Utility of Human Papillomavirus Testing for Cervical Cancer Screening in Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Cytology
2.3. Histological Diagnosis
2.4. HPV Detection by MyHPV CHIP™
2.5. HPV Detection by BD Onclarity™ HPV Assay
2.6. Statistical Analysis
3. Results
3.1. The Prevalence of HPV Infection
3.2. HPV Infection and Cytological Diagnosis
3.3. HPV Infection and Histological Diagnosis
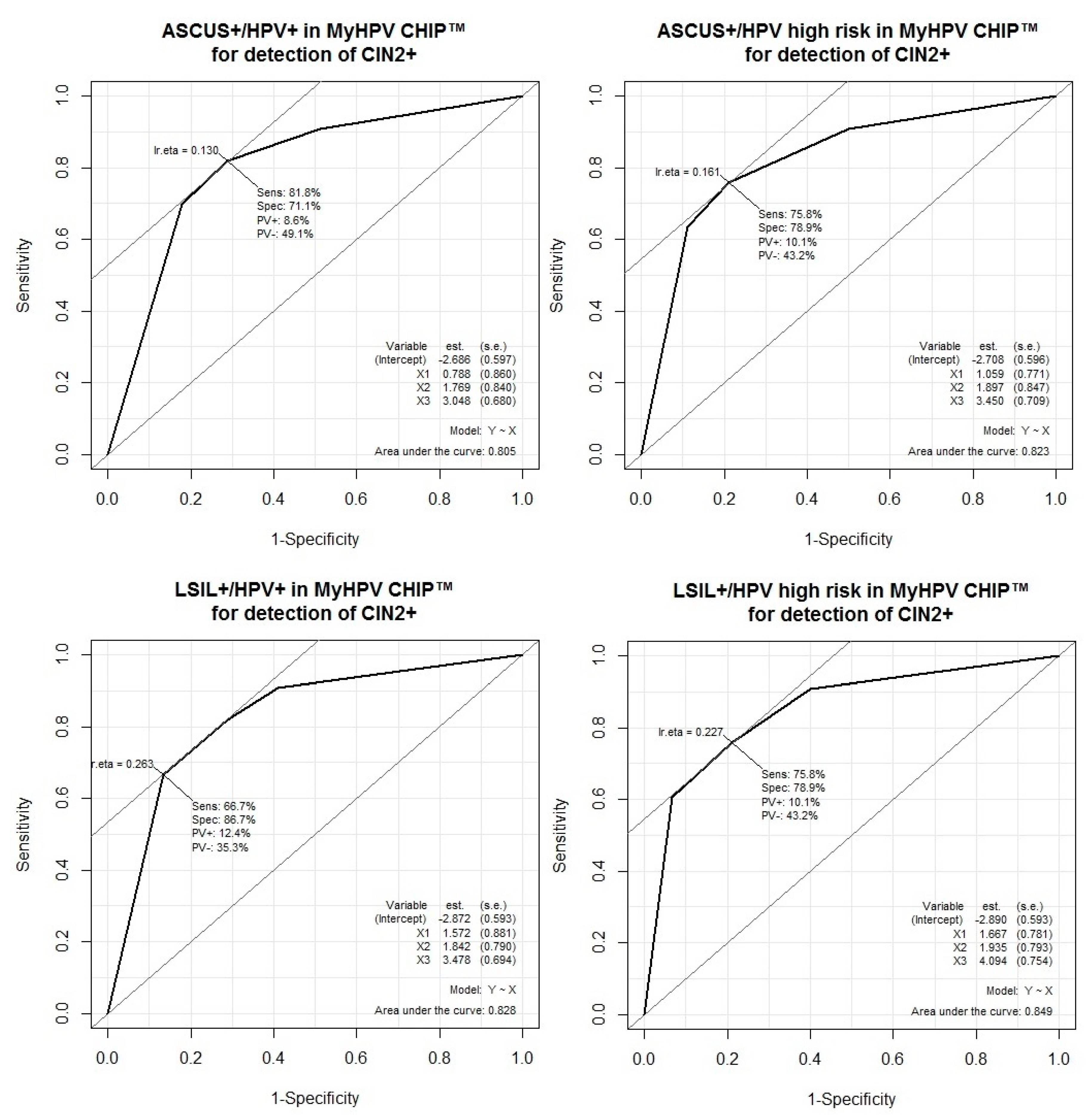
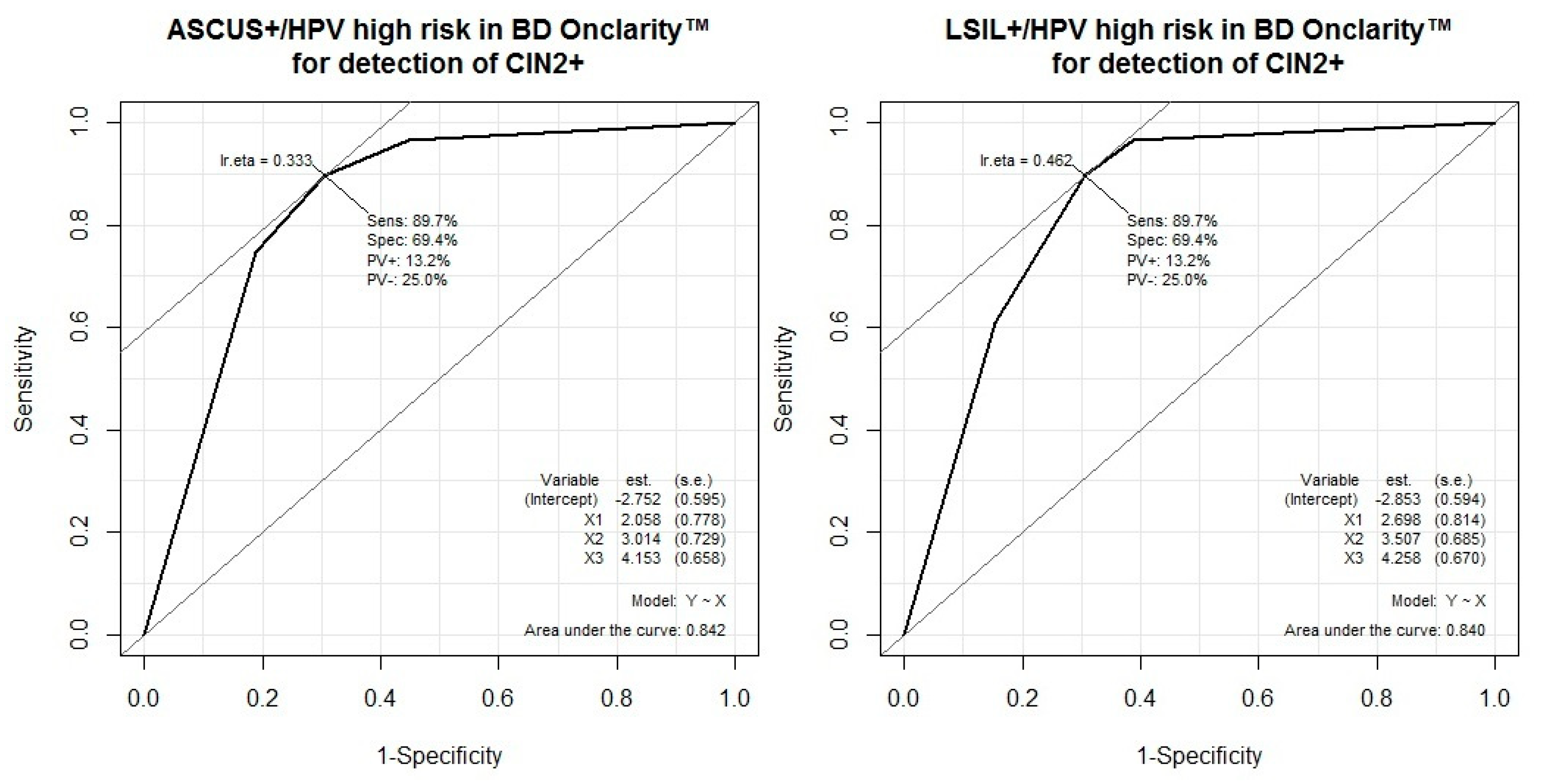
3.4. Performance of HPV Test Combinations and Cytology for the Detection of CIN2 or Worse
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jung, K.W.; Won, Y.J.; Kong, H.J.; Lee, E.S. Prediction of Cancer Incidence and Mortality in Korea, 2019. Cancer Res. Treat. 2019, 51, 431–437. [Google Scholar] [CrossRef]
- Jung, K.W.; Won, Y.J.; Oh, C.M.; Kong, H.J.; Lee, D.H.; Lee, K.H. Community of Population-Based Regional Cancer Registries. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2014. Cancer Res. Treat. 2017, 49, 292–305. [Google Scholar] [CrossRef]
- Zur Hausen, H. Papillomaviruses and Cancer: From Basic Studies to Clinical Application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Reid, R.; Stanhope, C.R.; Herschman, B.R.; Booth, E.; Phibbs, G.D.; Smith, J.P. Genital Warts and Cervical Cancer. I. Evidence of an Association between Subclinical Papillomavirus Infection and Cervical Malignancy. Cancer 1982, 50, 377–387. [Google Scholar] [CrossRef]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Munoz, N. Human Papillomavirus is a Necessary Cause of Invasive Cervical Cancer Worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Mayrand, M.H.; Duarte-Franco, E.; Rodrigues, I.; Walter, S.D.; Hanley, J.; Ferenczy, A.; Ratnam, S.; Coutlee, F.; Franco, E.L. Canadian Cervical Cancer Screening Trial Study Group. Human Papillomavirus DNA Versus Papanicolaou Screening Tests for Cervical Cancer. N. Engl. J. Med. 2007, 357, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Ronco, G.; Dillner, J.; Elfstrom, K.M.; Tunesi, S.; Snijders, P.J.; Arbyn, M.; Kitchener, H.; Segnan, N.; Gilham, C.; Giorgi-Rossi, P.; et al. Efficacy of HPV-Based Screening for Prevention of Invasive Cervical Cancer: Follow-Up of Four European Randomised Controlled Trials. Lancet 2014, 383, 524–532. [Google Scholar] [CrossRef]
- Ogilvie, G.S.; Krajden, M.; van Niekerk, D.J.; Martin, R.E.; Ehlen, T.G.; Ceballos, K.; Smith, L.W.; Kan, L.; Cook, D.A.; Peacock, S.; et al. Primary Cervical Cancer Screening with HPV Testing Compared with Liquid-Based Cytology: Results of Round 1 of a Randomised Controlled Trial-the HPV FOCAL Study. Br. J. Cancer 2012, 107, 1917–1924. [Google Scholar] [CrossRef]
- Gyllensten, U.; Gustavsson, I.; Lindell, M.; Wilander, E. Primary High-Risk HPV Screening for Cervical Cancer in Post-Menopausal Women. Gynecol. Oncol. 2012, 125, 343–345. [Google Scholar] [CrossRef]
- Malila, N.; Leinonen, M.; Kotaniemi-Talonen, L.; Laurila, P.; Tarkkanen, J.; Hakama, M. The HPV Test has Similar Sensitivity but More Overdiagnosis than the Pap Test-a Randomised Health Services Study on Cervical Cancer Screening in Finland. Int. J. Cancer 2013, 132, 2141–2147. [Google Scholar] [CrossRef]
- Arbyn, M.; Raifu, A.O.; Weiderpass, E.; Bray, F.; Anttila, A. Trends of Cervical Cancer Mortality in the Member States of the European Union. Eur. J. Cancer 2009, 45, 2640–2648. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Clavel, C.; Petry, K.U.; Meijer, C.J.; Hoyer, H.; Ratnam, S.; Szarewski, A.; Birembaut, P.; Kulasingam, S.; Sasieni, P.; et al. Overview of the European and North American Studies on HPV Testing in Primary Cervical Cancer Screening. Int. J. Cancer 2006, 119, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- van den Akker-van Marle, M.E.; van Ballegooijen, M.; van Oortmarssen, G.J.; Boer, R.; Habbema, J.D. Cost-Effectiveness of Cervical Cancer Screening: Comparison of Screening Policies. J. Natl. Cancer Inst. 2002, 94, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Forman, D.; de Martel, C.; Lacey, C.J.; Soerjomataram, I.; Lortet-Tieulent, J.; Bruni, L.; Vignat, J.; Ferlay, J.; Bray, F.; Plummer, M.; et al. Global Burden of Human Papillomavirus and Related Diseases. Vaccine 2012, 30 (Suppl. 5), F12–F23. [Google Scholar] [CrossRef] [PubMed]
- Asiaf, A.; Ahmad, S.T.; Mohammad, S.O.; Zargar, M.A. Review of the Current Knowledge on the Epidemiology, Pathogenesis, and Prevention of Human Papillomavirus Infection. Eur. J. Cancer Prev. 2014, 23, 206–224. [Google Scholar] [CrossRef]
- Kessis, T.D.; Slebos, R.J.; Nelson, W.G.; Kastan, M.B.; Plunkett, B.S.; Han, S.M.; Lorincz, A.T.; Hedrick, L.; Cho, K.R. Human Papillomavirus 16 E6 Expression Disrupts the p53-Mediated Cellular Response to DNA Damage. Proc. Natl. Acad. Sci. USA 1993, 90, 3988–3992. [Google Scholar] [CrossRef]
- Huh, W.K.; Ault, K.A.; Chelmow, D.; Davey, D.D.; Goulart, R.A.; Garcia, F.A.; Kinney, W.K.; Massad, L.S.; Mayeaux, E.J.; Saslow, D.; et al. Use of Primary High-Risk Human Papillomavirus Testing for Cervical Cancer Screening: Interim Clinical Guidance. Obstet. Gynecol. 2015, 125, 330–337. [Google Scholar] [CrossRef]
- Sasagawa, T.; Maehama, T.; Ideta, K.; Irie, T.; Fujiko Itoh J-HERS Study Group. Population-Based Study for Human Papillomavirus (HPV) Infection in Young Women in Japan: A Multicenter Study by the Japanese Human Papillomavirus Disease Education Research Survey Group (J-HERS). J. Med. Virol. 2016, 88, 324–335. [Google Scholar] [CrossRef]
- Wang, R.; Guo, X.L.; Wisman, G.B.; Schuuring, E.; Wang, W.F.; Zeng, Z.Y.; Zhu, H.; Wu, S.W. Nationwide Prevalence of Human Papillomavirus Infection and Viral Genotype Distribution in 37 Cities in China. BMC Infect. Dis. 2015, 15, 257. [Google Scholar] [CrossRef]
- Song, J.S.; Kim, E.J.; Choi, J.; Gong, G.; Sung, C.O. Significance of HPV-58 Infection in Women Who are HPV-Positive, Cytology-Negative and Living in a Country with a High Prevalence of HPV-58 Infection. PLoS ONE 2013, 8, e58678. [Google Scholar] [CrossRef]
- Bruni, L.; Diaz, M.; Castellsague, X.; Ferrer, E.; Bosch, F.X.; de Sanjose, S. Cervical Human Papillomavirus Prevalence in 5 Continents: Meta-Analysis of 1 Million Women with Normal Cytological Findings. J. Infect. Dis. 2010, 202, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.Y.; Lee, B.; Hwang, S.H.; Lee, D.O.; Sung, N.Y.; Park, J.Y.; Jun, J.K. Evaluation of Satisfaction with Three Different Cervical Cancer Screening Modalities: Clinician-Collected Pap Test Vs. HPV Test by Self-Sampling Vs. HPV Test by Urine Sampling. J. Gynecol. Oncol. 2019, 30, e76. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Ha, S.Y.; Cho, H.Y.; Chung, D.H.; Kim, N.R.; An, J.; Lee, S.; Seok, J.Y.; Jeong, J. Comparison of Papanicolaou Smear and Human Papillomavirus (HPV) Test as Cervical Screening Tools: Can we Rely on HPV Test Alone as a Screening Method? An 11-Year Retrospective Experience at a Single Institution. J. Pathol. Transl. Med. 2020, 54, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Kong, T.W.; Kim, M.; Kim, Y.H.; Kim, Y.B.; Kim, J.; Kim, J.W.; Park, M.H.; Park, J.H.; Rhee, J.H.; Lim, M.C.; et al. High-Risk Human Papillomavirus Testing as a Primary Screening for Cervical Cancer: Position Statement by the Korean Society of Obstetrics and Gynecology and the Korean Society of Gynecologic Oncology. J. Gynecol. Oncol. 2020, 31, e31. [Google Scholar] [CrossRef] [PubMed]
| HPV Test | Method | Genotypes |
|---|---|---|
| MyHPV CHIP™ | PCR and chip hybridization | 11 high-risk HPV: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, and 58 8 low-risk HPV: 6, 11, 34, 40, 42, 43, 44, and 54 |
| Other types of HPV: unspecified HPV | ||
| BD Onclarity™ HPV Assay | Real time PCR | 14 high-risk HPV: 16, 18, 31, 45, 51, 52, P1 (33/58), P2 (56/59/66), and P3 (35/39/68) |
| Genotypes | No. (%) | Genotypes | No. (%) | ||
|---|---|---|---|---|---|
| Sigle infection | 6 * | 16 (2.17) | Double infection | 6, 11 * | 2 (0.27) |
| 11 * | 4 (0.54) | 6, 16 | 2 (0.27) | ||
| 16 | 75 (10.18) | 6, 18 | 1 (0.135) | ||
| 18 | 30 (4.07) | 6, 39 | 1 (0.135) | ||
| 31 | 22 (2.99) | 6, 42 * | 1 (0.135) | ||
| 33 | 20 (2.71) | 6, 56 | 1 (0.135) | ||
| 34 * | 1 (0.135) | 11, 16 | 1 (0.135) | ||
| 35 | 9 (1.22) | 16, 18 | 3 (0.407) | ||
| 39 | 26 (3.53) | 16, 31 | 1 (0.135) | ||
| 40 * | 8 (1.09) | 16, 33 | 1 (0.135) | ||
| 42 * | 9 (1.22) | 16, 40 | 2 (0.27) | ||
| 43 * | 5 (0.68) | 16, 54 | 2 (0.27) | ||
| 44 * | 2 (0.27) | 16, 58 | 3 (0.407) | ||
| 45 | 8 (1.09) | 18, 33 | 1 (0.135) | ||
| 51 | 10 (1.35) | 18, 44 | 1 (0.135) | ||
| 52 | 36 (4.88) | 18, 45 | 1 (0.135) | ||
| 54 * | 30 (4.07) | 18, 52 | 1 (0.135) | ||
| 56 | 43 (5.83) | 18, 54 | 2 (0.27) | ||
| 58 | 59 (8.00) | 31, 44 | 1 (0.135) | ||
| Total | 413 (56.04) | 31, 54 | 1 (0.135) | ||
| Triple infection | 6, 16, 35 | 1 (0.135) | 31, 58 | 1 (0.135) | |
| 6, 42, 58 | 1 (0.135) | 33, 35 | 8 (1.09) | ||
| 6, 51, 52 | 1 (0.135) | 33, 40 | 1 (0.135) | ||
| 11, 33, 52 | 1 (0.135) | 33, 54 | 1 (0.135) | ||
| 16, 33, 54 | 1 (0.135) | 33, 58 | 9 (1.22) | ||
| 16, 33, 58 | 1 (0.135) | 34, 52 | 1 (0.135) | ||
| 16, 52, 56 | 1 (0.135) | 34, 58 | 1 (0.135) | ||
| 18, 33, 42 | 1 (0.135) | 35, 45 | 1 (0.135) | ||
| 33, 35, 39 | 1 (0.135) | 39, 54 | 1 (0.135) | ||
| 33, 35, 52 | 1 (0.135) | 39, 56 | 3 (0.407) | ||
| 33, 35, 56 | 1 (0.135) | 39, 58 | 2 (0.27) | ||
| 35, 58, 51 | 1 (0.135) | 40, 45 | 1 (0.135) | ||
| 43, 45, 56 | 1 (0.135) | 42, 43 * | 1 (0.135) | ||
| Total | 13 (1.76) | 42, 56 | 1 (0.135) | ||
| Quadruple | 16, 11, 33, 58 | 1 (0.135) | 43, 45 | 3 (0.407) | |
| infection | 16, 18, 33, 35 | 1 (0.135) | 43, 52 | 1 (0.135) | |
| 16, 33, 40, 58 | 1 (0.135) | 43, 54 * | 1 (0.135) | ||
| 16, 58, 40, 44 | 1 (0.135) | 43, 58 | 1 (0.135) | ||
| Total | 4 (0.54) | 45, 56 | 1 (0.135) | ||
| HPV-positive | High-risk | 427 (57.94) | 51, 54 | 1 (0.135) | |
| Low-risk | 80 (10.85) | 51, 58 | 1 (0.135) | ||
| Other | 230 (31.21) | 54, 56 | 4 (0.54) | ||
| Total | 737 (13.84) | 54, 58 | 2 (0.27) | ||
| HPV-negative | 4587 (86.16) | 56, 58 | 1 (0.135) | ||
| Total women | 5324 | Total | 77 (10.45) |
| Genotypes | No. (%) | Genotypes | No. (%) |
|---|---|---|---|
| 16 | 90 (8.0) | P1 (33/58), P2 (56/59/66) | 10 (0.89) |
| 18 | 20 (1.78) | P1 (33/58), P3 (35/39/68) | 7 (0.623) |
| 31 | 28 (2.49) | P2 (56/59/66), P3 (35/39/68) | 14 (1.25) |
| 45 | 19 (1.69) | 16, 18, P2 (56/59/66) | 1 (0.089) |
| 51 | 89 (7.92) | 16, 18, P3 (35/39/68) | 1 (0.089) |
| 52 | 165 (14.68) | 16, 31, P2 (56/59/66) | 1 (0.089) |
| P1 (33/58) | 125 (11.12) | 16, 45, P1 (33/58) | 1 (0.089) |
| P2 (56/59/66) | 163 (14.5) | 16, 45, P3 (35/39/68) | 1 (0.089) |
| P3 (35/39/68) | 249 (22.15) | 16, 52, P3 (35/39/68) | 1 (0.089) |
| 16, 18 | 1 (0.089) | 18, 31, P1 (33/58) | 2 (0.178) |
| 16, 31 | 1 (0.089) | 18, 52, P1 (33/58) | 1 (0.089) |
| 16, 51 | 3 (0.267) | 18, 51, P2 (56/59/66) | 1 (0.089) |
| 16, 52 | 6 (0.534) | 45, 51, P3 (35/39/68) | 1 (0.089) |
| 18, 51 | 4 (0.356) | 51, 52, P1 (33/58) | 2 (0.178) |
| 18, 52 | 5 (0.445) | 51, 52, P3 (35/39/68) | 1 (0.089) |
| 31, 45 | 1 (0.089) | 16, P1 (33/58), P3 (35/39/68) | 1 (0.089) |
| 31, 51 | 1 (0.089) | 16, P2 (56/59/66), P3 (35/39/68) | 1 (0.089) |
| 31, 52 | 2 (0.178) | 18, P2 (56/59/66), P3 (35/39/68) | 1 (0.089) |
| 45, 51 | 1 (0.089) | 31, P2 (56/59/66), P3 (35/39/68) | 1 (0.089) |
| 45, 52 | 1 (0.089) | 45, P2 (56/59/66), P3 (35/39/68) | 2 (0.178) |
| 51, 52 | 6 (0.534) | 51, P1 (33/58), P3 (35/39/68) | 1 (0.089) |
| 16, P1 (33/58) | 4 (0.356) | 52, P1 (33/58), P3 (35/39/68) | 2 (0.178) |
| 16, P2 (56/59/66) | 6 (0.534) | 51, P1 (33/58), P2 (56/59/66) | 1 (0.089) |
| 16, P3 (35/39/68) | 3 (0.267) | 52, P1 (33/58), P2 (56/59/66) | 1 (0.089) |
| 18, P2 (56/59/66) | 4 (0.356) | 51, P2 (56/59/66), P3 (35/39/68) | 1 (0.089) |
| 31, P1 (33/58) | 1 (0.089) | 52, P2 (56/59/66), P3 (35/39/68) | 2 (0.178) |
| 31, P2 (56/59/66) | 3 (0.267) | P1 (33/58), P2 (56/59/66), P3 (35/39/68) | 3 (0.267) |
| 31, P3 (35/39/68) | 2 (0.178) | 16, 18, 45, 51 | 1 (0.089) |
| 45, P1 (33/58) | 1 (0.089) | 31, 51, 52, P3(35/39/68) | 1 (0.089) |
| 45, P3 (35/39/68) | 2 (0.178) | 16, 18, P1 (33/58), P3 (35/39/68) | 1 (0.089) |
| 51, P1 (33/58) | 3 (0.267) | 45, 52, P2 (56/59/66), P3 (35/39/68) | 1 (0.089) |
| 51, P2 (56/59/66) | 3 (0.267) | 51, 52, P2 (56/59/66), P3 (35/39/68) | 1 (0.089) |
| 51, P3 (35/39/68) | 10 (0.89) | 16, 45, 31, P2 (56/59/66), P3 (35/39/68) | 1 (0.089) |
| 52, P1 (33/58) | 7 (0.623) | HPV-positive | 1124 (11.45) |
| 52, P2 (56/59/66) | 13 (1.16) | HPV-negative | 8693 (88.55) |
| 52, P3 (35/39/68) | 14 (1.25) | Total women | 9817 |
| Genotype | No. of Detection (%) |
|---|---|
| 6 * | 27 (3.17) |
| 11 * | 9 (1.06) |
| 16 | 98 (11.5) |
| 18 | 42 (4.93) |
| 31 | 26 (3.05) |
| 33 | 51 (5.99) |
| 34 * | 3 (0.35) |
| 35 | 24 (2.82) |
| 39 | 34 (3.99) |
| 40 * | 14 (1.64) |
| 42 * | 14 (1.64) |
| 43 * | 13 (1.53) |
| 44 * | 5 (0.59) |
| 45 | 16 (1.88) |
| 51 | 14 (1.64) |
| 52 | 43 (5.05) |
| 54 * | 46 (5.40) |
| 56 | 57 (6.69) |
| 58 | 86 (10.09) |
| Other | 230 (26.99) |
| Total | 852 |
| Genotype | No. of Detection (%) |
|---|---|
| 16 | 125 (9.3) |
| 18 | 43 (3.2) |
| 31 | 45 (3.35) |
| 45 | 33 (2.45) |
| 51 | 131 (9.75) |
| 52 | 232 (17.26) |
| P1 (33/58) | 174 (12.95) |
| P2 (56/59/66) | 235 (17.48) |
| P3 (35/39/68) | 326 (24.26) |
| Total | 1344 |
| Age | High-Risk HPV (%) | Total |
|---|---|---|
| <30 | 56 (12.3) | 454 |
| 30–39 | 109 (6.9) | 1574 |
| 40–49 | 124 (6.9) | 1799 |
| 50–59 | 85 (7.9) | 1081 |
| 60–69 | 35 (12.8) | 274 |
| ≥70 | 18 (12.7) | 142 |
| total | 427 (8.0) | 5324 |
| Age | High-Risk HPV (%) | Total |
|---|---|---|
| <30 | 199 (25.8) | 771 |
| 30–39 | 330 (11.0) | 2988 |
| 40–49 | 290 (8.3) | 3489 |
| 50–59 | 201 (11.1) | 1808 |
| 60–69 | 69 (12.4) | 556 |
| ≥70 | 35 (17.1) | 205 |
| total | 1124 (11.4) | 9817 |
| Unsatisfactory | NILM | Squamous Lesions | Glandular Lesions | Other Malignancies | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASCUS | ASCH | LSIL | HSIL | SCC | AGC | AIS | ADC | ||||||
| HPV-positive | LBC | 0 | 223 | 31 | 10 | 46 | 25 | 6 | 0 | 0 | 0 | 0 | 341 |
| Smear | 0 | 78 | 3 | 1 | 1 | 2 | 0 | 1 | 0 | 0 | 0 | 86 | |
| (high-risk) | Total (%) | 0 (0) | 301 (70.49) | 125 (29.27) | 1 (0.23) | 0 (0) | 427 (8.02) | ||||||
| HPV-positive | LBC | 0 | 183 | 23 | 1 | 23 | 5 | 0 | 1 | 0 | 0 | 0 | 236 |
| Smear | 0 | 70 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 74 | |
| (low-risk or other) | Total (%) | 0 (0) | 253 (81.61) | 56 (18.06) | 1 (0.32) | 0 (0) | 310 (5.82) | ||||||
| HPV-negative | LBC | 7 | 3049 | 85 | 10 | 23 | 7 | 6 | 7 | 0 | 2 | 2 | 3198 |
| Smear | 2 | 1379 | 4 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 1389 | |
| Total (%) | 9 (0.196) | 4428 (96.53) | 137 (2.99) | 11 (0.24) | 2 (0.044) | 4587 (86.16) | |||||||
| Total(%) | 9 (0.169) | 4982 (93.58) | 318 (5.97) | 13 (0.244) | 2 (0.037) | 5324 (100) | |||||||
| Unsatisfactory | NILM | Squamous Lesions | Glandular Lesions | Other Malignancies | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASCUS | ASCH | LSIL | HSIL | SCC | AGC | AIS | ADC | ||||||
| HPV-positive | LBC | 0 | 636 | 76 | 31 | 130 | 40 | 10 | 8 | 0 | 0 | 1 | 932 |
| Smear | 0 | 168 | 15 | 1 | 4 | 3 | 0 | 1 | 0 | 0 | 0 | 192 | |
| Total (%) | 0 (0) | 804 (71.53) | 310 (27.58) | 9 (0.80) | 1 (0.09) | 1124 (11.45) | |||||||
| HPV-negative | LBC | 11 | 6152 | 189 | 4 | 62 | 4 | 1 | 10 | 0 | 0 | 2 | 6435 |
| Smear | 0 | 2252 | 5 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2258 | |
| Total (%) | 11 (0.127) | 8404 (96.675) | 266 (3.06) | 10 (0.115) | 2 (0.023) | 8693 (88.55) | |||||||
| Total (%) | 11 (0.112) | 9208 (93.796) | 576 (5.867) | 19 (0.193) | 3 (0.031) | 9817 (100) | |||||||
| Cytology (threshold:ASCUS +) | HPV Infection (MyHPV CHIP™) | Negative | CIN1 | CIN2+ | Other Tumors | Endocervical Adenocarcinoma | Total | |
| Cytology (–) | HPV (–) | 39 | 3 | 3 | 2 | 0 | 47 | |
| (82.98) | (6.38) | (6.38) | (4.26) | (0) | (38.21) | |||
| HPV(+) | High-risk (%) | 0 | 9 | 4 | 0 | 0 | 13 | |
| (0) | (69.23) | (30.77) | (0) | (0) | (10.57) | |||
| Low-risk/ | 0 | 1 | 0 | 0 | 0 | 1 | ||
| Other types (%) | (0) | (100) | (0) | (0) | (0) | (0.81) | ||
| Cytology (+) | HPV (–) | 8 | 8 | 3 | 2 | 2 | 23 | |
| (34.78) | (34.78) | (13.04) | (8.7) | (8.7) | (18.7) | |||
| HPV (+) | High-risk (%) | 1 | 9 | 21 | 0 | 0 | 31 (25.2) | |
| (3.23) | (29.03) | (67.74) | (0) | (0) | ||||
| Low-risk/ | 0 | 6 | 2 | 0 | 0 | 8 | ||
| Other types (%) | (0) | (75) | (25) | (0) | (0) | (6.5) | ||
| Total (%) | 48 | 36 | 33 | 4 | 2 | 123 | ||
| (39.02) | (29.27) | (26.83) | (3.25) | (1.63) | (100) | |||
| Cytology (threshold:LSIL +) | HPV infection (MyHPV CHIP™) | Negative | CIN1 | CIN2+ | Other tumors | Endocervical adenocarcinoma | Total | |
| Cytology (–) | HPV (–) | 46 | 5 | 3 | 2 | 0 | 56 | |
| (82.14) | (8.93) | (5.36) | (3.57) | (0) | (45.53) | |||
| HPV (+) | High-risk (%) | 0 | 13 | 5 | 0 | 0 | 18 | |
| (0) | (72.22) | (27.78) | (0) | (0) | (14.63) | |||
| Low-risk/ | 0 | 1 | 0 | 0 | 0 | 1 | ||
| Other types (%) | (0) | (100) | 0 | (0) | (0) | (0.81) | ||
| Cytology (+) | HPV (–) | 1 | 6 | 3 | 2 | 2 | 14 | |
| (7.14) | (42.86) | (21.43) | (14.285) | (14.285) | (11.38) | |||
| HPV (+) | High-risk (%) | 1 | 5 | 20 | 0 | 0 | 26 | |
| (3.85) | (19.23) | (76.92) | (0) | (0) | (21.14) | |||
| Low-risk/ | 0 | 6 | 2 | 0 | 0 | 8 | ||
| Other types (%) | (0) | (75) | (25) | (0) | (0) | (6.5) | ||
| Total (%) | 48 | 36 | 33 | 4 | 2 | 123 | ||
| (39.02) | (29.27) | (26.83) | (3.25) | (1.63) | (100) | |||
| Cytology(threshold:ASCUS +) | HPV Infection(BD Onclarity™) | Negative | CIN1 | CIN2+ | OtherTumors | EndocervicalAdenocarcinoma | Total |
| Cytology (–) (%) | HPV (–) | 42 | 5 | 3 | 0 | 0 | 50 |
| (84) | (10) | (6) | (0) | (0) | (29.07) | ||
| HPV (+) | 3 | 7 | 13 | 0 | 0 | 23 | |
| (13.04) | (30.43) | (56.52) | (0) | (0) | (13.37) | ||
| Cytology (+) (%) | HPV (–) | 3 | 8 | 6 | 1 | 0 | 18 |
| (16.67) | (44.44) | (33.33) | (5.56) | (0) | (10.47) | ||
| HPV (+) | 2 | 14 | 65 | 0 | 0 | 81 | |
| (2.47) | (17.28) | (80.25) | (0) | (0) | (47.09) | ||
| Total (%) | 50 | 34 | 87 | 1 | 0 | 172 | |
| (29.07) | (19.77) | (50.58) | (0.58) | (0) | (100) | ||
| Cytology (threshold: ASCUS +) | HPV Infection(BD Onclarity™) | Negative | CIN1 | CIN2+ | Other tumors | Endocervical Adenocarcinoma | Total |
| Cytology (–) (%) | HPV (–) | 45 | 7 | 3 | 0 | 0 | 55 |
| (81.82) | (12.73) | (5.45) | (0) | (0) | (31.98) | ||
| HPV (+) | 5 | 8 | 25 | 0 | 0 | 38 | |
| (13.16) | (21.05) | (65.79) | (0) | (0) | (22.09) | ||
| Cytology (+) (%) | HPV (–) | 0 | 6 | 6 | 1 | 0 | 13 |
| (0) | (46.15) | (46.15) | (7.7) | (0) | (7.56) | ||
| HPV (+) | 0 | 13 | 53 | 0 | 0 | 66 | |
| (0) | (19.7) | (80.3) | (0) | (0) | (38.37) | ||
| Total (%) | Total | 50 | 34 | 87 | 1 | 0 | 172 |
| (29.07) | (19.77) | (50.58) | (0.58) | (0) | (100) |
| All women | Sensitivity% (95% CI) | Specificity% (95% CI) | PPV% (95% CI) | NPV% (95% CI) | PLR (95% CI) | NLR (95% CI) |
|---|---|---|---|---|---|---|
| HPV+, all types (MyHPV CHIP™) | 82 (65–93) | 71 (61–80) | 51 (37–65) | 91 (82–97) | 2.83 (1.97–4.07) | 0.26 (0.12, 0.53) |
| HPV+, High-risk (MyHPV CHIP™) | 76 (58–89) | 79 (69–87) | 57 (41–72) | 90 (81–96) | 3.59 (2.30–5.59) | 0.31 (0.17, 0.57) |
| HPV+, High-risk (BD Onclarity™) | 90 (81–95) | 69 (58–79) | 75 (66–83) | 87 (76–94) | 2.93 (2.11–4.07) | 0.15 (0.08, 0.28) |
| ASCUS or worse (ASCUS+) | 81 (73-87) | 63 (56–71) | 60 (52–68) | 83 (75–89) | 2.21 (1.79–2.74) | 0.30 (0.21, 0.44) |
| LSIL or worse (LSIL+) | 70 (61–78) | 75 (68–82) | 66 (57–74) | 79 (72–85) | 2.85 (2.14–3.79) | 0.40 (0.30–0.53) |
| HPV+ all types (MyHPV CHIP™) or ASCUS+ | 91 (76–98) | 49 (38–60) | 39 (28–51) | 94 (82–99) | 1.78 (1.41–2.24) | 0.19 (0.06–0.56) |
| HPV+, High-risk (MyHPV CHIP™) or ASCUS+ | 91 (76–98) | 50 (39–61) | 40 (29–52) | 94 (83–99) | 1.82 (1.44–2.30) | 0.18 (0.06–0.55) |
| HPV+, Hig- risk (BD Onclarity™) or ASCUS+ | 97 (90–99) | 55 (44–66) | 69 (60–77) | 94 (83–99) | 2.16 (1.70–2.74) | 0.06 (0.02–0.19) |
| HPV+ all types (MyHPV CHIP™) or LSIL+ | 91 (76–98) | 59 (48–69) | 45 (33–57) | 95 (85–99) | 2.21 (1.69–2.90) | 0.15 (0.05–0.46) |
| HPV+, High-risk (MyHPV CHIP™) or LSIL+ | 91 (76–98) | 60 (49–70) | 45 (33–58) | 95 (85–99) | 2.27 (1.73–2.99) | 0.15 (0.05–0.45) |
| HPV+, High-risk (BD Onclarity™) or LSIL+ | 97 (90–99) | 61 (50–72) | 72 (63–80) | 95 (85–99) | 2.49 (1.90–3.26) | 0.06 (0.02–0.17) |
| HPV+ all types (MyHPV CHIP™) and ASCUS+ | 70 (51–84) | 82 (73–89) | 59 (42–74) | 88 (79–94) | 3.92 (2.38–6.45) | 0.37 (0.22–0.62) |
| HPV+, High-risk (MyHPV CHIP™) and ASCUS+ | 64 (45–80) | 89 (81–95) | 68 (49–83) | 87 (78–93) | 5.73 (3.02–10.85) | 0.41 (0.26–0.65) |
| HPV+, High-risk (BD Onclarity™) and ASCUS+ | 75 (64–83) | 81 (71–89) | 80 (70–88) | 76 (66–84) | 3.97 (2.51–6.28) | 0.31 (0.21–0.45) |
| HPV+ all types (MyHPV CHIP™) and LSIL+ | 67 (48–82) | 87 (78–93) | 65 (46–80) | 88 (79–94) | 5.00 (2.80–8.92) | 0.38 (0.24–0.63) |
| HPV+, High-risk (MyHPV CHIP™) and LSIL+ | 61 (42–77) | 93 (86–98) | 77 (56–91) | 87 (78–93) | 9.09 (4.00–20.65) | 0.42 (0.28–0.65) |
| HPV+, High-risk (BD Onclarity™) and LSIL+ | 61 (50–71) | 85 (75–92) | 80 (69–89) | 68 (58–77) | 3.98 (2.35–6.75) | 0.46 (0.35–0.61) |
| Age < 45 | Sensitivity% (95% CI) | Specificity% (95% CI) | PPV% (95% CI) | NPV% (95% CI) | PLR (95% CI) | NLR (95% CI) |
|---|---|---|---|---|---|---|
| HPV+, all types (MyHPV CHIP™) | 89 (52–100) | 58 (37–78) | 44 (22–69) | 93 (68–100) | 2.13 (1.26–3.61) | 0.19 (0.03–1.25) |
| HPV+, High-risk (MyHPV CHIP™) | 67 (30–93) | 75 (53–90) | 50 (21–79) | 86 (64–97) | 2.67 (1.16–6.13) | 0.44 (0.17–1.15) |
| HPV+, High-risk (BD Onclarity™) | 97 (83–100) | 54 (25–81) | 83 (66–93) | 88 (47–100) | 2.09 (1.16–3.78) | 0.06 (0.01–0.45) |
| ASCUS or worse (ASCUS+) | 77 (61–89) | 70 (53–84) | 73 (57–.86) | 74 (57–88) | 2.59 (1.53–4.37) | 0.33 (0.18–0.60) |
| LSIL or worse (LSIL+) | 67 (50–81) | 81 (65–92) | 79 (61–91) | 70 (54–.83) | 3.52 (1.74–7.12) | 0.41 (0.26–0.66) |
| HPV+ all types (MyHPV CHIP™) or ASCUS+ | 89 (52–100) | 46 (26–67) | 38 (18–62) | 92 (62–100) | 1.64 (1.06–2.53) | 0.24 (0.04–1.62) |
| HPV+, High-risk (MyHPVCHIP™) or ASCUS+ | 89 (52–100) | 50 (29–71) | 40 (19–64) | 92 (64–100) | 1.78 (1.12–2.82) | 0.22 (0.03–1.47) |
| HPV+, High-risk (BD Onclarity™) or ASCUS+ | 97 (83–100) | 54 (25–81) | 83 (66–93) | 88 (47–100) | 2.09 (1.16–3.78) | 0.06 (0.01–0.45) |
| HPV+ all types (MyHPV CHIP™) or LSIL+ | 89 (52–100) | 58 (37–78) | 44 (22–69) | 93 (68–100) | 2.13 (1.26–3.61) | 0.19 (0.03–1.25) |
| HPV+, High-risk (MyHPV CHIP™) or LSIL+ | 89 (52–100) | 62 (41–81) | 47 (23–72) | 94 (70–100) | 2.37 (1.35–4.17) | 0.18 (0.03–1.16) |
| HPV+, High-risk (BD Onclarity™) or LSIL+ | 97 (83–100) | 54 (25–81) | 83 (66–93) | 88 (47–100) | 2.09 (1.16–3.78) | 0.06 (0.01–0.45) |
| HPV+ all types (MyHPV CHIP™) and ASCUS+ | 89 (52–100) | 79 (58–93) | 62 (32–86) | 95 (75–100) | 4.27 (1.89–9.62) | 0.14 (0.02–0.90) |
| HPV+, High-risk (MyHPV CHIP™) and ASCUS+ | 67 (30–93) | 92 (73–99) | 75 (35–97) | 88 (69–97) | 8.00 (1.96–32.60) | 0.36 (0.14–0.92) |
| HPV+, High-risk (BD Onclarity™) and ASCUS+ | 73 (54–88) | 77 (46–95) | 88 (69–97) | 56 (31–78) | 3.18 (1.15–8.77) | 0.35 (0.18–0.67) |
| HPV+ all types (MyHPV CHIP™) and LSIL+ | 89 (52–100) | 83 (63–95) | 67 (35–90) | 95 (76–100) | 5.33 (2.12–13.44) | 0.13 (0.02–0.85) |
| HPV+, High-risk (MyHPV CHIP™) and LSIL+ | 67 (30–93) | 96 (79–100) | 86 (42–100) | 88 (70–98) | 16.00 (2.22–115.14) | 0.35 (0.14–0.88) |
| HPV+, High-risk (BD Onclarity™) and LSIL+ | 60 (41–77) | 77 (46–95) | 86 (64–97) | 45 (24–68) | 2.60 (0.92–7.32) | 0.52 (0.31–0.88) |
| Age ≥ 45 | Sensitivity% (95% CI) | Specificity% (95% CI) | PPV% (95% CI) | NPV% (95% CI) | PLR (95% CI) | NLR (95% CI) |
|---|---|---|---|---|---|---|
| HPV+, all types (MyHPV CHIP™) | 79 (58–93) | 76 (64–85) | 54 (37–71) | 91 (80–97) | 3.27 (2.03–5.24) | 0.28 (0.12–0.61) |
| HPV+, High-risk (MyHPV CHIP™) | 79 (58–93) | 80 (69–89) | 59 (41–76) | 91 (81–97) | 4.02 (2.37–6.82) | 0.26 (0.12–0.57) |
| HPV+, High-risk (BD Onclarity™) | 86 (74–94) | 72 (60–82) | 71 (59–81) | 87 (75–94) | 3.09 (2.10–4.56) | 0.19 (0.10–0.38) |
| ASCUS or worse (ASCUS+) | 83 (73–90) | 62 (53–70) | 56 (46–65) | 86 (77–92) | 2.15 (1.71–2.72) | 0.28 (0.17–0.46) |
| LSIL or worse (LSIL+) | 72 (60–81) | 74 (66–81) | 62 (51–72) | 82 (74–88) | 2.74 (2.01–3.75) | 0.38 (0.27–0.55) |
| HPV+ all types (MyHPV CHIP™) or ASCUS+ | 92 (73–99) | 50 (37–63) | 40 (27–54) | 94 (81–99) | 1.83 (1.40–2.40) | 0.17 (0.04–0.64) |
| HPV+, High-risk (MyHPV CHIP™) or ASCUS+ | 92 (73–99) | 50 (37–63) | 40 (27–54) | 94 (81–99) | 1.83 (1.40–2.40) | 0.17 (0.04–0.64) |
| HPV+, High-risk (BD Onclarity™) or ASCUS+ | 96 (88–100) | 56 (43–67) | 63 (52–73) | 95 (84–99) | 2.17 (1.67–2.82) | 0.06 (0.02–0.25) |
| HPV+ all types (MyHPV CHIP™) or LSIL+ | 92 (73–99) | 59 (46–71) | 45 (31–60) | 95 (83–99) | 2.24 (1.64–3.07) | 0.14 (0.04–0.54) |
| HPV+, High-risk (MyHPV CHIP™) or LSIL+ | 92 (73–99) | 59 (46–71) | 45 (31–60) | 95 (83–99) | 2.24 (1.64–3.07) | 0.14 (0.04–0.54) |
| HPV+, High-risk (BD Onclarity™) or LSIL+ | 96 (88–100) | 62 (50–74) | 67 (56–77) | 96 (85–99) | 2.57 (1.90–3.48) | 0.06 (0.01–0.22) |
| HPV+ all types (MyHPV CHIP™) and ASCUS+ | 62 (41–81) | 83 (72–91) | 58 (37–77) | 86 (75–93) | 3.75 (2.01–6.99) | 0.45 (0.27–0.76) |
| HPV+, High-risk (MyHPV CHIP™) and ASCUS+ | 62 (41–81) | 88 (78–95) | 65 (43–84) | 87 (76–94) | 5.16 (2.51–10.59) | 0.43 (0.25–0.72) |
| HPV+, High-risk (BD Onclarity™) and ASCUS+ | 75 (62–86) | 82 (71–90) | 77 (64–87) | 81 (70–89) | 4.18 (2.50–6.98) | 0.30 (0.19–0.48) |
| HPV+ all types (MyHPV CHIP™) and LSIL+ | 58 (37–78) | 88 (78–95) | 64 (41–83) | 85 (75–93) | 4.81 (2.31–10.01) | 0.47 (0.29–0.77) |
| HPV+, High-risk(MyHPV CHIP™) and LSIL+ | 58 (37–78) | 92 (83–97) | 74 (49–91) | 86 (76–93) | 7.70 (3.11–19.09) | 0.45 (0.28–0.73) |
| HPV+, High-risk (BD Onclarity™) and LSIL+ | 61 (48–74) | 86 (76–93) | 78 (63–89) | 74 (63–83) | 4.42 (2.40–8.14) | 0.45 (0.32–0.63) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-s.; Lee, E.H.; Park, M.-i.; Lee, J.S.; Kim, K.; Roh, M.S.; Lee, H.W. Utility of Human Papillomavirus Testing for Cervical Cancer Screening in Korea. Int. J. Environ. Res. Public Health 2020, 17, 1726. https://doi.org/10.3390/ijerph17051726
Kim M-s, Lee EH, Park M-i, Lee JS, Kim K, Roh MS, Lee HW. Utility of Human Papillomavirus Testing for Cervical Cancer Screening in Korea. International Journal of Environmental Research and Public Health. 2020; 17(5):1726. https://doi.org/10.3390/ijerph17051726
Chicago/Turabian StyleKim, Mee-seon, Eun Hee Lee, Moon-il Park, Jae Seok Lee, Kisu Kim, Mee Sook Roh, and Hyoun Wook Lee. 2020. "Utility of Human Papillomavirus Testing for Cervical Cancer Screening in Korea" International Journal of Environmental Research and Public Health 17, no. 5: 1726. https://doi.org/10.3390/ijerph17051726
APA StyleKim, M.-s., Lee, E. H., Park, M.-i., Lee, J. S., Kim, K., Roh, M. S., & Lee, H. W. (2020). Utility of Human Papillomavirus Testing for Cervical Cancer Screening in Korea. International Journal of Environmental Research and Public Health, 17(5), 1726. https://doi.org/10.3390/ijerph17051726





