Stroke to Dementia Associated with Environmental Risks—A Semi-Markov Model
Abstract
1. Introduction
2. Materials and Methods
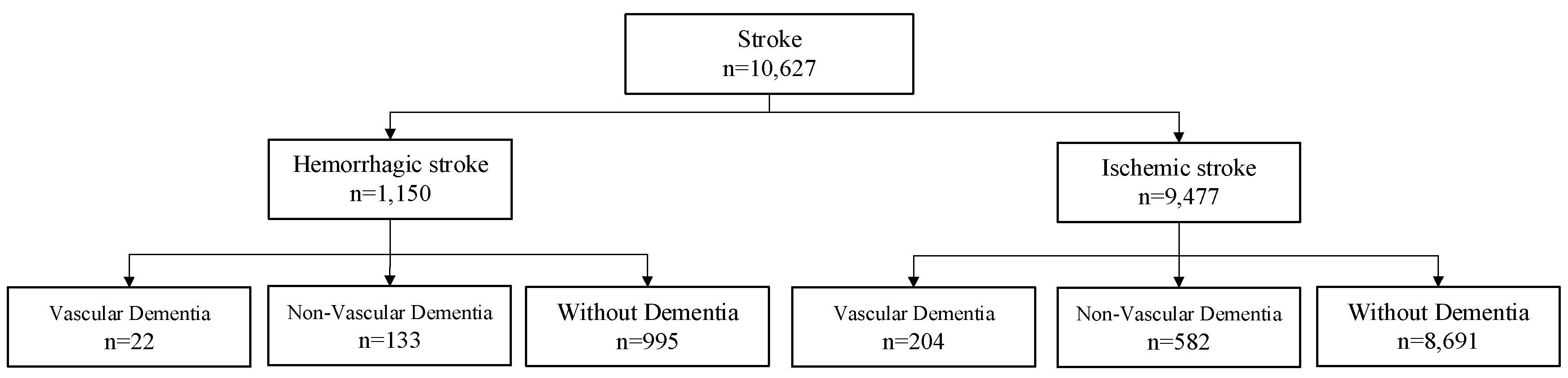
2.1. Population
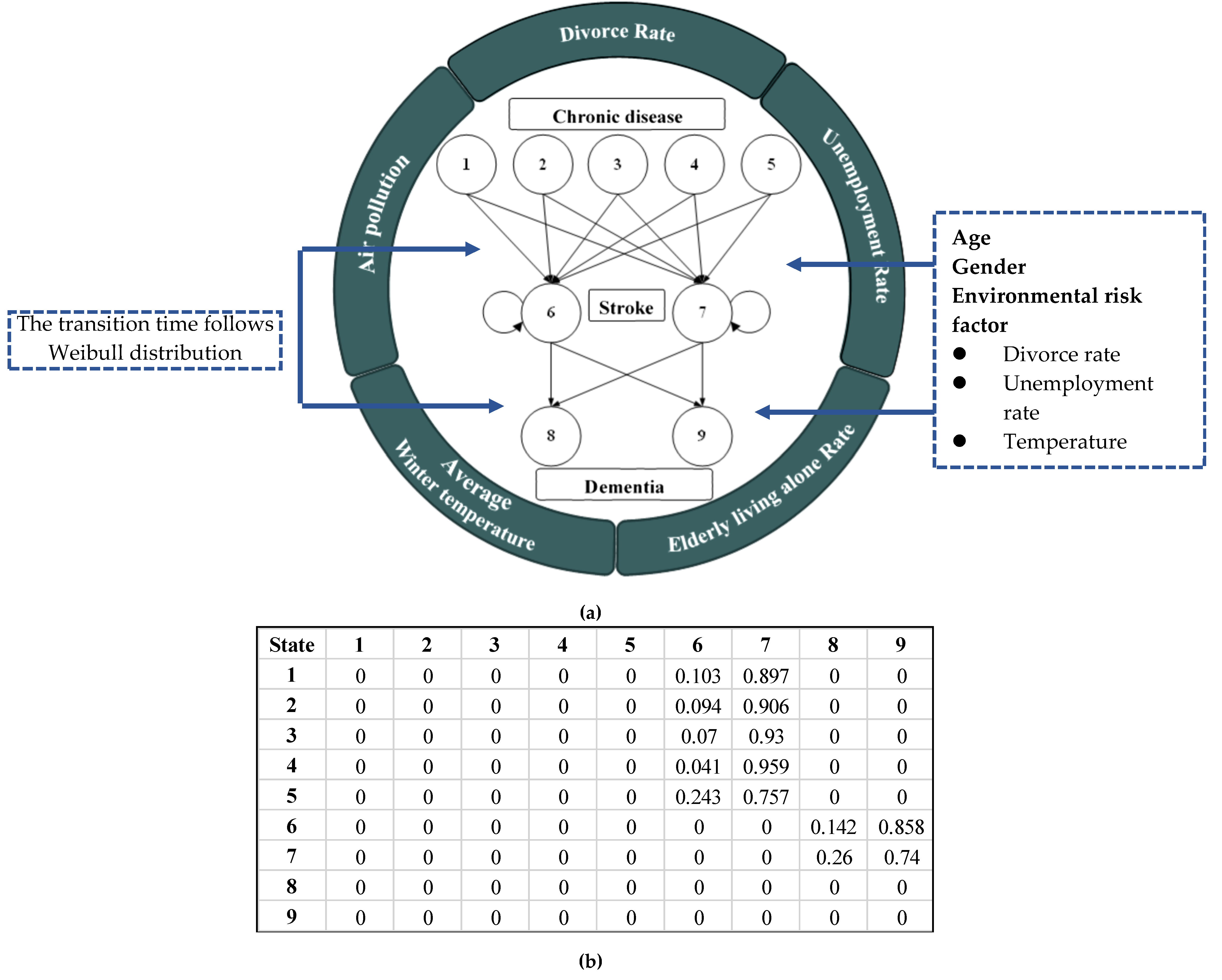
2.2. Markovian-Based Modeling
3. Results and Discussion
3.1. Multivariate Analysis
3.2. Findings in SMP
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arslan, A.K.; Colak, C.; Sarihan, M.E. Different medical data mining approaches based prediction of ischemic stroke. Comput. Methods Programs Biomed. 2016, 130, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Arboix, A. Cardiovascular risk factors for acute stroke: Risk profiles in the different subtypes of ischemic stroke. World J. Clin. Cases 2015, 3, 418. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart disease and stroke statistics—2018 update: A report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Blank, R.H. Alzheimer’s Disease and Other Dementias: An Introduction. In Social & Public Policy of Alzheimer’s Disease in the United States; Palgrave Pivot: Singapore, 2019; pp. 1–26. [Google Scholar]
- Bordone, M.P.; Salman, M.M.; Titus, H.E.; Amini, E.; Andersen, J.V.; Chakraborti, B.; Diuba, A.V.; Dubouskaya, T.G.; Ehrke, E.; de Freitas, A.E.; et al. The energetic brain—A review from students to students. J. Neurochem. 2019, 151, 139–165. [Google Scholar] [CrossRef]
- Cao, Q.; Buskens, E.; Feenstra, T.; Jaarsma, T.; Hillege, H.; Postmus, D. Continuous-time semi-Markov models in health economic decision making: An illustrative example in heart failure disease management. Med. Decis. Mak. 2016, 36, 59–71. [Google Scholar] [CrossRef]
- Chen, R.; Wang, C.; Meng, X.; Chen, H.I.; Thach, T.Q.; Wong, C.M.; Kan, H. Both low and high temperature may increase the risk of stroke mortality. Neurology 2013, 81, 1064–1070. [Google Scholar] [CrossRef]
- Cheng, C.L.; Kao, Y.H.Y.; Lin, S.J.; Lee, C.H.; Lai, M.L. Validation of the national health insurance research database with ischemic stroke cases in Taiwan. Pharmacoepidemiol. Drug Saf. 2011, 20, 236–242. [Google Scholar] [CrossRef]
- Cox, D.R. Regression models and life-tables. J. R. Stat. Soc. Ser. B 1972, 34, 187–220. [Google Scholar] [CrossRef]
- Desmond, D.W.; Moroney, J.T.; Sano, M.; Stern, Y. Incidence of dementia after ischemic stroke: Results of a longitudinal study. Stroke 2002, 33, 2254–2262. [Google Scholar] [CrossRef]
- Dichgans, M. Dementia risk after transient ischaemic attack and stroke. Lancet Neurol. 2019, 18, 223–225. [Google Scholar] [CrossRef]
- Eichler, T.S.; Hoffmann, W.; Hertel, J.; Richter, S.; Wucherer, D.; Michalowsky, B.; Dreier, A.; Thyrian, J.R. Living alone with dementia: Prevalence, correlates and the utilization of health and nursing care services. J. Alzheimers Dis. 2016, 52, 619–629. [Google Scholar] [CrossRef]
- Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- Feigin, V.L.; Wiebers, D.O. Environmental factors and stroke: A selective review. J. Stroke Cerebrovasc. Dis. 1997, 6, 108–113. [Google Scholar] [CrossRef]
- Feigin, V.L.; Forouzanfar, M.H.; Krishnamurthi, R.; Mensah, G.A.; Connor, M.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.; Truelsen, T.; et al. Global and regional burden of stroke during 1990–2010: Findings from the global burden of disease study 2010. Lancet 2014, 383, 245–255. [Google Scholar] [CrossRef]
- Franklin, S.S.; Wong, N.D. Hypertension and cardiovascular disease: Contributions of the Framingham Heart Study. Glob. Heart 2013, 8, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Zheng, M.; Feng, W.; Wu, J.; Deng, C.; Luo, G.; Wang, L.; Pan, B.; Liu, H. Effects of ambient temperature on stroke hospital admissions: Results from a time-series analysis of 104,432 strokes in Guangzhou, China. Sci. Total Environ. 2017, 580, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Hachinski, V.; Einhäupl, K.; Ganten, D.; Alladi, S.; Brayne, C.; Stephan, B.C.; Sweeney, M.D.; Zlokovic, B.; Iturria-Medina, Y.; Iadecola, C.; et al. Preventing dementia by preventing stroke: The Berlin Manifesto. Alzheimers Dement. 2019, 15, 961–984. [Google Scholar] [CrossRef] [PubMed]
- Henon, H.; Durieu, I.; Guerouaou, D.; Lebert, F.; Pasquier, F.; Leys, D. Poststroke dementia incidence and relationship to prestroke cognitive decline. Neurology 2001, 57, 1216–1222. [Google Scholar] [CrossRef]
- Hu, G.C.; Chen, Y.M. Post-stroke dementia: Epidemiology, mechanisms and management. Int. J. Gerontol. 2017, 11, 210–214. [Google Scholar] [CrossRef]
- Hsieh, F.I.; Chiou, H.Y. Stroke: Morbidity, risk factors, and care in taiwan. J. Stroke 2014, 16, 59. [Google Scholar] [CrossRef]
- Ivan, C.S.; Seshadri, S.; Beiser, A.; Au, R.; Kase, C.S.; Kelly-Hayes, M.; Wolf, P.A. Dementia after stroke: The Framingham Study. Stroke 2004, 35, 1264–1268. [Google Scholar] [CrossRef] [PubMed]
- Kapetanakis, V.; Matthews, F.E.; Hout, A. A semi-Markov model for stroke with piecewise-constant hazards in the presence of left, right and interval censoring. Stat. Med. 2013, 32, 697–713. [Google Scholar] [CrossRef] [PubMed]
- Kurichi, J.E.; Kwong, P.L.; Xie, D.; Bogner, H.R. Predictive indices for functional improvement and deterioration, institutionalization, and death among elderly medicare beneficiaries. PmR 2017, 9, 1065–1076. [Google Scholar] [CrossRef][Green Version]
- Lee, J.W.; Lim, H.S.; Kim, D.W.; Shin, S.A.; Kim, J.; Yoo, B.; Cho, K.H. The development and implementation of stroke risk prediction model in National Health Insurance Service’s personal health record. Comput. Methods Programs Biomed. 2018, 153, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Horton, R.M.; Bader, D.A.; Liu, F.; Sun, Q.; Kinney, P.L. Long-term projections of temperature-related mortality risks for ischemic stroke, hemorrhagic stroke, and acute ischemic heart disease under changing climate in Beijing, China. Environ. Int. 2018, 112, 1–9. [Google Scholar] [CrossRef]
- Lim, J.S.; Kwon, H.M.; Kim, S.E.; Lee, J.; Lee, Y.S.; Yoon, B.W. Effects of temperature and pressure on acute stroke incidence assessed using a Korean nationwide insurance database. J. Stroke 2017, 19, 295. [Google Scholar] [CrossRef]
- Listwon, A.; Saint-Pierre, P. Semimarkov: An R package for parametric estimation in multi-state semi-markov models. J. Stat. Softw. 2015, 66, 784. [Google Scholar]
- Listwon, A.; Saint-Pierre, P.; Listwon, M.A. Package ‘SemiMarkov’ 2013. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.408.2595&rep=rep1&type=pdf (accessed on 1 December 2019).
- Ljungman, P.L.; Mittleman, M.A. Ambient air pollution and stroke. Stroke 2014, 45, 3734–3741. [Google Scholar] [CrossRef]
- Madsen, T.E.; Roberts, E.T.; Kuczynski, H.; Goldmann, E.; Parikh, N.S.; Boden-Albala, B. Gender, social networks, and stroke preparedness in the stroke warning information and faster treatment Study. J. Stroke Cerebrovasc. Dis. 2017, 26, 2734–2741. [Google Scholar] [CrossRef]
- Makond, B.; Wang, K.J.; Wang, K.M. Probabilistic modeling of short survival in patients with brain metastasis from lung cancer. Comput. Methods Programs Biomed. 2015, 119, 142–162. [Google Scholar] [CrossRef]
- Mijajlović, M.D.; Pavlović, A.; Brainin, M.; Heiss, W.D.; Quinn, T.J.; Ihle-Hansen, H.B.; Hermann, D.M.; Assayag, E.B.; Richard, E.; Thiel, A.; et al. Post-stroke dementia—A comprehensive review. BMC Med. 2017, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Moulin, S.; Labreuche, J.; Bombois, S.; Rossi, C.; Boulouis, G.; Hénon, H.; Duhamel, A.; Leys, D.; Cordonnier, C. Dementia risk after spontaneous intracerebral haemorrhage: A prospective cohort study. Lancet Neurol. 2016, 15, 820–829. [Google Scholar] [CrossRef]
- National Health Insurance Administration. 2019. Available online: https://www.nhi.gov.tw/english/ (accessed on 1 December 2019).
- Rothwell, P.; Slattery, J.; Warlow, C.P.; Wroe, S.J. Is stroke incidence related to season or temperature? Lancet 1996, 347, 934–936. [Google Scholar] [CrossRef]
- Roy, M.K.; Ray, A. Effect of body temperature on mortality of acute stroke. J. Assoc. Physicians India 2004, 52, 959–961. [Google Scholar]
- Wang, K.J.; Adrian, A.M.; Chen, K.-H.; Wang, K.M. A hybrid classifier combining Borderline-SMOTE with AIRS algorithm for estimating brain metastasis from lung cancer: A case study in Taiwan. Comput. Methods Programs Biomed. 2015, 119, 63–76. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, C.; Liu, H.; Li, W.; Zhao, Y.; Xu, G.; Yan, C.; Lin, H.; Lang, L. Hypertension modifies the short-term effects of temperature on morbidity of hemorrhagic stroke. Sci. Total Environ. 2017, 598, 198–203. [Google Scholar] [CrossRef]
- World Health Organization. 2019. Available online: http://www.who.int/mediacentre/factsheets/fs310/en/ (accessed on 1 December 2019).
- World Heart Federation Stroke. 2019. Available online: http://www.world-heart-federation.org/cardiovascular-health/stroke/ (accessed on 1 December 2019).
- Yang, W.S.; Wang, X.; Deng, Q.; Fan, W.Y.; Wang, W.Y. An evidence-based appraisal of global association between air pollution and risk of stroke. Int. J. Cardiol. 2014, 175, 307–313. [Google Scholar] [CrossRef]
- Yousufuddin, M.; Bartley, A.C.; Alsawas, M.; Sheely, H.L.; Shultz, J.; Takahashi, P.Y.; Young, N.P.; Murad, M.H. Impact of multiple chronic conditions in patients hospitalized with stroke and transient ischemic attack. J. Stroke Cerebrovasc. Dis. 2017, 26, 1239–1248. [Google Scholar] [CrossRef]
| Disease Type | Disease Name | ICD-9-CM |
|---|---|---|
| Chronic disease | Hypertension | 401–405 |
| Diabetes mellitus | 250 | |
| Hyperlipidemia | 272 | |
| Chronic lung disease | 490–496 | |
| Hyperthyroidism | 242 | |
| Chronic kidney disease | 585 | |
| Heart failure | 428 | |
| Atrial fibrillation | 42731 | |
| Sleep apnea | 780.51, 780.53, 780.57 | |
| Gout | 274 | |
| Peripheral artery disease | 437.3, 440, 441, 443.1, 443.2, 443.8, 443.9, 447.1, 447.8, 447.9, 445 | |
| Stroke | Hemorrhagic stroke | 430, 432 |
| Ischemic stroke | 433, 437 | |
| Dementia | Vascular dementia | 2904 |
| Non-vascular dementia | 294, 337 |
| 0 (Base Group) | 1 | |
|---|---|---|
| Gender | Female | Male |
| Age | Otherwise | >65 years old |
| Divorce rate | Otherwise | High divorce rate |
| Unemployment rate | Otherwise | High unemployment rate |
| Elderly living alone rate | Otherwise | High elderly living alone rate |
| Temperature | Otherwise | Low winter temperature |
| Air pollution | Otherwise | High rate of PSI > 100 |
| Environmental Type | Definition |
|---|---|
| Divorce rate | |
| Unemployment rate | |
| Air pollution | |
| Elderly living alone rate | |
| Temperature | Average of temperature in January, February and December |
| State | Type | Description |
|---|---|---|
| 1 | Chronic disease | Diagnosed only with hypertension or with one metabolic risk factor after having hypertension |
| 2 | Diagnosed only with diabetes mellitus | |
| 3 | Diagnosed only with hyperlipidemia or with one metabolic risk factor after having hyperlipidemia | |
| 4 | Diagnosed with more than four metabolic risk factors after having other 8 chronic diseases | |
| 5 | Diagnosed other than 11 types of chronic disease or without chronic | |
| 6 | Stroke | Hemorrhagic stroke |
| 7 | Ischemic stroke | |
| 8 | Dementia | Vascular dementia |
| 9 | Non-vascular dementia |
| Model A: Environmental Risk | Model B: Medication and Rehabilitation | Model C: All Factors | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transition | Estimation β | Relative Risk | p-Value | Transition | Estimation β | Relative Risk | p-Value | Transition | Estimation β | Relative Risk | p-Value | |
| Gender | β16 (1→6) | 0.214 | 1.24 | 0.0042 * | β16 (1→6) | 0.183 | 1.20 | 0.0143 * | β16 (1→6) | 0.096 | 1.10 | 0.0108 |
| β36 (3→6) | 0.493 | 1.64 | 0.02 * | β36 (3→6) | 0.509 | 1.66 | 0.0164 * | β36 (3→6) | 0.490 | 1.63 | 0.0206 | |
| Age | β17 (1→7) | −0.117 | 0.89 | <0.0001 * | β17 (1→7) | −0.097 | 0.91 | 0.0002 * | β17 (1→7) | −0.124 | 0.88 | <0.0001 |
| β56 (5→6) | −2.366 | 0.09 | <0.0001 * | β56 (5→6) | −2.382 | 0.09 | <0.0001 * | β56 (5→6) | −2.374 | 0.09 | <0.0001 | |
| β57 (5→7) | −2.538 | 0.08 | <0.0001 * | β57 (5→7) | −2.538 | 0.08 | <0.0001 * | β57 (5→7) | −2.541 | 0.08 | <0.0001 | |
| β69 (6→9) | −0.394 | 0.67 | 0.0259 * | β69 (6→9) | −0.394 | 0.67 | 0.0259 * | β69 (6→9) | −0.393 | 0.68 | 0.0259 | |
| β79 (7→9) | −0.372 | 0.69 | 0.0008 * | β79 (7→9) | −0.389 | 0.68 | 0.0005 * | β79 (7→9) | −0.366 | 0.69 | 0.001 | |
| Divorce rate | β26 (2→6) | 1.364 | 3.91 | 0.0122 * | β26 (2→6) | 1.364 | 3.91 | 0.0123 | ||||
| Unemployment rate | β16 (1→6) | −0.434 | 0.65 | 0.0004 * | β16 (1→6) | −0.447 | 0.64 | 0.0003 | ||||
| β17 (1→7) | −0.369 | 0.69 | <0.0001 * | β17 (1→7) | −0.365 | 0.69 | <0.0001 | |||||
| β36 (3→6) | −0.673 | 0.51 | 0.0379 * | β36 (3→6) | −0.673 | 0.51 | 0.0377 | |||||
| β37 (3→7) | −0.486 | 0.62 | <0.0001 * | β37 (3→7) | −0.493 | 0.61 | <0.0001 | |||||
| β47 (4→7) | −0.904 | 0.40 | <0.0001 * | β47 (4→7) | −0.915 | 0.40 | <0.0001 | |||||
| Temperature | β16 (1→6) | 0.237 | 1.27 | 0.0088 * | β16 (1→6) | 0.222 | 1.25 | 0.0142 | ||||
| β17 (1→7) | 0.408 | 1.50 | <0.0001 * | β17 (1→7) | 0.397 | 1.49 | <0.0001 | |||||
| β27 (2→7) | 0.540 | 1.72 | <0.0001 * | β27 (2→7) | 0.625 | 1.87 | <0.0001 | |||||
| β37 (3→7) | 0.237 | 1.27 | 0.0015 * | β37 (3→7) | 0.227 | 1.25 | 0.0023 | |||||
| β78 (7→8) | −0.316 | 0.73 | 0.0411 * | β78 (7→8) | −0.316 | 0.73 | 0.0409 | |||||
| β79 (7→9) | −0.444 | 0.64 | <0.0001 * | β79 (7→9) | −0.446 | 0.64 | <0.0001 | |||||
| Air pollution | β17 (1→7) | 0.071 | 1.07 | 0.0057 * | β17 (1→7) | 0.079 | 1.08 | 0.0035 | ||||
| β47 (4→7) | 0.272 | 1.31 | 0.0059 * | β47 (4→7) | 0.260 | 1.30 | 0.0085 | |||||
| Blood pressure lowing drug | β16 (1→6) | −0.275 | 0.76 | 0.0042 * | β16 (1→6) | −0.318 | 0.73 | 0.0009 | ||||
| β17 (1→7) | −0.458 | 0.63 | <0.0001 * | β17 (1→7) | −0.469 | 0.63 | <0.0001 | |||||
| β37 (3→7) | −0.218 | 0.80 | 0.0006 * | β37 (3→7) | −0.215 | 0.81 | 0.0008 | |||||
| β47 (4→7) | −0.331 | 0.72 | 0.0309 * | β47 (4→7) | −0.351 | 0.70 | 0.0222 | |||||
| β68 (6→8) | −0.822 | 0.44 | <0.0001 * | - | - | - | - | |||||
| Blood lipid lowing drug | β17 (1→7) | −0.261 | 0.77 | <0.0001 * | β17 (1→7) | −0.250 | 0.78 | <0.0001 | ||||
| Glycemic lowing drug | β17 (1→7) | −0.132 | 0.88 | 0.0168 * | β17 (1→7) | −0.150 | 0.89 | 0.0068 | ||||
| β27 (2→7) | −0.254 | 0.78 | 0.0119 * | β27 (2→7) | −0.349 | 0.71 | 0.0007 | |||||
| Thrombus prevention drug | β16 (1→6) | −0.237 | 0.79 | 0.0053 * | β16 (1→6) | 0.096 | 1.10 | 0.0108 | ||||
| Rehabilitation | β79 (7→9) | −0.187 | 0.83 | 0.0243 * | - | - | - | - | ||||
| Overall Sojourn Time Prediction | ||||||||||
| Chronic to Stroke | Hemorrhagic Stroke | Ischemic Stroke | ||||||||
| Mean (Year) | SD | R2 * | Mean | SD | R2 | |||||
| State 1: Hypertension | 2.94 | 2.94 | 92.3% | 2.72 | 2.89 | 87.0% | ||||
| State 2: Diabetes mellitus | 2.48 | 2.50 | 90.2% | 1.96 | 2.36 | 86.1% | ||||
| State 3: Hyperlipidemia | 2.93 | 2.94 | 95.5% | 2.63 | 2.80 | 92.9% | ||||
| State 4: Other 8 chronic diseases | 4.67 | 3.74 | 92.3% | 4.77 | 3.71 | 97.5% | ||||
| State 5: Other than above 11 types of chronic disease or without chronic | 25.53 | 20.24 | 90.3% | 29.03 | 22.65 | 91.3% | ||||
| Stroke to Dementia | State 8: Vascular dementia | State 9: Non-vascular dementia | ||||||||
| Mean | SD | R2 | Mean | SD | R2 | |||||
| State 6: Hemorrhagic stroke | 1.94 | 2.23 | 77.3% | 1.29 | 2.02 | 80.0% | ||||
| State 7: Ischemic stroke | 2.61 | 2.85 | 83.4% | 2.71 | 2.79 | 88.6% | ||||
| Sojourn Time Prediction by Different Covariates Models | ||||||||||
| Covariate Transition | A: Environmental Risk | B: Medication and Rehabilitation | C: All Factors | |||||||
| Hemorrhagic stroke | Mean (Year) | SD | R2 | Mean (Year) | SD | R2 | Mean (Year) | SD | R2 | |
| β16 (1→6) | 2.79 | 2.86 | 95.34% | 3.19 | 3.06 | 93.11% | 3.12 | 3.05 | 94.07% | |
| β26 (2→6) | 2.36 | 2.45 | 90.92% | 2.37 | 2.43 | 91.81% | 2.24 | 2.36 | 89.23% | |
| β36 (3→6) | 2.80 | 2.88 | 95.06% | 2.68 | 2.79 | 94.28% | 2.97 | 3.01 | 93.61% | |
| β46 (4→6) | 7.03 | 3.56 | 91.73% | 6.83 | 3.68 | 91.49% | 7.04 | 3.58 | 90.82% | |
| β56 (5→6) | 30.22 | 22.70 | 90.33% | 29.82 | 22.62 | 90.35% | 30.19 | 22.68 | 92.31% | |
| Ischemic stroke | Mean (yr) | SD | R2 | Mean (yr) | SD | R2 | Mean (yr) | SD | R2 | |
| β17 (1→7) | 2.71 | 2.90 | 92.63% | 3.15 | 3.10 | 87.83% | 3.11 | 3.09 | 92.30% | |
| β27 (2→7) | 1.86 | 2.30 | 88.28% | 1.97 | 2.37 | 87.47% | 1.93 | 2.35 | 86.08% | |
| β37 (3→7) | 2.55 | 2.76 | 93.98% | 2.65 | 2.81 | 93.26% | 2.64 | 2.82 | 93.06% | |
| β47 (4→7) | 5.03 | 3.88 | 96.04% | 5.56 | 3.93 | 97.07% | 5.69 | 4.02 | 96.34% | |
| β57 (5→7) | 37.15 | 25.55 | 91.22% | 36.97 | 25.51 | 91.23% | 37.15 | 25.55 | 91.57% | |
| Vascular dementia | Mean (yr) | SD | R2 | Mean (yr) | SD | R2 | Mean (yr) | SD | R2 | |
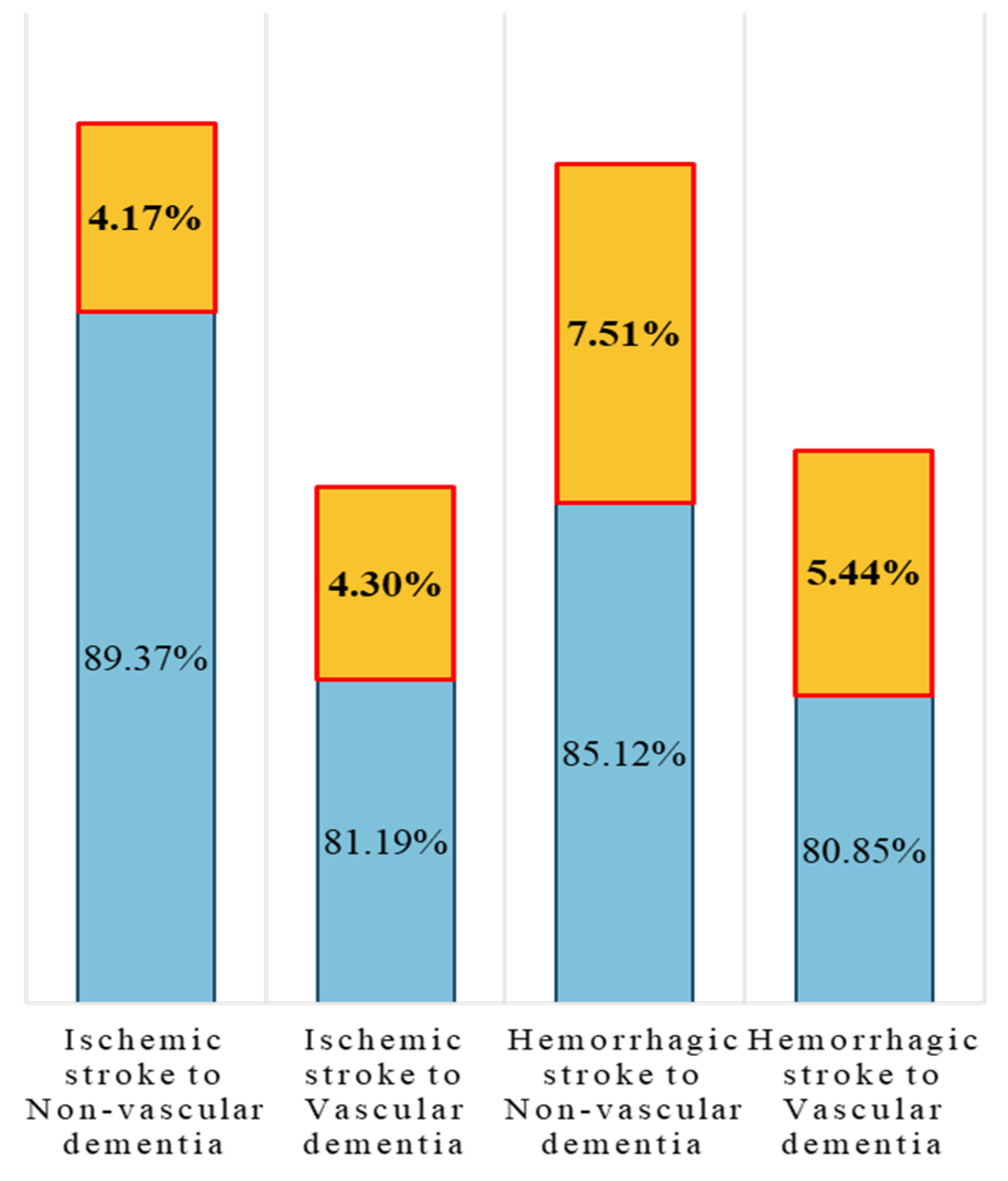
| β68 (6→8) | 1.68 | 2.15 | 91.79% | 2.25 | 2.36 | 80.85% | 2.39 | 2.38 | 86.29% | |
| β69 (6→9) | 3.01 | 3.05 | 87.23% | 3.10 | 3.07 | 85.12% | 3.16 | 3.07 | 92.63% | |
| Non-vascular dementia | Mean (yr) | SD | R2 | Mean (yr) | SD | R2 | Mean (yr) | SD | R2 | |
| β78 (7→8) | 1.44 | 2.13 | 78.96% | 1.42 | 2.12 | 81.19% | 1.44 | 2.13 | 85.49% | |
| β79 (7→9) | 3.07 | 2.99 | 90.07% | 3.11 | 3.00 | 89.37% | 3.17 | 3.03 | 93.54% | |
| Hemorrhagic Stroke | Linear Regression | Decision Tree | Random Forest | SVM | SMP |
| Environmental risk | 88.52% | 91.35% | 88.84% | 91.93% | 92.66% |
| Medication and Rehabilitation | 88.41% | 91.87% | 88.49% | 90.22% | 92.22% |
| All factors | 88.50% | 92.00% | 91.60% | 90.19% | 92.01% |
| Ischemic stroke | |||||
| Environmental risk | 85.63% | 88.57% | 85.09% | 89.40% | 92.42% |
| Medication and Rehabilitation | 85.36% | 90.06% | 85.81% | 90.00% | 91.38% |
| All factors | 85.60% | 90.35% | 91.57% | 90.56% | 91.88% |
| Vascular dementia | Linear Regression | Decision Tree | Random Forest | SVM | SMP Model |
| Environmental risk | 75.74% | 79.61% | 81.18% | 73.28% | 89.51% |
| Medication and Rehabilitation | 77.46% | 82.61% | 82.88% | 75.54% | 83.00% |
| All factors | 78.76% | 85.27% | 86.91% | 78.52% | 89.46% |
| Non-vascular dementia | |||||
| Environmental risk | 75.80% | 76.44% | 77.70% | 73.60% | 84.52% |
| Medication and Rehabilitation | 77.87% | 81.11% | 81.29% | 76.77% | 85.28% |
| All factors | 75.66% | 82.96% | 84.45% | 81.67% | 89.52% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.-J.; Lee, C.-M.; Hu, G.-C.; Wang, K.-M. Stroke to Dementia Associated with Environmental Risks—A Semi-Markov Model. Int. J. Environ. Res. Public Health 2020, 17, 1944. https://doi.org/10.3390/ijerph17061944
Wang K-J, Lee C-M, Hu G-C, Wang K-M. Stroke to Dementia Associated with Environmental Risks—A Semi-Markov Model. International Journal of Environmental Research and Public Health. 2020; 17(6):1944. https://doi.org/10.3390/ijerph17061944
Chicago/Turabian StyleWang, Kung-Jeng, Chia-Min Lee, Gwo-Chi Hu, and Kung-Min Wang. 2020. "Stroke to Dementia Associated with Environmental Risks—A Semi-Markov Model" International Journal of Environmental Research and Public Health 17, no. 6: 1944. https://doi.org/10.3390/ijerph17061944
APA StyleWang, K.-J., Lee, C.-M., Hu, G.-C., & Wang, K.-M. (2020). Stroke to Dementia Associated with Environmental Risks—A Semi-Markov Model. International Journal of Environmental Research and Public Health, 17(6), 1944. https://doi.org/10.3390/ijerph17061944




