Association of Cesarean Birth with Body Mass Index Trajectories in Adolescence
Abstract
1. Introduction
2. Methods
2.1. Population
2.2. Outcomes
2.3. Statistical Methods
3. Results
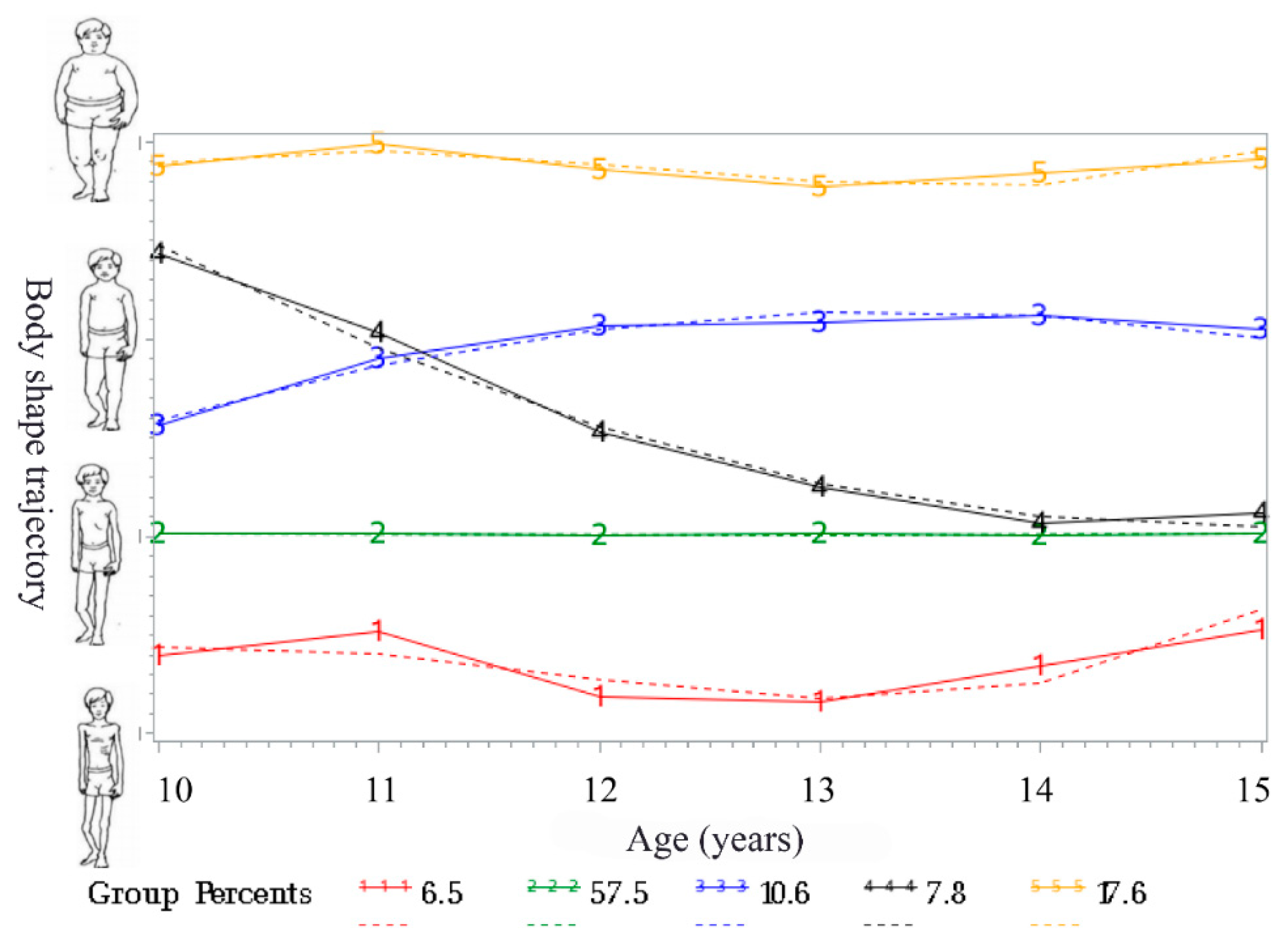
3.1. Body Shape Trajectories
3.2. Association of Delivery Mode with Body Shape Trajectory Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- (NCD-RisC) NRFC. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- Tun, H.M.; Bridgman, S.L.; Chari, R.; Field, C.J.; Guttman, D.S.; Becker, A.B.; Scott, J.A. Roles of Birth Mode and Infant Gut Microbiota in Intergenerational Transmission of Overweight and Obesity from Mother to Offspring. JAMA Pediatr. 2018, 172, 368–377. [Google Scholar] [CrossRef]
- Landberg, A.; Fält, A.; Montgomery, S.; Sundqvist, P.; Fall, K. Overweight and obesity during adolescence increases the risk of renal cell carcinoma. Int. J. Cancer 2019, 145, 1232–1237. [Google Scholar] [CrossRef]
- Ohlsson, C.; Bygdell, M.; Sondén, A.; Rosengren, A.; Kindblom, J.M. Association between excessive BMI increase during puberty and risk of cardiovascular mortality in adult men: A population-based cohort study. Lancet Diab. Endocrinol. 2016, 4, 1017–1024. [Google Scholar] [CrossRef]
- Black, M.; Bhattacharya, S.; Philip, S.; Norman, J.E.; McLernon, D.J. Planned Cesarean Delivery at Term and Adverse Outcomes in Childhood Health. JAMA 2015, 314, 2271–2279. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.T.; Mao, G.; Bennet, W.L.; Hourigan, S.K.; Dominguez-Bello, M.G.; Appel, L.J.; Wang, X. Does vaginal delivery mitigate or strengthen the intergenerational association of overweight and obesity? Findings from the Boston Birth Cohort. Int. J. Obes. 2017, 41, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Hu, Y.; Bruner, D.W. Composition of gut microbiota and its association with body mass index and lifestyle factors in a cohort of 7-18 years old children from the American Gut Project. Pediatr. Obes. 2019, 14, e12480. [Google Scholar] [CrossRef] [PubMed]
- Rutayisire, E.; Huang, K.; Liu, Y.; Tao, F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: A systematic review. BMC Gastroenterol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Hyde, M.J.; Mostyn, A.; Modi, N.; Kemp, P.R. The health implications of birth by Caesarean section. Biol. Rev. 2012, 87, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Li, H.T.; Luo, S.; Trasande, L.; Hellerstein, S.; Kang, C.; Li, J.X.; Blustein, J. Geographic Variations and Temporal Trends in Cesarean Delivery Rates in China, 2008–2014. JAMA 2017, 317, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Buscot, M.J.; Thomson, R.J.; Juonala, M.; Sabin, M.A.; Burgner, D.P.; Lehtimäki, T.; Laitinen, T. BMI Trajectories Associated With Resolution of Elevated Youth BMI and Incident Adult Obesity. Pediatrics 2018, 141, e20172003. [Google Scholar] [CrossRef] [PubMed]
- Charakida, M.; Deanfield, J.E. BMI trajectories from childhood: The slippery slope to adult obesity and cardiovascular disease. Eur. Heart J. 2018, 39, 2271–2273. [Google Scholar] [CrossRef] [PubMed]
- Oluwagbemigun, K.; Buyken, A.E.; Alexy, U.; Schmid, M.; Herder, C.; Nöthlings, U. Developmental trajectories of body mass index from childhood into late adolescence and subsequent late adolescence-young adulthood cardiometabolic risk markers. Cardiovasc. Diabetol. 2019, 18, 9. [Google Scholar] [CrossRef]
- Widhalm, K. Prevention of Obesity in Childhood and Adolescence. Obes. Facts 2018, 11, 232–233. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.; Zhang, Q.; Zhang, Y.; Wang, T. Prevalence of overweight and obesity among children and adolescents increased rapidly in Chinese rural regions while level off in urban areas. Int. J. Cardiol. 2016, 223, 61–62. [Google Scholar] [CrossRef] [PubMed]
- Tomeo, C.A.; Rich-Edwards, J.W.; Michels, K.B.; Berkey, C.S.; Hunter, D.J.; Frazier, A.L.; Buka, S.L. Reproducibility and validity of maternal recall of pregnancy-related events. Epidemiology 1999, 10, 774–777. [Google Scholar] [CrossRef] [PubMed]
- The Institute of Child and Adolescent Health Peking University; The Institute of Nutrition and Health Chinese Center for Disease Control and Prevention; The National Center for Maternal and Child Health Chinese Center for Disease Control and Prevention. Screening for Overweight and Obesity among School-Age Children and Adolescents; China Standards Press: Beijing, China, 2018. [Google Scholar]
- Jones, B.L.; Nagin, D.S. Advances in Group-Based Trajectory Modeling and an SAS Procedure for Estimating Them. Sociol. Methods Res. 2007, 35, 542–571. [Google Scholar] [CrossRef]
- Zheng, Y.; Song, M.; Manson, J.E.; Giovannucci, E.L.; Hu, F.B. Group-Based Trajectory of Body Shape From Ages 5 to 55 Years and Cardiometabolic Disease Risk in 2 US Cohorts. Am. J. Epidemiol. 2017, 186, 1246–1255. [Google Scholar] [CrossRef]
- Nagin, D.S.; Odgers, C.L. Group-based trajectory modeling in clinical research. Annu. Rev. Clin. Psychol. 2010, 6, 109–138. [Google Scholar] [CrossRef]
- Kwon, S.; Janz, K.F.; Letuchy, E.M.; Burns, T.L.; Levy, S.M. Association between body mass index percentile trajectories in infancy and adiposity in childhood and early adulthood. Obesity 2017, 25, 166–171. [Google Scholar] [CrossRef]
- Péneau, S.; Giudici, K.V.; Gusto, G.; Goxe, D.; Lantieri, O.; Hercberg, S.; Rolland-Cachera, M.F. Growth Trajectories of Body Mass Index during Childhood: Associated Factors and Health Outcome at Adulthood. J. Pediatr. 2017, 186, 64–71.e1. [Google Scholar] [CrossRef]
- Rzehak, P.; Oddy, W.H.; Mearin, M.L.; Grote, V.; Mori, T.A.; Szajewska, H.; Huang, R.C. Infant feeding and growth trajectory patterns in childhood and body composition in young adulthood. Am. J. Clin. Nutr. 2017, 106, 568–580. [Google Scholar] [CrossRef]
- Buscot, M.J.; Thomson, R.J.; Juonala, M.; Sabin, M.A.; Burgner, D.P.; Lehtimäki, T.; Magnussen, C.G. Distinct child-to-adult body mass index trajectories are associated with different levels of adult cardiometabolic risk. Eur. Heart J. 2018, 39, 2263–2270. [Google Scholar] [CrossRef] [PubMed]
- Bichteler, A.; Gershoff, E.T. Identification of Children’s BMI Trajectories and Prediction from Weight Gain in Infancy. Obesity 2018, 26, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Gungor, N.K. Overweight and obesity in children and adolescents. J. Clin. Res. Pediatr. Endocrinol. 2014, 6, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Gaskins, A.J.; Blaine, A.I.; Zhang, C.; Gillman, M.W.; Missmer, S.A.; Chavarro, J.E. Association between Cesarean Birth and Risk of Obesity in Offspring in Childhood, Adolescence, and Early Adulthood. JAMA Pediatr. 2016, 170, e162385. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, M.; Maher, G.M.; Boland, F.; Fitzgerald, A.P.; Murray, D.M.; Biesma, R. Group-based trajectory modelling for BMI trajectories in childhood: A systematic review. Obes. Rev. 2019, 20, 998–1015. [Google Scholar] [CrossRef] [PubMed]
- Almgren, M.; Schlinzig, T.; Gomez-Cabrero, D.; Gunnar, A.; Sundin, M.; Johansson, S.; Ekström, T.J. Cesarean delivery and hematopoietic stem cell epigenetics in the newborn infant: Implications for future health? Am. J. Obstet. Gynecol. 2014, 211, 502.e1–502.e8. [Google Scholar] [CrossRef]
- Barros, F.C.; Matijasevich, A.; Hallal, P.C.; Horta, B.L.; Barros, A.J.; Menezes, A.B.; Victora, C.G. Cesarean section and risk of obesity in childhood, adolescence, and early adulthood: Evidence from 3 Brazilian birth cohorts. Am. J. Clin. Nutr. 2012, 95, 465–470. [Google Scholar] [CrossRef]
- Meo, S.A.; Altuwaym, A.A.; Alfallaj, R.M.; Alduraibi, K.A.; Alhamoudi, A.M.; Alghamdi, S.M.; Akram, A. Effect of Obesity on Cognitive Function among School Adolescents: A Cross-Sectional Study. Obes. Facts 2019, 12, 150–156. [Google Scholar] [CrossRef]
- Masukume, G.; McCarthy, F.P.; Baker, P.N.; Kenny, L.C.; Morton, S.M.; Murray, D.M.; Khashan, A.S. Association between caesarean section delivery and obesity in childhood: A longitudinal cohort study in Ireland. BMJ Open 2019, 9, e025051. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Loy, S.L.; Tan, K.H.; Godfrey, K.M.; Gluckman, P.D.; Chong, Y.S.; Chan, S.Y. Association of Elective and Emergency Cesarean Delivery With Early Childhood Overweight at 12 Months of Age. JAMA Netw. Open 2018, 1, e185025. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ye, R.; Pei, L.; Ren, A.; Zheng, X.; Liu, J. Caesarean delivery, caesarean delivery on maternal request and childhood overweight: A Chinese birth cohort study of 181 380 children. Pediatr. Obes. 2014, 9, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.G.; Martin, R.M.; Whincup, P.H.; Davey-Smith, G.; Gillman, M.W.; Cook, D.G. The effect of breastfeeding on mean body mass index throughout life: A quantitative review of published and unpublished observational evidence. Am. J. Clin. Nutr. 2005, 82, 1298–1307. [Google Scholar] [CrossRef]
- Isganaitis, E.; Venditti, S.; Matthews, T.J.; Lerin, C.; Demerath, E.W.; Fields, D.A. Maternal obesity and the human milk metabolome: Associations with infant body composition and postnatal weight gain. Am. J. Clin. Nutr. 2019, 110, 111–120. [Google Scholar] [CrossRef] [PubMed]
| Variable | N (% of Sample) | Persistent Underweight (Trajectory 1) | Persistent Healthy Weight (Trajectory 2) | Progressive Overweight (Trajectory 3) | Obesity to Healthy Weight (Trajectory 4) | Progressive Obesity (Trajectory 5) | pa |
|---|---|---|---|---|---|---|---|
| Child characteristics | |||||||
| N | 569 | 37 | 327 | 62 | 43 | 100 | |
| Sex | <0.001 | ||||||
| Girls (%) | 294 (52.3%) | 13 (35.1%) | 195 (59.6%) | 27 (43.5%) | 19 (44.2%) | 40 (40.0%) | |
| Boys (%) | 275 (47.7%) | 24 (64.9%) | 132 (40.4%) | 35 (56.5%) | 24 (55.8%) | 60 (60.0%) | |
| Delivery mode (%) | 0.003 | ||||||
| Vaginal (%) | 407 (71.7%) | 29 (78.4%) | 243 (75.4%) | 44 (70.5%) | 33 (76.7%) | 58 (56.0%) | |
| Cesarean (%) | 162 (28.3%) | 8 (21.6%) | 80 (24.6%) | 19 (29.5%) | 10 (23.3%) | 45 (44.0%) | |
| Gestational age at birth (weeks, mean (SD)) | 38.9 (1.4) | 39 (0.9) | 38.9 (1.5) | 38.9 (1.5) | 38.8 (1.4) | 38.9 (1.4) | 0.699 |
| Birth weight (%) | 0.519 | ||||||
| LBW | 24 (4.5%) | 4 (11.1%) | 13 (4.2%) | 1 (1.6%) | 1 (2.5%) | 5 (5.3%) | |
| NBW | 415 (77.1%) | 25 (69.5%) | 240 (78.4%) | 48 (78.7%) | 33 (82.5%) | 69 (72.6%) | |
| HBW | 99 (18.4%) | 7 (19.4%) | 53 (17.3%) | 12 (19.7%) | 6 (15.0%) | 21 (22.1%) | |
| History of hypertension (%) | 0.039 | ||||||
| Yes | 235 (42.9%) | 14 (38.9%) | 125 (39.4%) | 27 (45.8%) | 14 (36.8%) | 55 (56.2%) | |
| No | 313 (57.1%) | 22 (61.1%) | 192 (60.6%) | 32 (54.2%) | 24 (62.2%) | 43 (43.8%) | |
| Household income (per year) | |||||||
| <60,000 ¥ | 259 (46.5%) | 16 (44.4%) | 148 (46.3%) | 23 (37.7%) | 20 (46.5%) | 52 (53.6%) | 0.415 |
| ≥60,000 ¥ | 298(53.5%) | 20 (55.6%) | 172 (53.7) | 38 (62.3%) | 23 (53.5%) | 45 (46.4%) | |
| Maternal characteristics | |||||||
| Maternal pregnancy age (y, mean (SD)) | 26.5 (2.9) | 26.8 (2.9) | 26.5 (2.6) | 25.6 (3.6) | 27.1 (3.2) | 26.5 (3.1) | |
| Mother schooling (%) | 0.007 | ||||||
| ≤9 years | 272 (47.8%) | 17 (45.9%) | 158 (48.3%) | 36 (58.1%) | 27 (62.8%) | 34 (39.1%) | |
| >9 years | 297 (52.2%) | 20 (54.1%) | 169 (51.7%) | 26 (41.9%) | 16 (37.2%) | 53 (60.9%) | |
| Maternal BMI status (%) | 0.289 | ||||||
| Underweight | 30 (5.5%) | 1 (2.9%) | 17 (5.5%) | 4 (6.9%) | 4 (9.8%) | 4 (4.1%) | |
| Healthy weight | 349 (64.3%) | 25 (71.4%) | 201 (64.6%) | 35 (60.3%) | 31 (75.6%) | 57 (58.2%) | |
| Overweight/Obesity | 164 (30.2%) | 9 (25.7%) | 93 (29.9%) | 19 (32.8%) | 6 (14.6%) | 37 (37.7%) |
| Variable | Trajectory 1 vs. Trajectory 2 | Trajectory 3 vs. Trajectory 2 | Trajectory 4 vs. Trajectory 2 | Trajectory 5 vs. Trajectory 2 | ||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Both sexes | ||||||||
| Vaginal delivery | 1.00 | - | 1.00 | - | 1.00 | - | 1.00 | - |
| Cesarean delivery 1 | 0.85 | 0.37–1.92 | 1.28 | 0.70–2.35 | 0.93 | 0.44–1.97 | 2.41 | 1.51–3.85 |
| Cesarean delivery 2 | 1.06 | 0.42–2.68 | 1.11 | 0.55–2.27 | 0.72 | 0.27–1.88 | 2.50 | 1.42–4.41 |
| Boys | ||||||||
| Vaginal delivery | 1.00 | - | 1.00 | - | 1.00 | - | 1.00 | - |
| Cesarean delivery 1 | 0.61 | 0.19–1.91 | 1.21 | 0.53–2.79 | 0.61 | 0.19–1.91 | 2.32 | 1.21–4.43 |
| Cesarean delivery 2 | 0.53 | 0.13–2.19 | 1.08 | 0.38–3.05 | 0.35 | 0.06–1.87 | 2.42 | 1.10-5.33 |
| Girls | ||||||||
| Vaginal delivery | 1.00 | - | 1.00 | - | 1.00 | - | 1.00 | - |
| Cesarean delivery 1 | 1.37 | 0.40–4.66 | 1.37 | 0.56–3.36 | 1.42 | 0.51–3.96 | 2.52 | 1.25–5.11 |
| Cesarean delivery 2 | 2.42 | 0.61–9.56 | 1.26 | 0.46–3.44 | 1.17 | 0.33–4.14 | 2.38 | 1.01–5.62 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Zhang, Y.; Sun, Y.; Zhang, D. Association of Cesarean Birth with Body Mass Index Trajectories in Adolescence. Int. J. Environ. Res. Public Health 2020, 17, 2003. https://doi.org/10.3390/ijerph17062003
Zhou Y, Zhang Y, Sun Y, Zhang D. Association of Cesarean Birth with Body Mass Index Trajectories in Adolescence. International Journal of Environmental Research and Public Health. 2020; 17(6):2003. https://doi.org/10.3390/ijerph17062003
Chicago/Turabian StyleZhou, Yunping, Yanqing Zhang, Yun Sun, and Dongfeng Zhang. 2020. "Association of Cesarean Birth with Body Mass Index Trajectories in Adolescence" International Journal of Environmental Research and Public Health 17, no. 6: 2003. https://doi.org/10.3390/ijerph17062003
APA StyleZhou, Y., Zhang, Y., Sun, Y., & Zhang, D. (2020). Association of Cesarean Birth with Body Mass Index Trajectories in Adolescence. International Journal of Environmental Research and Public Health, 17(6), 2003. https://doi.org/10.3390/ijerph17062003






