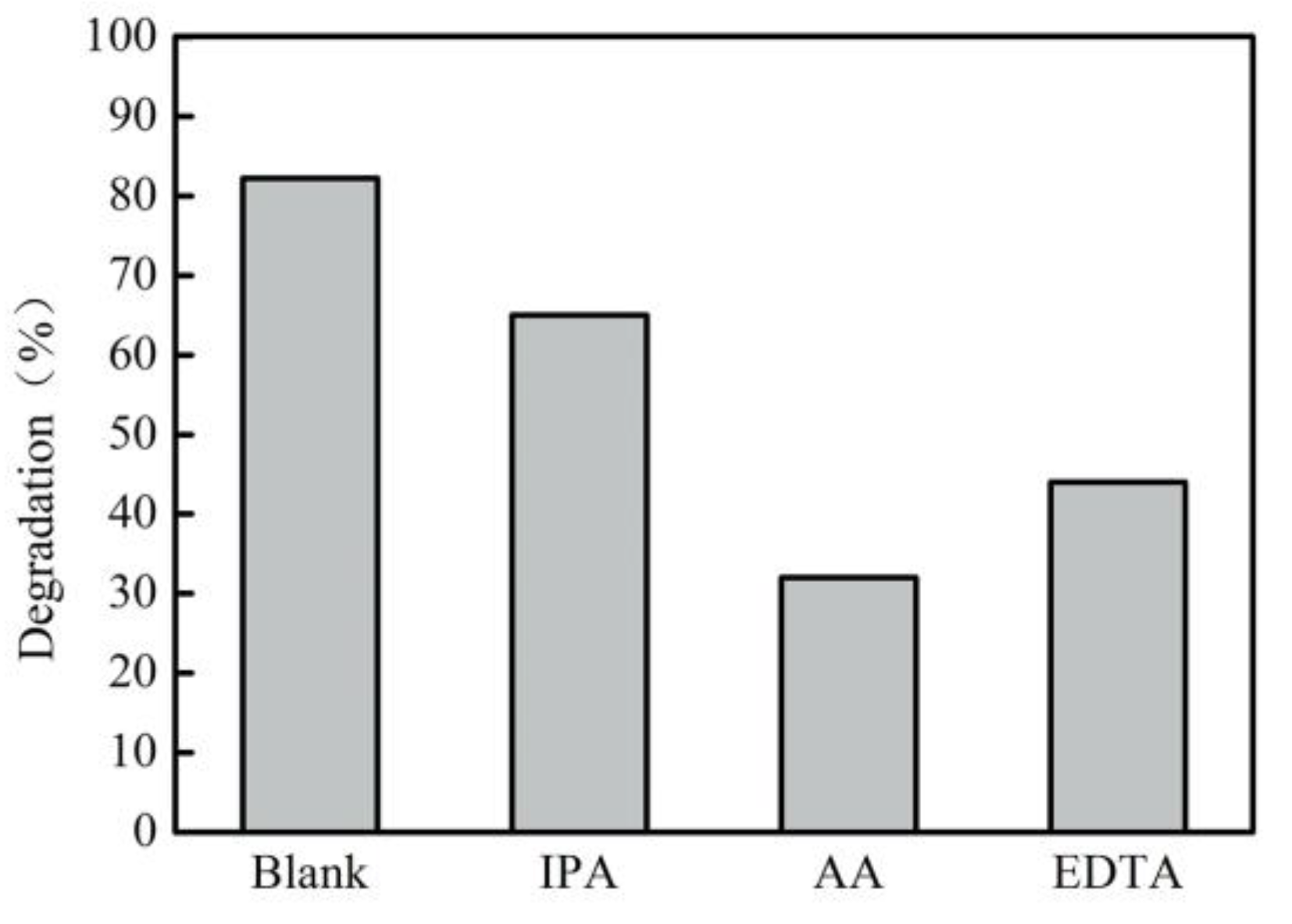
3.4.1. Trapping Experiments and Formation of Active Species
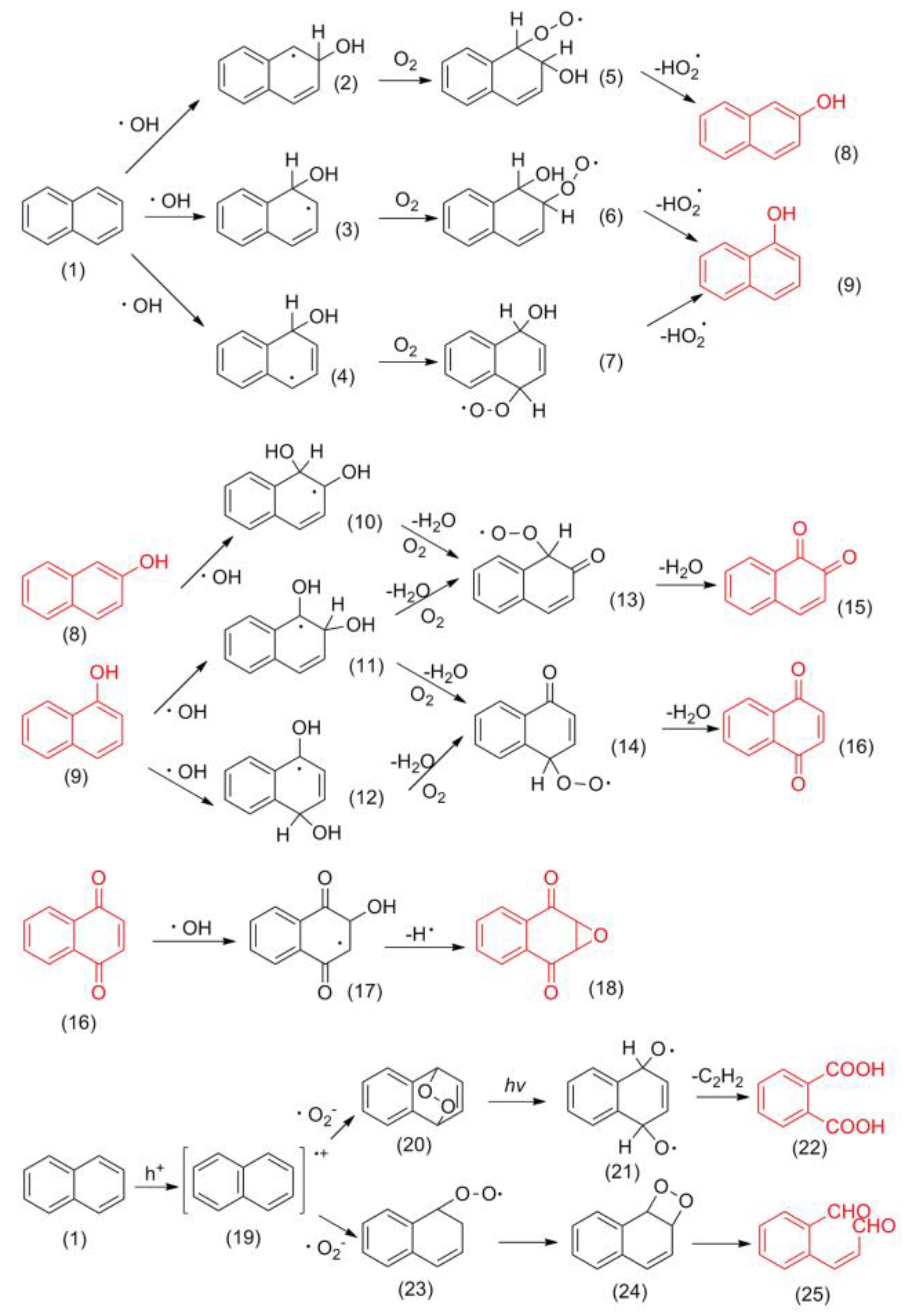
3.4.2. Intermediates of Naphthalene Degradation
3.4.3. Reaction Pathway
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Essabri, A.M.A.; Aydinlik, N.P.; Williams, N.E. Bioaugmentation and biostimulation of total petroleum hydrocarbon degradation in a petroleum-contaminated soil with fungi isolated from olive oil effluent. Water Air Soil Pollut. 2019, 230, 76. [Google Scholar] [CrossRef]
- Douglas, G.S.; Hardenstine, J.H.; Liu, B.; Uhler, A.D. Laboratory and field verification of a method to estimate the extent of petroleum biodegradation in soil. Environ. Sci. Technol. 2012, 46, 8279–8287. [Google Scholar] [CrossRef] [PubMed]
- Masakorala, K.; Yao, J.; Chandankere, R.; Yuan, H.; Liu, H.; Yu, C.; Cai, M. Effects of petroleum hydrocarbon contaminated soil on germination, metabolism and early growth of green gram, Vigna radiata L. Bull. Environ. Contam. Toxicol. 2013, 91, 224–230. [Google Scholar] [CrossRef]
- Cai, S.S.; Syage, J.A.; Hanold, K.A.; Balogh, M.P. Ultra performance liquid chromatography atmospheric pressure photoionization tandem mass spectrometry for high-sensitivity and high throughput analysis of U.S. Environmental Protection Agency 16 priority pollutants polynuclear aromatic hydrocarbons. Anal. Chem. 2009, 81, 2123–2128. [Google Scholar] [CrossRef]
- Cabal, B.; Budinova, T.; Ania, C.O.; Tsyntsarski, B.; Parra, J.B.; Petrova, B. Adsorption of naphthalene from aqueous solution on activated carbons obtained from bean pods. J. Hazard. Mater. 2009, 161, 1150–1156. [Google Scholar] [CrossRef]
- Rubio-Clemente, A.; Torres-Palma, R.A.; Penuela, G.A. Removal of polycyclic aromatic hydrocarbons in aqueous environment by chemical treatments: A review. Sci. Total Environ. 2014, 478, 201–225. [Google Scholar] [CrossRef]
- Seo, J.S.; Keum, Y.S.; Li, Q.X. Bacterial degradation of aromatic compounds. Int. J. Environ. Res. Public Health 2009, 6, 278–309. [Google Scholar] [CrossRef]
- Jing, L.; Chen, B.; Zhang, B. Modeling of UV-induced photodegradation of naphthalene in marine oily wastewater by artificial neural networks. Water Air Soil Pollut. 2014, 225, 1906. [Google Scholar] [CrossRef]
- Iovino, P.; Canzano, S.; Capasso, S.; Di Natale, M.; Erto, A.; Lama, A.; Musmarra, D. Single and competitive adsorption of toluene and naphthalene onto activated carbon. Chem. Eng. Trans. 2013, 32, 67–72. [Google Scholar]
- Bogan, B.W.; Trbovic, V.; Paterek, J.R. Inclusion of vegetable oils in Fenton’s chemistry for remediation of PAH contaminated soils. Chemosphere 2003, 50, 15–21. [Google Scholar] [CrossRef]
- Musat, F.; Galushko, A.; Jacob, J.; Widdel, F.; Kube, M.; Reinhardt, R.; Wilkes, H.; Schink, B.; Rabus, R. Anaerobic degradation of naphthalene and 2-methylnaphthalene by strains of marine sulfate-reducing bacteria. Environ. Microbiol. 2009, 11, 209–219. [Google Scholar] [CrossRef]
- Zou, Z.; Ye, J.; Sayama, K.; Arakawa, H. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature 2001, 414, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.M.; Kim, J.-S. Solvothermal synthesis of anatase TiO2-graphene oxide nanocomposites and their photocatalytic performance. J. Alloys Compd. 2016, 688, 123–129. [Google Scholar] [CrossRef]
- Xu, J.; Shen, K.; Xue, B.; Li, Y.-X.; Cao, Y. Synthesis of three-dimensional mesostructured graphitic carbon nitride materials and their application as heterogeneous catalysts for knoevenagel condensation reactions. Catal. Lett. 2013, 143, 600–609. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Wu, M.; Du, Y.; Fan, X.; Wang, M.; Zhang, L.; Kong, Q.; Shi, J. Mesostructured CeO2/g-C3N4 nanocomposites: Remarkably enhanced photocatalytic activity for CO2 reduction by mutual component activations. Nano Energy 2016, 19, 145–155. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Z.; Zhu, Y. Enhanced visible-light-driven photocatalytic disinfection performance and organic pollutant degradation activity of porous g-C3N4 nanosheets. ACS Appl. Mater. Interfaces 2017, 9, 27727–27735. [Google Scholar] [CrossRef]
- Wang, X.; Feng, J.; Bai, Y.; Zhang, Q.; Yin, Y. Synthesis, properties, and applications of hollow micro-/nanostructures. Chem. Rev. 2016, 116, 10983–11060. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Mohan, R.; Kim, S.J. Graphene oxide as a photocatalytic material. Appl. Phys. Lett. 2011, 98, 244101. [Google Scholar] [CrossRef]
- Song, X.; Tao, H.; Chen, L.; Sun, Y. Synthesis of Fe/g-C3N4 composites with improved visible light photocatalytic activity. Mater. Lett. 2014, 116, 265–267. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, M.; Ye, X.; Qiu, X.; Lin, S.; Wang, X. Iodine modified carbon nitride semiconductors as visible light photocatalysts for hydrogen evolution. Adv. Mater. 2014, 26, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Takanabe, K.; Kamata, K.; Wang, X.; Antonietti, M.; Kubota, J.; Domen, K. Photocatalytic hydrogen evolution on dye-sensitized mesoporous carbon nitride photocatalyst with magnesium phthalocyanine. Phys. Chem. Chem. Phys. 2010, 12, 13020–13025. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.K.; Lee, J.Y.; Selvam, N.C.S. Synthesis of MoS2 nanosheets loaded ZnO-g-C3N4 nanocomposites for enhanced photocatalytic applications. Chem. Eng. J. 2016, 289, 306–318. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Fu, X.; Antonietti, M.; Wang, X. Fe-g-C3N4-catalyzed oxidation of benzene to phenol using hydrogen peroxide and visible light. J. Am. Chem. Soc. 2009, 131, 11658–11659. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, S.; Zhang, Y.; Fang, J.; Zhou, Y.; Yuan, S.; Zhang, C.; Chen, W. One-pot synthesis of K-doped g-C3N4 nanosheets with enhanced photocatalytic hydrogen production under visible-light irradiation. Appl. Surf. Sci. 2018, 440, 258–265. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, X.; Liu, D.; Wang, J.; Zhu, Y. Enhanced catalytic activity of potassium-doped graphitic carbon nitride induced by lower valence position. Appl. Catal. B Environ. 2015, 164, 77–81. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.H.; Li, J.Y.; Tang, Z.R.; Xu, Y.J. 3D graphene-based gel photocatalysts for environmental pollutants degradation. Environ. Pollut. 2019, 253, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Du, A.; Sanvito, S.; Li, Z.; Wang, D.; Jiao, Y.; Liao, T.; Sun, Q.; Ng, Y.H.; Zhu, Z.; Amal, R.; et al. Hybrid graphene and graphitic carbon nitride nanocomposite: Gap opening, electron-hole puddle, interfacial charge transfer, and enhanced visible light response. J. Am. Chem. Soc. 2012, 134, 4393–4397. [Google Scholar] [CrossRef]
- Bai, X.; Yan, S.; Wang, J.; Wang, L.; Jiang, W.; Wu, S.; Sun, C.; Zhu, Y. A simple and efficient strategy for the synthesis of a chemically tailored g-C3N4 material. J. Mater. Chem. A 2014, 2, 17521–17529. [Google Scholar] [CrossRef]
- Nartey, O.D.; Zhao, B. Biochar preparation, characterization, and adsorptive capacity and its effect on bioavailability of contaminants: An overview. Adv. Mater. Sci. Eng. 2014, 2014, 715398. [Google Scholar] [CrossRef]
- Hu, S.; Ma, L.; Wang, H.; Zhang, L.; Zhao, Y.; Wu, G. Properties and photocatalytic performance of polypyrrole and polythiophene modified g-C3N4 nanocomposites. RSC Adv. 2015, 5, 31947–31953. [Google Scholar] [CrossRef]
- Han, Q.; Wang, B.; Gao, J.; Cheng, Z.; Zhao, Y.; Zhang, Z.; Qu, L. Atomically thin mesoporous nanomesh of graphitic C3N4 for high-efficiency photocatalytic hydrogen evolution. ACS Nano 2016, 10, 2745–2751. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Zhou, S.; Lin, W.; Kong, Y. A facile one-step synthesis of Fe-doped g-C3N4 nanosheets and their improved visible-light photocatalytic performance. ChemCatChem 2017, 9, 1708–1715. [Google Scholar] [CrossRef]
- Hossain, M.K.; Strezov, V.; Chan, K.Y.; Ziolkowski, A.; Nelson, P.F. Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. J. Environ. Manag. 2011, 92, 223–228. [Google Scholar] [CrossRef]
- Yan, S.; Li, Z.; Zou, Z. Photodegradation performance of g-C3N4 fabricated by directly heating melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef]
- Barbara, J.; Elisabeth, I.; Jurgen, S.; Peter, K.; Helen, M.; Wolfgang, S. Melem (2,5,8-triamino-tri-s-triazine), an important intermediate during condensation of melamine rings to graphitic carbon nitride: Synthesis, structure determination by X-ray powder diffractometry, solid-state NMR, and theoretical studies. J. Am. Chem. Soc. 2003, 125, 10288–10300. [Google Scholar]
- Gao, H.; Yan, S.; Wang, J.; Huang, Y.; Wang, P.; Li, Z.; Zou, Z. Towards efficient solar hydrogen production by intercalated carbon nitride photocatalyst. Phys. Chem. Chem. Phys. 2013, 15, 18077–18084. [Google Scholar] [CrossRef]
- Li, G.; Yang, N.; Wang, W.; Zhang, W. Synthesis, photophysical and photocatalytic properties of N-doped sodium niobate sensitized by carbon nitride. J. Phys. Chem. C 2009, 113, 14829–14833. [Google Scholar] [CrossRef]
- Yang, S.; Gong, Y.; Zhang, J.; Zhan, L.; Ma, L.; Fang, Z.; Vajtai, R.; Wang, X.; Ajayan, P.M. Exfoliated graphitic carbon nitride nanosheets as efficient catalysts for hydrogen evolution under visible light. Adv. Mater. 2013, 25, 2452–2456. [Google Scholar] [CrossRef]
- Nakajima, H.; Mori, T.; Shen, Q.; Toyoda, T. Photoluminescence study of mixtures of anatase and rutile TiO2 nanoparticles: Influence of charge transfer between the nanoparticles on their photoluminescence excitation bands. Chem. Phys. Lett. 2005, 409, 81–84. [Google Scholar] [CrossRef]
- Wang, W.; An, T.; Li, G.; Xia, D.; Zhao, H.; Yu, J.C.; Wong, P.K. Earth-abundant Ni2P/g-C3N4 lamellar nanohydrids for enhanced photocatalytic hydrogen evolution and bacterial inactivation under visible light irradiation. Appl. Catal. B Environ. 2017, 217, 570–580. [Google Scholar] [CrossRef]
- Shalom, M.; Inal, S.; Fettkenhauer, C.; Neher, D.; Antonietti, M. Improving carbon nitride photocatalysis by supramolecular preorganization of monomers. J. Am. Chem. Soc. 2013, 135, 7118–7121. [Google Scholar] [CrossRef]
- Liu, D.; Wu, Z.; Tian, F.; Ye, B.-C.; Tong, Y. Synthesis of N and La co-doped TiO2/AC photocatalyst by microwave irradiation for the photocatalytic degradation of naphthalene. J. Alloys Compd. 2016, 676, 489–498. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Chen, M. Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater. Bioresour. Technol. 2016, 214, 836–851. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, A.; Sharma, G.; Al-Muhtaseb, A.H.; Naushad, M.; Ghfar, A.A.; Guo, C.; Stadler, F.J. Biochar-templated g-C3N4/Bi2O2CO3/CoFe2O4 nano-assembly for visible and solar assisted photo-degradation of paraquat, nitrophenol reduction and CO2 conversion. Chem. Eng. J. 2018, 339, 393–410. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Dong, F.; Reshak, A.H.; Ye, L.; Pinna, N.; Zeng, C.; Zhang, T.; Huang, H. Chlorine intercalation in graphitic carbon nitride for efficient photocatalysis. Appl. Catal. B Environ. 2017, 203, 465–474. [Google Scholar] [CrossRef]
- Zhu, Z.; Tang, X.; Kang, S.; Huo, P.; Song, M.; Shi, W.; Lu, Z.; Yan, Y. Constructing of the magnetic photocatalytic nanoreactor MS@FCN for cascade catalytic degrading of tetracycline. J. Phys. Chem. C 2016, 120, 27250–27258. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Theurich, J.; Bahnemann, D.W.; Vogel, R.; Ehamed, F.E.; Alhakimi, G.; Rajab, I. Photocatalytic degradation of naphthalene and anthracene: GC-MS analysis of the degradation pathway. Res. Chem. Intermed. 1997, 23, 247–274. [Google Scholar] [CrossRef]
- Vialaton, D.; Richard, C.; Baglio, D.; Paya-Perez, A.-B. Mechanism of the photochemical transformation of naphthalene in water. J. Photochem. Photobiol. A Chem. 1999, 123, 15–19. [Google Scholar] [CrossRef]
- Guillard, C.; Delprat, H.; Hoang-van, C.; Pichat, P. Laboratory study of the rates and products of the phototransformations of naphthalene adsorbed on samples of titanium dioxide, ferric oxide, muscovite, and fly ash. J. Atmos. Chem. 1993, 16, 47–59. [Google Scholar] [CrossRef]


| Nos. | Structures | Names | Nos. | Structures | Names |
|---|---|---|---|---|---|
| 1 |  | 2-naphthol | 2 | 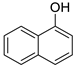 | 1-naphthol |
| 3 | 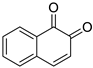 | 1,2-naphthalenedione | 4 | 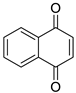 | 1,4-naphthalenedione |
| 5 | 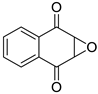 | 2,3-dihydro-2,3-epoxy-1,4-naphthoquinone | 6 |  | 1,2-dicarboxybenzene |
| 7 | 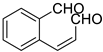 | 2-formylcinnamaldehyde |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Lin, M. Synthesis of Biochar-Supported K-doped g-C3N4 Photocatalyst for Enhancing the Polycyclic Aromatic Hydrocarbon Degradation Activity. Int. J. Environ. Res. Public Health 2020, 17, 2065. https://doi.org/10.3390/ijerph17062065
Li F, Lin M. Synthesis of Biochar-Supported K-doped g-C3N4 Photocatalyst for Enhancing the Polycyclic Aromatic Hydrocarbon Degradation Activity. International Journal of Environmental Research and Public Health. 2020; 17(6):2065. https://doi.org/10.3390/ijerph17062065
Chicago/Turabian StyleLi, Fayun, and Meixia Lin. 2020. "Synthesis of Biochar-Supported K-doped g-C3N4 Photocatalyst for Enhancing the Polycyclic Aromatic Hydrocarbon Degradation Activity" International Journal of Environmental Research and Public Health 17, no. 6: 2065. https://doi.org/10.3390/ijerph17062065
APA StyleLi, F., & Lin, M. (2020). Synthesis of Biochar-Supported K-doped g-C3N4 Photocatalyst for Enhancing the Polycyclic Aromatic Hydrocarbon Degradation Activity. International Journal of Environmental Research and Public Health, 17(6), 2065. https://doi.org/10.3390/ijerph17062065




