Cancer Mortality and Deprivation in the Proximity of Polluting Industrial Facilities in an Industrial Region of Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Mortality Data
2.2. Socioeconomic Level
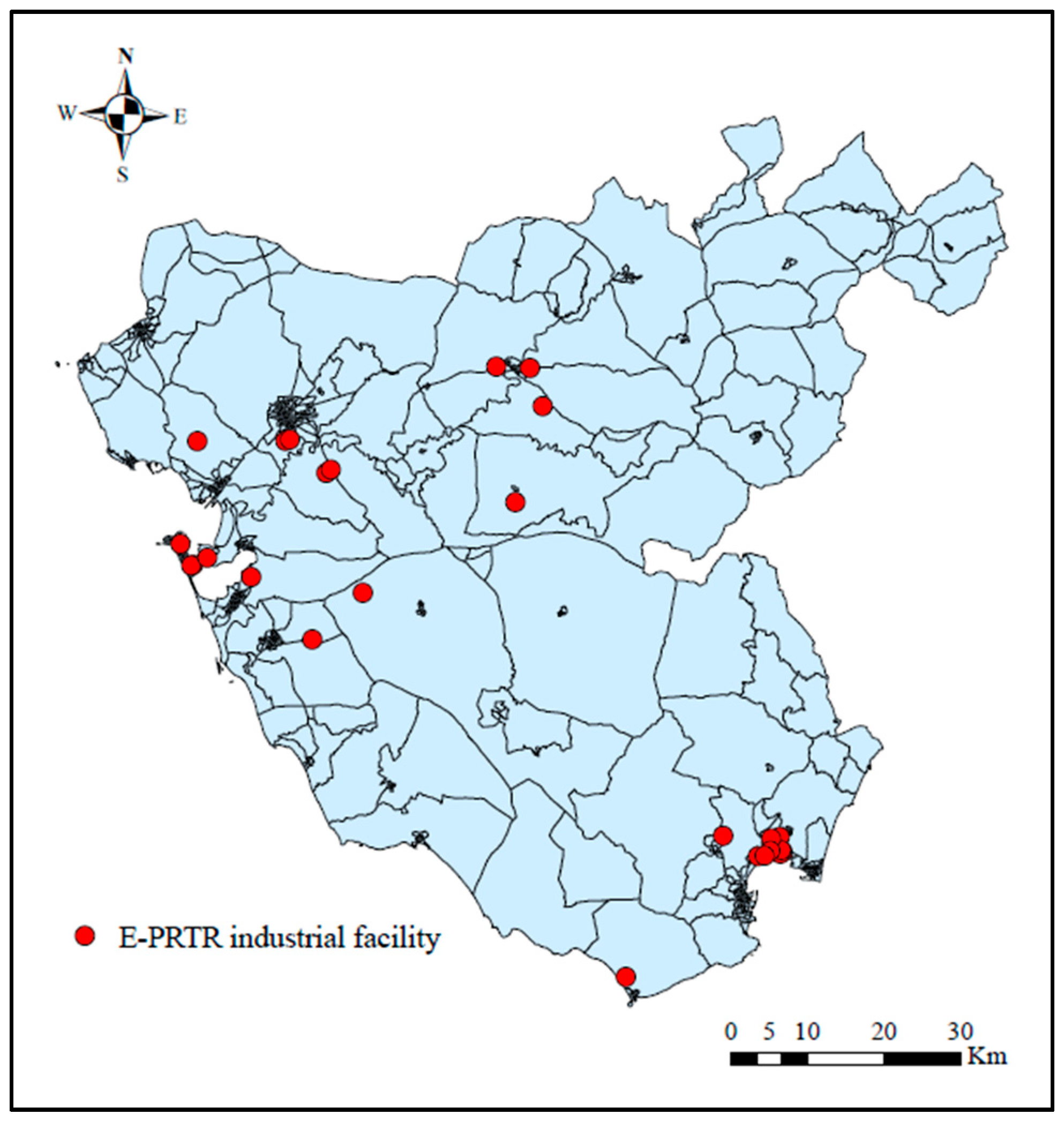
2.3. Industrial Pollution Exposure Data
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Lung Cancer
4.2. Laryngeal Cancer
4.3. Bladder Cancer
4.4. Kidney Cancer
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Disclaimer
References
- Instituto Nacional de Estadística. Defunciones según la Causa de Muerte. 2017. Available online: https://www.ine.es/dynt3/inebase/es/index.htm?padre=5449&capsel=5450 (accessed on 16 January 2019).
- López-Abente, G.; Ramis, R.; Pollán, M.; Aragonés, N.; Pérez-Gómez, B.; Gómez-Barroso, D.; Carrasco, J.M.; Lope, V.; García-Pérez, J.; Boldo, E.; et al. Atlas Municipal de Mortalidad Por Cáncer En España, 1989–1998; Instituto de Salud Carlos III: Madrid, Spain, 2006. [Google Scholar]
- Benítez-Rodríguez, E.; Sanz-García, M.J.; Mejías-Márquez, C.; Vilches-Campos, M.L.; Doménech-Torrejón, R.; Machado-Ruguero, M.A.; Cruzado-Morillas, M.J. Situación del cáncer en la provincia de Cádiz. periodo 2007–2009. In La Salud en Cádiz y sus Determinantes; Córdoba Doña, J.A., Martínez Nieto, J.M., Almenara Barrios, J., Eds.; Editorial UCA. Servicio de Publicaciones de la Universidad de Cádiz: Cádiz, Spain, 2015; pp. 245–262. [Google Scholar]
- Instituto de Salud Carlos III. Servidor Interactivo de Información Epidemiológica (ARIADNA). 2020. Available online: http://ariadna.cne.isciii.es (accessed on 5 March 2020).
- Moore, J.X.; Akinyemiju, T.; Wang, H.E. Pollution and regional variations of lung cancer mortality in the United States. Cancer Epidemiol. 2017, 49, 118–127. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, G.; Pollán, M.; Boldo, E.; Pérez-Gómez, B.; Aragonés, N.; Lope, V.; Ramis, R.; Vidal, E.; López-Abente, G. Mortality due to lung, laryngeal and bladder cancer in towns lying in the vicinity of combustion installations. Sci. Total Environ. 2009, 407, 2593–2602. [Google Scholar] [CrossRef] [PubMed]
- Cong, X. Air Pollution from Industrial Waste Gas Emissions Is Associated With Cancer Incidences in Shanghai, China. Environ. Sci. Pollut. Res. Int. 2018, 25, 13067–13078. [Google Scholar] [CrossRef] [PubMed]
- EPER. European Pollutant Emission Register. 2020. Available online: https://prtr.eea.europa.eu/#/home (accessed on 15 October 2019).
- Im, J.; Kim, H.; Kim, B.; Yun, J.; Lee, J.; Lee, C. A study on the characteristics of pollutant release and transfer registers (PRTRs) and cancer incidence rates in Korea. Environ. Sci. Pollut. Res. 2019, 26, 17080–17090. [Google Scholar] [CrossRef]
- Ramis, R.; Diggle, P.; Cambra, K.; López-Abente, G. Prostate cancer and industrial pollution: Risk around putative focus in a multi-source scenario. Environ. Int. 2011, 37, 577–585. [Google Scholar] [CrossRef]
- García-Pérez, J.; Boldo, E.; Ramis, R.; Pollán, M.; Pérez-Gómez, B.; Aragonés, M.; López-Abente, G. Description of industrial pollution in Spain. BMC Public Health 2007, 7, 40. [Google Scholar] [CrossRef]
- Puigpinós-Riera, R.; Marí-Dell’Olmo, M.; Gotsens, M.; Cano-Serral, G.; Ascaso, C.; Borrell, C.; Daponte, A.; Domínguez-Berjón, F.M.; Esnaola, S.; Gandarillas, A.; et al. Cancer mortality inequalities in urban areas: A Bayesian small area analysis in Spanish cities. Int. J. Health Geogr. 2011, 10, 6. [Google Scholar] [CrossRef]
- Cambra, K.; Martínez-Rueda, T.; Alonso-Fustel, E.; Cirarda, F.B.; Audicana, C.; Esnaola, S.; Ibáñez, B. Association of proximity to polluting industries, deprivation and mortality in small areas of the Basque Country (Spain). Eur. J. Public Health 2013, 23, 171–176. [Google Scholar] [CrossRef]
- Havard, S.; Deguen, S.; Zmirou-Navier, D.; Schillinger, C.; Bard, D. Traffic-related air pollution and socioeconomic status: A spatial autocorrelation study to assess environmental equity on a small-area scale. Epidemiology 2009, 20, 223–230. [Google Scholar] [CrossRef]
- Observatorio de Desigualdad de Andalucía. I Informe. 2017. Available online: https://observatoriodesigualdadandalucia.org/sites/default/files/i_informe_oda_0.pdf (accessed on 17 July 2019).
- Fernández-Ajuria, A.; Ocaña-Riola, R.; Sánchez-Cantalejo, C.; Sánchez-Villegas, P.; Daponte-Codina, A. Estudio Sobre la Mortalidad por Municipios en la Provincia de Cádiz y Estudio Sobre la Situación de Salud en la Provincia de Cádiz. Escuela Andaluza de Salud Pública. 2004. Available online: https://www.juntadeandalucia.es/export/drupaljda/salud_5af9587a082ec_mortalidad_salud_cadiz.pdf (accessed on 7 April 2019).
- Grupo de Trabajo de la Sociedad Española de Epidemiología. Dictamen realizado por Encargo del Defensor del Pueblo Andaluz Sobre El Exceso de Mortalidad y Morbilidad Detectado en Varias Investigaciones en El Campo de Gibraltar. 2013. Available online: https://www.seepidemiologia.es/documents/dummy/DICTAMEN_CampoGibraltar-Definitiva.pdf (accessed on 27 May 2019).
- Junta de Andalucía. Geocoder Software. 2019. Available online: https://www.juntadeandalucia.es/repositorio/usuario/listado/fichacompleta.jsf;jsessionid=32672964E117FF07126FCC02C7318F87?linkDummyForm:_idcl=_id153&idProyecto=679& (accessed on 23 July 2019).
- Instituto Nacional de Estadística. Callejero del Censo Electoral. Available online: https://www.ine.es/ss/Satellite?L=es_ES&c=Page&cid=1259952026632&p=1259952026632&pagename=ProductosYServicios%2FPYSLayout (accessed on 18 February 2019).
- Ruiz-Ramos, M.; Escolar-Pujolar, A.; Sánchez Perea, J.; Garrucho Rivero, G. Evolución de las desigualdades sociales en la mortalidad general de la ciudad de Sevilla (1994–2002). Gac. Sanit. 2006, 20, 303–310. [Google Scholar] [CrossRef]
- Córdoba-Doña, J.A.; Novalbos-Ruiz, J.P.; Suárez-Farfante, J.; Andérica-Frías, G.; Escolar-Pujolar, A. Social inequalities in HIV-TB and non-HIV-TB patients in two urban areas in southern Spain: Multilevel analysis. Int. J. Tuberc. Lung Dis. 2012, 16, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, G.J.; Escolar-Pujolar, A.; Córdoba-Doña, J.A. Evolución de las desigualdades sociales en la mortalidad general de la ciudad de Cádiz (1992–2007). Gac. Sanit. 2014, 28, 313–315. [Google Scholar] [CrossRef]
- Dorling, D.; Mitchell, R.; Shaw, M.; Orford, S.; Smith, G.D. The ghost of Christmas past: Health effects of poverty in London in 1896 and 1991. BMJ 2000, 321, 1547–1551. [Google Scholar] [CrossRef]
- Garcia-Perez, J.; Boldo, E.; Ramis, R.; Vidal, E.; Aragonés, N.; Perez-Gomez, B.; Pollán, M.; López-Abente, G. Validation of the geographic position of EPER-Spain industries. Int. J. Health Geogr. 2008, 7, 1. [Google Scholar] [CrossRef]
- UNSCEAR. UNSCEAR 2006 Report: Volume I: Annex A: Epidemiological Studies of Radiation and Cancer. 2006. Available online: http://www.unscear.org/unscear/en/publications.html (accessed on 10 December 2018).
- Besag, J.; York, J.; Mollié, A. Bayesian image restoration with two applications in spatial statistics. Ann. Inst. Stat. Math. 1991, 43, 1–20. [Google Scholar] [CrossRef]
- Rue, H.; Martino, S.; Chopin, N. Approximate Bayesian inference for latent Gaussian models using integrated nested Laplace approximations (with discussion). J. R. Stat. Soc. 2009, 71, 319–392. [Google Scholar] [CrossRef]
- The R-INLA Project. Available online: http://www.r-inla.org/ (accessed on 22 November 2018).
- Schiaffino, A.; Fernandez, E.; Borrell, C.; Salto, E.; Garcia, M.; Borras, J.M. Gender and educational differences in smoking initiation rates in Spain from 1948 to 1992. Eur. J. Public Health 2003, 13, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, J.; Malvezzi, M.; Negri, E.; La Vecchia, C.; Boffetta, P. Risk Factors for Lung Cancer Worldwide. Eur. Respir. J. 2016, 48, 889–902. [Google Scholar] [CrossRef]
- Drewnowski, A. Obesity, Diets, and Social Inequalities. Nutr. Rev. 2009, 67 (Suppl. S1), S36–S39. [Google Scholar] [CrossRef]
- Yau, A.; Adams, J.; Monsivais, P. Time trends in adherence to UK dietary recommendations and associated sociodemographic inequalities, 1986–2012: A repeated cross-sectional analysis. Eur. J. Clin. Nutr. 2019, 73, 997–1005. [Google Scholar] [CrossRef]
- Benedetti, M.; Iavarone, I.; Comba, P. Cancer risk associated with residential proximity to industrial sites: A review. Arch. Environ. Health 2001, 56, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, K.; Grittner, U.; Kramer, S.; Gmel, G. Social inequalities in alcohol consumption and alcohol-related problems in the study countries of the EU concerted action ‘Gender, Culture and Alcohol Problems: A Multi-national Study’. Alcohol Alcohol. Suppl. 2006, 41, i26–i36. [Google Scholar] [CrossRef] [PubMed]
- Khalil, D.; Corsten, M.J.; Holland, M.; Balram, A.; McDonald, J.T.; Johnson-Obaseki, S. Does Socioeconomic Status Affect Stage at Presentation for Larynx Cancer in Canada’s Universal Health Care System? Otolaryngol. Head Neck Surg. 2019, 160, 488–493. [Google Scholar] [CrossRef]
- Michelozzi, P.; Fusco, D.; Forastiere, F.; Ancona, C.; Dell’Orco, V.; Perucci, C.A. Small area study of mortality among people living near multiple sources of air pollution. Occup. Environ. Med. 1998, 55, 611–615. [Google Scholar] [CrossRef]
- López-Abente, G.; Aragonés, N.; Ramis, R.; Hernández-Barrera, V.; Pérez-Gómez, B.; Escolar-Pujolar, A.; Pollan, M. Municipal distribution of bladder cancer mortality in Spain: Possible role of mining and industry. BMC Public Health 2006, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.H.; Goycolea, M.; Haque, R.; Biggs, M.L. Marked increase in bladder and lung cancer mortality in a region of Northern Chile due to arsenic in drinking water. Am. J. Epidemiol. 1998, 147, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Minatel, B.C.; Sage, A.P.; Anderson, C.; Hubaux, R.; Marshall, E.A.; Lam, W.L.; Martinez, V.D. Environmental arsenic exposure: From genetic susceptibility to pathogenesis. Environ. Int. 2018, 112, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.V.; Pal, S.; Mohammed, A.; Farooqui, M.; Doescher, M.P.; Asch, A.S.; Yamada, H.Y. Biological effects and epidemiological consequences of arsenic exposure, and reagents that can ameliorate arsenic damage in vivo. Oncotarget 2017, 8, 57605–57621. [Google Scholar] [CrossRef]
- Kogevinas, M.; Mannetje, A.; Cordier, S.; Ranft, U.; Gonzalez, C.A.; Vineis, P.; Chang-Claude, J.; Lynge, E.; Wahrendorf, J.; Tzonou, A.; et al. Occupation and bladder cancer among men in Western Europe. Cancer Causes Control 2003, 14, 907–914. [Google Scholar] [CrossRef]
- Letašiová, S.; Medveďová, A.; Šovčíková, A.; Dušinská, M.; Volkovová, K.; Mosoiu, C.; Bartonová, A. Bladder cancer, a review of the environmental risk factors. Environ. Health 2012, 11 (Suppl. S1), S11. [Google Scholar]
- Menvielle, G.; Rey, G.; Jougla, E.; Luce, D. Diverging trends in educational inequalities in cancer mortality between men and women in the 2000s in France. BMC Public Health 2013, 13, 823. [Google Scholar] [CrossRef] [PubMed]
- Hoebel, J.; Kroll, L.E.; Fiebig, J.; Lampert, T.; Katalinic, A.; Barnes, B.; Kraywinkel, K. Socioeconomic Inequalities in Total and Site-Specific Cancer Incidence in Germany: A Population-Based Registry Study. Front. Oncol. 2018, 8, 402. [Google Scholar] [CrossRef] [PubMed]
- Song, J.K.; Luo, H.; Yin, X.H.; Huang, G.L.; Luo, S.Y.; Lin du, R.; Yuan, D.B.; Zhang, W.; Zhu, J.G. Association between cadmium exposure and renal cancer risk: A meta-analysis of observational studies. Sci. Rep. 2015, 5, 17976. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.B.; Shim, J.Y.; Park, B.; Lee, Y.J. Long-Term Exposure to Air Pollutants and Cancer Mortality: A Meta-Analysis of Cohort Studies. Int. J. Environ. Res. Public Health 2018, 15, 2608. [Google Scholar] [CrossRef] [PubMed]
- Clayton, D.G.; Bernardinelli, L.; Montomoli, C. Spatial correlation in ecological analysis. Int. J. Epidemiol. 1993, 22, 1193–1202. [Google Scholar] [CrossRef]
- Diggle, P.J.; Elliott, P. Disease risk near point sources: Statistical issues for analyses using individual or spatially aggregated data. J. Epidemiol. Community Health 1995, 49, 20–27. [Google Scholar] [CrossRef]
| Cause | DI | 1 km | 2 km | 3 km | 4 km | 5 km | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposed CT = 46 | Exposed CT = 151 | Exposed CT = 224 | Exposed CT = 297 | Exposed CT = 413 | ||||||||||||
| n | RR | 95% CI | n | RR | 95% CI | n | RR | 95% CI | n | RR | 95% CI | n | RR | 95% CI | ||
| Lung cancer | 1 | 174 | 1.00 | - | 390 | 1.00 | - | 542 | 1.00 | - | 694 | 1.00 | - | 1026 | 1.00 | - |
| 2 | 74 | 1.20 | 1.09–1.32 | 372 | 1.19 | 1.08–1.32 | 662 | 1.19 | 1.08–1.31 | 876 | 1.19 | 1.07–1.31 | 1233 | 1.19 | 1.07–1.31 | |
| 3 | 65 | 1.26 | 1.14–1.39 | 418 | 1.25 | 1.13–1.38 | 610 | 1.24 | 1.13–1.37 | 803 | 1.25 | 1.13–1.37 | 1085 | 1.25 | 1.13–1.38 | |
| 4 | 125 | 1.34 | 1.21–1.47 | 448 | 1.33 | 1.20–1.46 | 536 | 1.33 | 1.21–1.47 | 704 | 1.33 | 1.20–1.47 | 934 | 1.33 | 1.21–1.47 | |
| 5 | 143 | 1.37 | 1.24–1.51 | 306 | 1.36 | 1.23–1.50 | 412 | 1.36 | 1.23–1.50 | 512 | 1.36 | 1.23–1.50 | 607 | 1.37 | 1.24–1.51 | |
| Laryngeal cancer | 1 | 18 | 1.00 | - | 38 | 1.00 | - | 52 | 1.00 | - | 68 | 1.00 | - | 94 | 1.00 | - |
| 2 | 5 | 1.34 | 1.06–1.70 | 37 | 1.33 | 1.05–1.68 | 74 | 1.33 | 1.05–1.68 | 95 | 1.33 | 1.05–1.68 | 129 | 1.33 | 1.05–1.68 | |
| 3 | 10 | 1.70 | 1.36–2.14 | 50 | 1.69 | 1.35–2.12 | 73 | 1.69 | 1.35–2.12 | 97 | 1.69 | 1.35–2.12 | 142 | 1.69 | 1.35–2.12 | |
| 4 | 20 | 1.85 | 1.47–2.31 | 53 | 1.84 | 1.47–2.31 | 73 | 1.84 | 1.47–2.30 | 101 | 1.83 | 1.46–2.29 | 121 | 1.84 | 1.47–2.30 | |
| 5 | 11 | 2.09 | 1.67–2.62 | 33 | 2.09 | 1.66–2.61 | 46 | 2.08 | 1.66–2.61 | 61 | 2.07 | 1.65–2.59 | 76 | 2.08 | 1.66–2.61 | |
| Bladder cancer | 1 | 45 | 1.00 | - | 100 | 1.00 | - | 145 | 1.00 | - | 188 | 1.00 | - | 265 | 1.00 | - |
| 2 | 16 | 1.12 | 0.96–1.30 | 98 | 1.11 | 0.95–1.29 | 170 | 1.10 | 0.95–1.28 | 215 | 1.10 | 0.95–1.28 | 282 | 1.10 | 0.95–1.28 | |
| 3 | 19 | 1.20 | 1.03–1.39 | 91 | 1.18 | 1.02–1.37 | 138 | 1.18 | 1.02–1.37 | 174 | 1.18 | 1.02–1.37 | 250 | 1.18 | 1.02–1.37 | |
| 4 | 32 | 1.20 | 1.03–1.39 | 99 | 1.19 | 1.02–1.38 | 127 | 1.19 | 1.02–1.38 | 168 | 1.18 | 1.02–1.38 | 221 | 1.19 | 1.02–1.38 | |
| 5 | 29 | 1.04 | 0.89–1.21 | 70 | 1.03 | 0.88–1.20 | 89 | 1.02 | 0.88–1.20 | 103 | 1.03 | 0.88–1.20 | 123 | 1.03 | 0.88–1.21 | |
| Kidney cancer | 1 | 15 | 1.00 | - | 31 | 1.00 | - | 41 | 1.00 | - | 54 | 1.00 | - | 76 | 1.00 | - |
| 2 | 5 | 0.99 | 0.76–1.28 | 17 | 0.97 | 0.74–1.25 | 38 | 0.96 | 0.74–1.24 | 56 | 0.95 | 0.74–1.24 | 76 | 0.96 | 0.74–1.24 | |
| 3 | 9 | 1.10 | 0.86–1.43 | 38 | 1.08 | 0.84–1.39 | 47 | 1.08 | 0.83–1.39 | 56 | 1.08 | 0.83–1.39 | 69 | 1.08 | 0.84–1.39 | |
| 4 | 7 | 0.96 | 0.73–1.25 | 23 | 0.94 | 0.72–1.22 | 30 | 0.94 | 0.72–1.23 | 37 | 0.94 | 0.72–1.22 | 53 | 0.94 | 0.72–1.23 | |
| 5 | 7 | 0.80 | 0.61–1.07 | 13 | 0.79 | 0.60–1.05 | 16 | 0.79 | 0.60–1.05 | 23 | 0.79 | 0.59–1.05 | 29 | 0.80 | 0.60–1.06 | |
| Cause | DI | 1 km | 2 km | 3 km | 4 km | 5 km | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposed CT = 46 | Exposed CT = 151 | Exposed CT = 224 | Exposed CT = 297 | Exposed CT = 413 | ||||||||||||
| n | RR | 95% CI | n | RR | 95% CI | n | RR | 95% CI | n | RR | 95% CI | n | RR | 95% CI | ||
| Lung cancer | 1 | 38 | 1.00 | - | 72 | 1.00 | - | 89 | 1.00 | - | 110 | 1.00 | - | 151 | 1.00 | - |
| 2 | 3 | 0.91 | 0.75–1.11 | 30 | 0.89 | 0.74–1.08 | 63 | 0.89 | 0.73–1.08 | 93 | 0.89 | 0.73–1.07 | 124 | 0.89 | 0.73–1.07 | |
| 3 | 11 | 0.81 | 0.67–0.99 | 37 | 0.80 | 0.65–0.97 | 55 | 0.79 | 0.65–0.97 | 71 | 0.80 | 0.65–0.97 | 96 | 0.80 | 0.65–0.97 | |
| 4 | 10 | 0.77 | 0.63–0.94 | 36 | 0.75 | 0.62–0.92 | 44 | 0.76 | 0.62–0.92 | 61 | 0.75 | 0.62–0.92 | 76 | 0.76 | 0.62–0.92 | |
| 5 | 9 | 0.63 | 0.51–0.79 | 22 | 0.63 | 0.50–0.78 | 27 | 0.63 | 0.50–0.78 | 32 | 0.62 | 0.50–0.78 | 39 | 0.63 | 0.51–0.78 | |
| Laryngeal cancer | 1 | 2 | 1.00 | - | 2 | 1.00 | - | 3 | 1.00 | - | 3 | 1.00 | - | 7 | 1.00 | - |
| 2 | 0 | 0.79 | 0.27–2.28 | 1 | 0.78 | 0.27–2.25 | 2 | 0.80 | 0.28–2.31 | 3 | 0.81 | 0.28–2.34 | 4 | 0.80 | 0.28–2.31 | |
| 3 | 0 | 0.88 | 0.30–2.57 | 2 | 0.90 | 0.31–2.60 | 4 | 0.90 | 0.31–2.60 | 4 | 0.90 | 0.31–2.60 | 5 | 0.90 | 0.31–2.59 | |
| 4 | 0 | 0.81 | 0.26–2.49 | 1 | 0.83 | 0.27–2.55 | 1 | 0.81 | 0.26–2.51 | 2 | 0.83 | 0.27–2.55 | 3 | 0.81 | 0.26–2.51 | |
| 5 | 0 | 1.19 | 0.40–3.56 | 0 | 1.23 | 0.41–3.69 | 1 | 1.21 | 0.40–3.62 | 1 | 1.25 | 0.41–3.76 | 2 | 1.20 | 0.40–3.57 | |
| Bladder cancer | 1 | 14 | 1.00 | - | 22 | 1.00 | - | 33 | 1.00 | - | 42 | 1.00 | - | 52 | 1.00 | - |
| 2 | 3 | 0.84 | 0.58–1.19 | 19 | 0.80 | 0.56–1.14 | 30 | 0.79 | 0.56–1.13 | 34 | 0.79 | 0.55–1.13 | 40 | 0.80 | 0.56–1.13 | |
| 3 | 5 | 1.14 | 0.82–1.59 | 19 | 1.10 | 0.79–1.53 | 25 | 1.09 | 0.78–1.51 | 34 | 1.10 | 0.79–1.53 | 46 | 1.10 | 0.79–1.53 | |
| 4 | 3 | 1.03 | 0.73–1.45 | 13 | 1.01 | 0.72–1.41 | 16 | 1.01 | 0.72–1.41 | 24 | 1.01 | 0.72–1.41 | 35 | 1.01 | 0.72–1.42 | |
| 5 | 6 | 0.87 | 0.60–1.26 | 8 | 0.85 | 0.59–1.23 | 10 | 0.84 | 0.58–1.22 | 13 | 0.85 | 0.58–1.23 | 17 | 0.86 | 0.59–1.25 | |
| Kidney cancer | 1 | 8 | 1.00 | - | 17 | 1.00 | - | 23 | 1.00 | - | 30 | 1.00 | - | 47 | 1.00 | - |
| 2 | 5 | 1.18 | 0.85–1.65 | 16 | 1.15 | 0.83–1.60 | 25 | 1.15 | 0.83–1.60 | 34 | 1.15 | 0.83–1.60 | 48 | 1.15 | 0.83–1.60 | |
| 3 | 1 | 0.83 | 0.58–1.20 | 12 | 0.81 | 0.57–1.17 | 13 | 0.82 | 0.57–1.18 | 23 | 0.81 | 0.57–1.17 | 37 | 0.81 | 0.57–1.17 | |
| 4 | 7 | 0.86 | 0.60–1.23 | 18 | 0.84 | 0.59–1.21 | 22 | 0.85 | 0.59–1.22 | 28 | 0.85 | 0.59–1.22 | 31 | 0.85 | 0.59–1.22 | |
| 5 | 3 | 0.68 | 0.45–1.02 | 6 | 0.67 | 0.45–1.00 | 6 | 0.68 | 0.45–1.02 | 8 | 0.68 | 0.45–1.01 | 11 | 0.67 | 0.45–1.01 | |
| Cause | 1 km | 2 km | 3 km | 4 km | 5 km | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposed CT = 46 | Exposed CT = 151 | Exposed CT = 224 | Exposed CT = 297 | Exposed CT = 413 | |||||||||||
| n | RR | 95% CI | n | RR | 95% CI | n | RR | 95% CI | n | RR | 95% CI | n | RR | 95% CI | |
| Lung cancer | |||||||||||||||
| Men | 581 | 1.24 | 1.08–1.41 | 1934 | 1.14 | 1.05–1.24 | 2762 | 1.12 | 1.04–1.20 | 3589 | 1.11 | 1.04–1.19 | 4885 | 1.09 | 1.02–1.16 |
| Women | 71 | 1.18 | 0.91–1.54 | 197 | 0.95 | 0.80–1.13 | 278 | 0.92 | 0.79–1.08 | 367 | 0.92 | 0.80–1.07 | 486 | 0.88 | 0.77–1.01 |
| Laryngeal cancer | |||||||||||||||
| Men | 64 | 1.19 | 0.88–1.59 | 211 | 1.07 | 0.89–1.28 | 318 | 1.11 | 0.95–1.31 | 422 | 1.13 | 0.98–1.31 | 562 | 1.09 | 0.95–1.25 |
| Women | 2 | 1.67 | 0.36–7.78 | 6 | 1.48 | 0.53–4.14 | 11 | 1.89 | 0.78–4.55 | 13 | 1.72 | 0.73–4.02 | 21 | 1.99 | 0.91–4.37 |
| Bladder cancer | |||||||||||||||
| Men | 141 | 1.32 | 1.08–1.60 | 458 | 1.18 | 1.05–1.34 | 669 | 1.18 | 1.06–1.31 | 848 | 1.14 | 1.03–1.26 | 1141 | 1.11 | 1.01–1.22 |
| Women | 31 | 1.91 | 1.28–2.86 | 81 | 1.37 | 1.03–1.82 | 114 | 1.36 | 1.05–1.76 | 147 | 1.32 | 1.04–1.69 | 190 | 1.24 | 0.98–1.57 |
| Kidney cancer | |||||||||||||||
| Men | 43 | 1.55 | 1.12–2.17 | 122 | 1.23 | 0.99–1.54 | 172 | 1.18 | 0.97–1.44 | 226 | 1.18 | 0.98–1.42 | 303 | 1.14 | 0.95–1.35 |
| Women | 24 | 1.59 | 1.02–2.50 | 69 | 1.27 | 0.94–1.72 | 89 | 1.13 | 0.85–1.50 | 123 | 1.18 | 0.91–1.53 | 174 | 1.20 | 0.94–1.53 |
| IARC Group 1 * | |||||||||||||||
| Cause | 1 km | 2 km | 3 km | 4 km | 5 km | ||||||||||
| Exposed CT = 46 | Exposed CT = 145 | Exposed CT = 211 | Exposed CT = 284 | Exposed CT = 393 | |||||||||||
| n | RR | 95% CI | n | RR | 95% CI | n | RR | 95% CI | n | RR | 95% CI | n | RR | 95% CI | |
| Lung cancer | |||||||||||||||
| Men | 581 | 1.23 | 1.07–1.40 | 1865 | 1.14 | 1.05–1.23 | 2164 | 1.11 | 1.03–1.19 | 3420 | 1.09 | 1.02–1.17 | 4663 | 1.08 | 1.01–1.15 |
| Women | 71 | 1.20 | 0.92–1.56 | 192 | 0.97 | 0.81–1.15 | 269 | 0.96 | 0.82–1.12 | 357 | 0.95 | 0.82–1.09 | 471 | 0.90 | 0.79–1.03 |
| Laryngeal cancer | |||||||||||||||
| Men | 64 | 1.20 | 0.89–1.61 | 204 | 1.09 | 0.91–1.31 | 304 | 1.14 | 0.97–1.34 | 413 | 1.17 | 1.01–1.35 | 542 | 1.12 | 0.98–1.28 |
| Women | 2 | 1.43 | 0.31–6.52 | 6 | 1.31 | 0.48–3.54 | 10 | 1.55 | 0.66–3.65 | 12 | 1.40 | 0.62–3.19 | 19 | 1.60 | 0.75–3.39 |
| Bladder cancer | |||||||||||||||
| Men | 141 | 1.31 | 1.08–1.60 | 443 | 1.18 | 1.04–1.34 | 635 | 1.18 | 1.06–1.31 | 809 | 1.13 | 1.02–1.25 | 1088 | 1.10 | 1.00–1.21 |
| Women | 31 | 1.89 | 1.26–2.82 | 78 | 1.35 | 1.02–1.80 | 111 | 1.37 | 1.06–1.77 | 142 | 1.30 | 1.02–1.66 | 183 | 1.22 | 0.97–1.54 |
| Kidney cancer | |||||||||||||||
| Men | 43 | 1.59 | 1.14–2.21 | 120 | 1.28 | 1.03–1.60 | 170 | 1.27 | 1.04–1.54 | 221 | 1.24 | 1.03–1.49 | 295 | 1.19 | 1.00–1.41 |
| Women | 24 | 1.53 | 0.98–2.40 | 67 | 1.22 | 0.90–1.65 | 85 | 1.09 | 0.82–1.45 | 117 | 1.11 | 0.86–1.44 | 164 | 1.13 | 0.89–1.44 |
| IARC Group 2A ** | |||||||||||||||
| Cause | 1 km | 2 km | 3 km | 4 km | 5 km | ||||||||||
| Exposed CT = 26 | Exposed CT = 87 | Exposed CT = 158 | Exposed CT = 240 | Exposed CT = 370 | |||||||||||
| n | RR | 95% CI | n | RR | 95% CI | n | RR | 95% CI | n | RR | 95% CI | n | RR | 95% CI | |
| Lung cancer | |||||||||||||||
| Men | 318 | 1.11 | 0.93–1.32 | 1076 | 1.06 | 0.96–1.17 | 1872 | 1.04 | 0.96–1.13 | 2793 | 1.07 | 0.99–1.15 | 4355 | 1.08 | 1.01–1.15 |
| Women | 41 | 1.19 | 0.85–1.65 | 109 | 0.93 | 0.75–1.16 | 203 | 0.95 | 0.80–1.13 | 301 | 0.95 | 0.82–1.11 | 447 | 0.90 | 0.79–1.03 |
| Laryngeal cancer | |||||||||||||||
| Men | 42 | 1.27 | 0.89–1.82 | 122 | 1.05 | 0.84–1.30 | 213 | 1.07 | 0.89–1.28 | 342 | 1.16 | 1.00–1.35 | 504 | 1.10 | 0.96–1.26 |
| Women | 2 | 2.60 | 0.57–11.85 | 3 | 1.17 | 0.32–4.18 | 7 | 1.52 | 0.58–3.95 | 10 | 1.48 | 0.62–3.51 | 19 | 1.80 | 0.85–3.83 |
| Bladder cancer | |||||||||||||||
| Men | 92 | 1.46 | 1.15–1.86 | 257 | 1.14 | 0.98–1.32 | 461 | 1.14 | 1.01–1.29 | 659 | 1.10 | 0.99–1.23 | 1022 | 1.09 | 0.99–1.20 |
| Women | 15 | 1.72 | 1.00–2.95 | 35 | 1.14 | 0.78–1.66 | 80 | 1.40 | 1.05–1.85 | 116 | 1.31 | 1.01–1.68 | 174 | 1.22 | 0.97–1.54 |
| Kidney cancer | |||||||||||||||
| Men | 25 | 1.51 | 1.00–2.30 | 79 | 1.37 | 1.06–1.77 | 137 | 1.34 | 1.09–1.66 | 194 | 1.29 | 1.07–1.56 | 279 | 1.19 | 1.00–1.41 |
| Women | 13 | 1.49 | 0.83–2.67 | 36 | 1.18 | 0.81–1.71 | 61 | 1.08 | 0.79–1.47 | 98 | 1.15 | 0.87–1.50 | 160 | 1.19 | 0.94–1.51 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos-Sánchez, V.; Córdoba-Doña, J.A.; García-Pérez, J.; Escolar-Pujolar, A.; Pozzi, L.; Ramis, R. Cancer Mortality and Deprivation in the Proximity of Polluting Industrial Facilities in an Industrial Region of Spain. Int. J. Environ. Res. Public Health 2020, 17, 1860. https://doi.org/10.3390/ijerph17061860
Santos-Sánchez V, Córdoba-Doña JA, García-Pérez J, Escolar-Pujolar A, Pozzi L, Ramis R. Cancer Mortality and Deprivation in the Proximity of Polluting Industrial Facilities in an Industrial Region of Spain. International Journal of Environmental Research and Public Health. 2020; 17(6):1860. https://doi.org/10.3390/ijerph17061860
Chicago/Turabian StyleSantos-Sánchez, Vanessa, Juan Antonio Córdoba-Doña, Javier García-Pérez, Antonio Escolar-Pujolar, Lucia Pozzi, and Rebeca Ramis. 2020. "Cancer Mortality and Deprivation in the Proximity of Polluting Industrial Facilities in an Industrial Region of Spain" International Journal of Environmental Research and Public Health 17, no. 6: 1860. https://doi.org/10.3390/ijerph17061860
APA StyleSantos-Sánchez, V., Córdoba-Doña, J. A., García-Pérez, J., Escolar-Pujolar, A., Pozzi, L., & Ramis, R. (2020). Cancer Mortality and Deprivation in the Proximity of Polluting Industrial Facilities in an Industrial Region of Spain. International Journal of Environmental Research and Public Health, 17(6), 1860. https://doi.org/10.3390/ijerph17061860







