Influence of the Menstrual Cycle on Blood Markers of Muscle Damage and Inflammation Following Eccentric Exercise
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
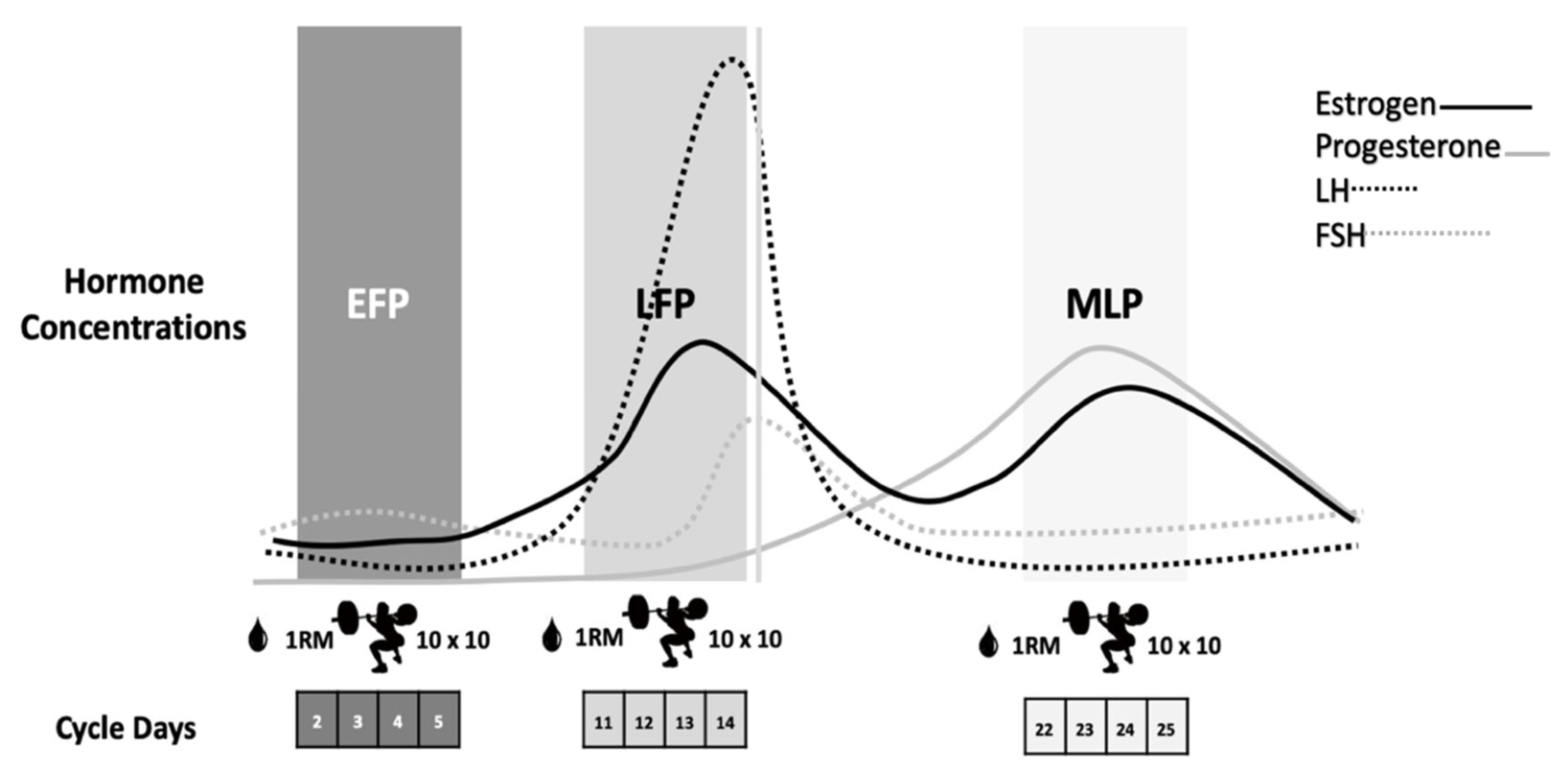
2.2. Menstrual Cycle Phase Determination
2.3. Screening Protocol
2.4. Eccentric-Exercise Sessions
2.5. Blood Sampling and Biochemical Analysis
2.6. Statistical Analysis
3. Results
3.1. Sex Hormones and Strength Assessment
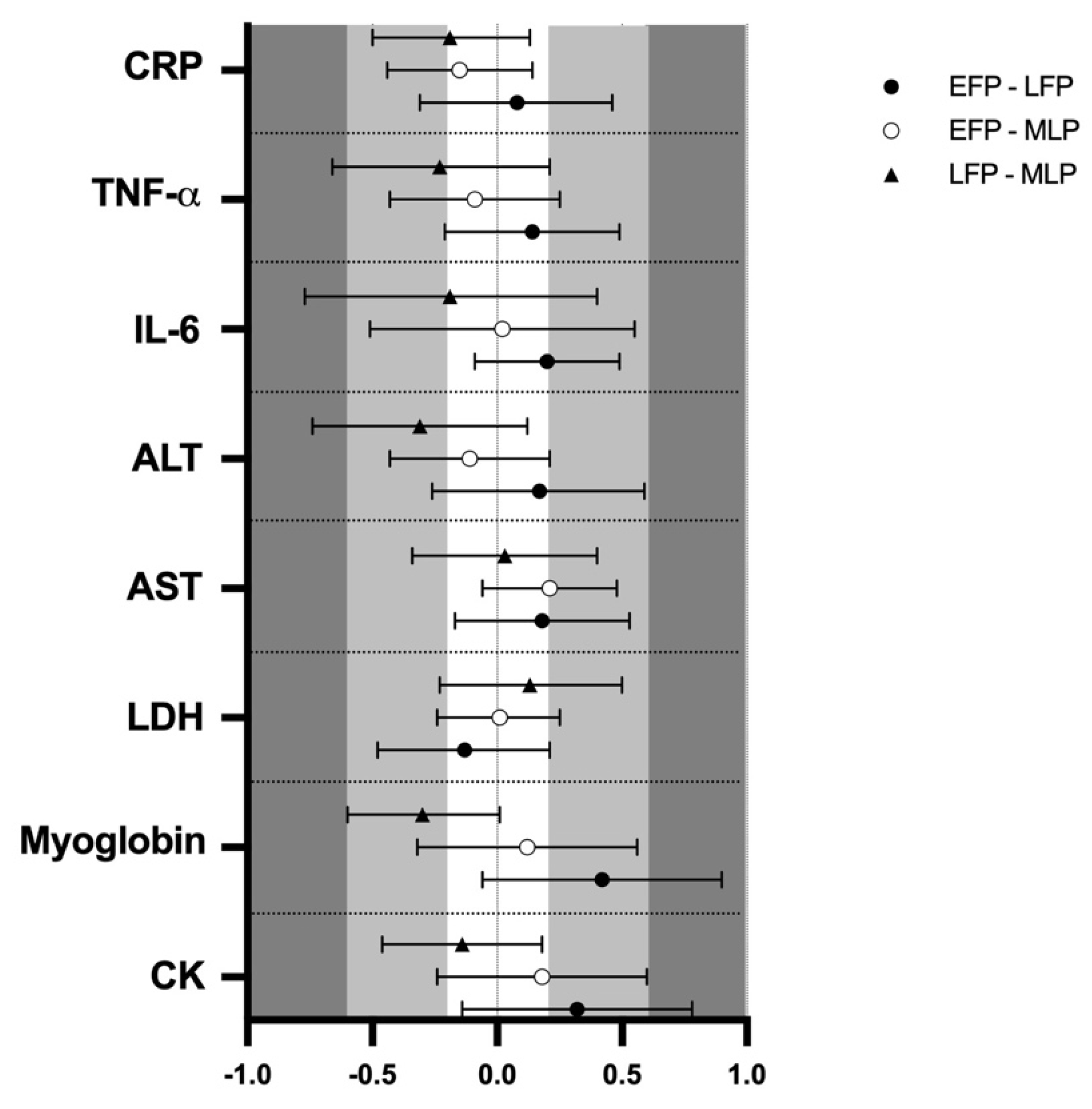
3.2. Muscle Damage
3.3. Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peake, J.M.; Neubauer, O.; Della Gatta, P.A.; Nosaka, K. Muscle damage and inflammation during recovery from exercise. J. Appl. Phys. 2017, 122, 559–570. [Google Scholar] [CrossRef]
- Koch, A.J.; Pereira, R.; Machado, M. The creatine kinase response to resistance exercise. J. Musculoskelet. Neuronal Interact. 2014, 14, 68–77. [Google Scholar] [PubMed]
- Brancaccio, P.; Maffulli, N.; Limongelli, F.M. Creatine kinase monitoring in sport medicine. Br. Med. Bull. 2007, 81–82, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Kendall, B.; Eston, R. Exercise-induced muscle damage and the potential protective role of estrogen. Sports Med. 2002, 32, 103–123. [Google Scholar] [CrossRef]
- Enns, D.L.; Tiidus, P.M. The influence of estrogen on skeletal muscle. Sports Med. 2010, 40, 41–58. [Google Scholar] [CrossRef]
- Rankin, P.; Lawlor, M.J.; Hills, F.A.; Bell, P.G.; Stevenson, E.J.; Cockburn, E. The effect of milk on recovery from repeat-sprint cycling in female team-sport athletes. Appl. Physiol. Nutr. Metab. 2018, 43, 113–122. [Google Scholar] [CrossRef]
- Greising, S.M.; Baltgalvis, K.A.; Lowe, D.A.; Warren, G.L. Hormone therapy and skeletal muscle strength: A meta-analysis. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2009, 64, 1071–1081. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Spektor, T.M.; Rice, J.C.; Schroeder, E.T. Hormone therapy attenuates exercise-induced skeletal muscle damage in postmenopausal women. J. Appl. Physiol. 2009, 107, 853–858. [Google Scholar] [CrossRef]
- Bruinvels, G.; Burden, R.J.; McGregor, A.J.; Ackerman, K.E.; Dooley, M.; Richards, T.; Pedlar, C. Sport, exercise and the menstrual cycle: Where is the research? Br. J. Sports Med. 2017, 51, 487–488. [Google Scholar] [CrossRef]
- Hackney, A.C.; Kallman, A.L.; Ağgön, E. Female sex hormones and the recovery from exercise: Menstrual cycle phase affects responses. Biomed. Hum. Kinet. 2019, 11, 87–89. [Google Scholar] [CrossRef]
- Janse de Jonge, X.A.K. Effects of the menstrual cycle on exercise performance. Sports Med. 2003, 33, 833–851. [Google Scholar] [CrossRef]
- Anderson, L.J.; Baker, L.L.; Schroeder, E.T. Blunted myoglobin and quadriceps soreness after electrical stimulation during the luteal phase or oral contraception. Res. Q. Exerc. Sport 2017, 88, 193–202. [Google Scholar] [CrossRef]
- Oosthuyse, T.; Bosch, A. The effect of gender and menstrual phase on serum creatine kinase activity and muscle soreness following downhill Running. Antioxidants 2017, 6, 16. [Google Scholar] [CrossRef]
- Williams, T.; Walz, E.; Lane, A.R.; Pebole, M.; Hackney, A.C. The effect of estrogen on muscle damage biomarkers following prolonged aerobic exercise in eumenorrheic women. Biol. Sport 2015, 32, 193–198. [Google Scholar] [CrossRef]
- Chaffin, M.E.; Davis, J.E.; Berg, K.E.; French, J.A.; Meendering, J.R.; Llewellyn, T.L. Interleukin-6 and delayed onset muscle soreness do not vary during the menstrual cycle. Res. Q. Exerc. Sport 2011, 82, 693–701. [Google Scholar] [CrossRef]
- Sipavičienė, S.; Daniusevičiutė, L.; Klizienė, I.; Kamandulis, S.; Skurvydas, A. Effects of estrogen fluctuation during the menstrual cycle on the response to stretch-shortening exercise in females. BioMed Res. Int. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Hicks, K.M.; Onambele-Pearson, G.L.; Winwood, K.; Morse, C.I. Muscle-tendon unit properties during eccentric exercise correlate with the creatine kinase response. Front. Physiol. 2017, 8, 657. [Google Scholar] [CrossRef]
- Minahan, C.; Joyce, S.; Bulmer, A.C.; Cronin, N.; Sabapathy, S. The influence of estradiol on muscle damage and leg strength after intense eccentric exercise. Eur. J. Appl. Physiol. 2015, 115, 1493–1500. [Google Scholar] [CrossRef]
- Hicks, K.M.; Onambélé, G.L.; Winwood, K.; Morse, C.I. Muscle Damage following Maximal Eccentric Knee Extensions in Males and Females. PLoS ONE 2016, 11, e0150848. [Google Scholar] [CrossRef]
- Joyce, S.; Sabapathy, S.; Bulmer, A.C.; Minahan, C. The effect of prior eccentric exercise on heavy-intensity cycling: The role of gender and oral contraceptives. Eur. J. Appl. Physiol. 2014, 114, 995–1003. [Google Scholar] [CrossRef]
- Brown, M.A.; Howatson, G.; Keane, K.; Stevenson, E.J. Undefined, Exercise-induced muscle damage following dance and sprint specific exercise in females. J. Sports Med. Phys. Fit. 2016, 56, 1376–1383. [Google Scholar]
- Nikolaidis, M.G.; Paschalis, V.; Giakas, G.; Fatouros, I.G.; Sakellariou, G.K.; Theodorou, A.A.; Koutedakis, Y.; Jamurtas, A.Z. Favorable and prolonged changes in blood lipid profile after muscle-damaging exercise. Med. Sci. Sports Exerc. 2008, 40, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Stupka, N.; Lowther, S.; Chorneyko, K.; Bourgeois, J.M.; Hogben, C.; Tarnopolsky, M.A. Gender differences in muscle inflammation after eccentric exercise. J. Appl. Physiol. 2000, 89, 2325–2332. [Google Scholar] [CrossRef] [PubMed]
- Janse de Jonge, X.A.K.; Thompson, B.; Han, A. Methodological Recommendations for Menstrual Cycle Research in Sports and Exercise. Med. Sci. Sports Exerc. 2019, 51, 2610–2617. [Google Scholar] [CrossRef]
- Lebrun, C.M.; McKenzie, D.C.; Prior, J.C.; Taunton, J.E. Effects of menstrual cycle phase on athletic performance. Med. Sci. Sports Exerc. 1995, 27, 437–444. [Google Scholar] [CrossRef]
- Miller, P.B.; Soules, M.R. The usefulness of a urinary LH kit for ovulation prediction during menstrual cycles of normal women. Obstet. Gynecol. 1996, 87, 13–17. [Google Scholar] [CrossRef]
- Balsalobre-Fernández, C.; Marchante, D.; Baz-Valle, E.; Alonso-Molero, I.; Jiménez, S.L.; Muñóz-López, M. Analysis of wearable and smartphone-based technologies for the measurement of barbell velocity in different resistance training exercises. Front. Physiol. 2017, 8, 649. [Google Scholar] [CrossRef]
- Balsalobre-Fernández, C.; Marchante, D.; Muñoz-López, M.; Jiménez, S.L. Validity and reliability of a novel iPhone app for the measurement of barbell velocity and 1RM on the bench-press exercise. J. Sports Sci. 2018, 36, 64–70. [Google Scholar] [CrossRef]
- Macdonald, G.Z.; Button, D.C.; Drinkwater, E.J.; Behm, D.G. Foam rolling as a recovery tool after an intense bout of physical activity. Med. Sci. Sports Exerc. 2014, 46, 131–142. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef]
- Hyldahl, R.D.; Chen, T.C.; Nosaka, K. Mechanisms and Mediators of the Skeletal Muscle Repeated Bout Effect. Exerc. Sport Sci. Rev. 2017, 45, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Kamandulis, S.; Skurvydas, A.; Snieckus, A.; Masiulis, N.; Aagaard, P.; Dargeviciute, G.; Brazaitis, M. Monitoring markers of muscle damage during a 3 week periodized drop-jump exercise programme. J. Sports Sci. 2011, 29, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; de Paula Vieira, R.; Bischof, F.; Walter, M.; Movassaghi, M.; Berchtold, N.C.; Niess, A.M.; Cotman, C.W.; Northoff, H. Sex-specific variation in signaling pathways and gene expression patterns in human leukocytes in response to endotoxin and exercise. J. Neuroinflamm. 2016, 13, 289. [Google Scholar] [CrossRef] [PubMed]
- Fish, E.N. The X-files in immunity: Sex-based differences predispose immune responses. Nat. Rev. Immunol. 2008, 8, 737–744. [Google Scholar] [CrossRef]
- Northoff, H.; Symons, S.; Zieker, D.; Schaible, E.V.; Schaefer, K.; Thoma, S.; Loeffler, M.; Abbasi, A.; Simon, P.; Niess, A.M.; et al. Gender- and menstrual phase dependent regulation of inflammatory gene expression in response to aerobic exercise. Exerc. Immunol. Rev. 2008, 14, 86–103. [Google Scholar]
- Timmons, B.W.; Hamadeh, M.J.; Devries, M.C.; Tarnopolsky, M.A. Influence of gender, menstrual phase, and oral contraceptive use on immunological changes in response to prolonged cycling. J. Appl. Physiol. 2005, 99, 979–985. [Google Scholar] [CrossRef]
- Oosthuyse, T.; Bosch, A.N. The Effect of the Menstrual Cycle on Exercise Metabolism. Sports Med. 2010, 40, 207–227. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Fischer, C.P. Beneficial health effects of exercise—The role of IL-6 as a myokine. Trends Pharmacol. Sci. 2007, 28, 152–156. [Google Scholar] [CrossRef]
- Pedersen, B.K. Anti-inflammatory effects of exercise: Role in diabetes and cardiovascular disease. Eur. J. Clin. Investig. 2017, 47, 600–611. [Google Scholar] [CrossRef]
- Liu, Z.; Que, S.; Xu, J.; Peng, T. Alanine aminotransferase-old biomarker and new concept: A review. Int. J. Med. Sci. 2014, 11, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Bekkelund, S.I.; Jorde, R. Alanine Aminotransferase and Body Composition in Obese Men and Women. Dis. Markers 2019, 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Chaki, B.; Chattopadhyay, S.; Bandyopadhyay, A. High-Intensity Exercise Induced Oxidative Stress and Skeletal Muscle Damage in Postpubertal Boys and Girls. J. Strength Cond. Res. 2018, 32, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.L.; Hsiao, C.Y.; Li, Y.H.; Meng, F.B.; Huang, C.C.; Chen, Y.M. Nanobubbles Water Curcumin Extract Reduces Injury Risks on Drop Jumps in Women: A Pilot Study. Evid. Based Complement. Altern. Med. 2019, 2019. [Google Scholar] [CrossRef]

| EFP | LFP | MLP | TOTAL | |||||
|---|---|---|---|---|---|---|---|---|
| CK (U·L−1) | ||||||||
| Baseline | 108.6 | (48.0) | 105.7 | (33.1) | 100.7 | (29.9) | 105.0 | (37.3) |
| 2 h | 151.6 | (70.0) | 155.1 | (44.9) | 150.6 | (43.8) | 152.4 * | (53.3) |
| 24 h | 154.1 | (69.3) | 195.5 | (95.3) | 172.1 | (85.8) | 173.9 a | (84.4) |
| 48 h | 117.3 | (40.1) | 130.6 | (47.7) | 128.8 | (49.5) | 125.6 a,c | (45.5) |
| Total | 132.9 | (60.7) | 146.7 | (67.7) | 138.1 | (61.1) | ||
| Myoglobin (µg·L) | ||||||||
| Baseline | 62.8 | (8.2) | 60.4 | (7.2) | 60.1 | (10.6) | 61.1 | (8.9) |
| 2 h | 105.5 | (43.9) | 129.1 | (56.3) | 107.9 | (41.2) | 115.0 ** | (48.7) |
| 24 h | 64.5 | (9.6) | 64.9 | (7.9) | 66.1 | (10.7) | 65.2 | (9.6) |
| 48 h | 59.8 | (7.4) | 63.8 | (9.0) | 62.2 | (8.1) | 61.9 | (8.5) |
| Total | 73.2 | (30.0) | 79.6 | (41.0) | 74.1 | (30.1) | ||
| LDH (U·L−1) | ||||||||
| Baseline | 166.5 | (14.7) | 164.3 | (22.6) | 163.9 | (13.4) | 164.9 | (17.4) |
| 2 h | 187.1 | (20.3) | 181.5 | (26.8) | 184.4 | (16.3) | 184.3 ** | (21.3) |
| 24 h | 166.2 | (15.9) | 170.2 | (23.9) | 165.7 | (17.4) | 167.4 | (19.1) |
| 48 h | 163.8 | (16.5) | 161.4 | (16.2) | 167.9 | (16.9) | 164.4 | (16.5) |
| Total | 170.9 | (19.1) | 169.3 | (23.6) | 170.7 | (18.9) | ||
| IL-6 (pg/mL) | ||||||||
| Baseline | 1.7 | (0.7) | 1.7 | (0.5) | 1.6 | (0.3) | 1.7 | (0.6) |
| 2 h | 1.8 | (0.7) | 1.7 | (0.6) | 2.0 | (1.3) | 1.9 | (0.9) |
| 24 h | 1.6 | (0.2) | 1.7 | (0.7) | 1.5 | (0.0) | 1.6 b | (0.4) |
| 48 h | 1.6 | (0.5) | 1.9 | (0.7) | 1.5 | (0.1) | 1.7 | (0.4) |
| Total | 1.7 | (0.5) | 1.7 | (0.6) | 1.7 | (0.7) | ||
| TNF-α(pg/mL) | ||||||||
| Baseline | 4.9 | (1.1) | 5.3 | (1.9) | 4.9 | (2.0) | 5.0 | (1.7) |
| 2 h | 5.2 | (1.4) | 5.3 | (1.6) | 4.9 | (1.4) | 5.2 | (1.5) |
| 24 h | 4.8 | (1.4) | 4.8 | (1.0) | 4.6 | (0.9) | 4.7 ## | (1.1) |
| 48 h | 4.5 | (0.9) | 4.6 | (0.9) | 4.7 | (1.2) | 4.6 | (1.0) |
| Total | 4.8 | (1.2) | 5.0 | (1.4) | 4.8 | (1.4) | ||
| CRP (mg/L) | ||||||||
| Baseline | 0.6 | (0.3) | 0.6 | (0.3) | 0.5 | (0.3) | 0.5 | (0.3) |
| 2 h | 0.5 | (0.3) | 0.5 | (0.3) | 0.5 | (0.3) | 0.5 | (0.3) |
| 24 h | 0.6 | (0.4) | 0.5 | (0.3) | 0.5 | (0.3) | 0.5 ## | (0.3) |
| 48 h | 0.5 | (0.3) | 0.5 | (0.3) | 0.5 | (0.3) | 0.5 b,# | (0.3) |
| Total | 0.5 | (0.4) | 0.5 | (0.3) | 0.5 | (0.3) | ||
| AST (Ui/L) | ||||||||
| Baseline | 21.2 | (4.4) | 21.8 | (5.0) | 22.6 | (5.1) | 21.8 | (4.8) |
| 2 h | 22.8 | (4.2) | 23.4 | (4.7) | 23.8 | (4.2) | 23.4 a | (4.3) |
| 24 h | 23.1 | (4.7) | 24.3 | (5.1) | 23.8 | (4.0) | 23.7 a | (4.6) |
| 48 h | 22.4 | (5.2) | 23.3 | (5.4) | 22.8 | (4.6) | 22.8 | (5.0) |
| Total | 22.4 | (4.6) | 23.2 | (5.0) | 23.3 | (4.5) | ||
| ALT (Ui/L) | ||||||||
| Baseline | 16.0 | (4.5) | 16.8 | (5.5) | 14.7 | (4.9) | 15.8 | (5.1) |
| 2 h | 16.3 | (4.4) | 17.3 | (5.4) | 15.6 | (4.1) | 16.4 | (4.7) |
| 24 h | 16.4 | (4.5) | 17.4 | (5.3) | 15.8 | (3.9) | 16.6 | (4.6) |
| 48 h | 16.4 | (5.1) | 17.5 | (5.9) | 15.9 | (4.4) | 16.6 | (5.1) |
| Total | 16.3 | (4.5) | 17.3 | (5.4) | 15.5 § | (4.4) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Parra, N.; Barba-Moreno, L.; Rael, B.; Alfaro-Magallanes, V.M.; Cupeiro, R.; Díaz, Á.E.; Calderón, F.J.; Peinado, A.B. Influence of the Menstrual Cycle on Blood Markers of Muscle Damage and Inflammation Following Eccentric Exercise. Int. J. Environ. Res. Public Health 2020, 17, 1618. https://doi.org/10.3390/ijerph17051618
Romero-Parra N, Barba-Moreno L, Rael B, Alfaro-Magallanes VM, Cupeiro R, Díaz ÁE, Calderón FJ, Peinado AB. Influence of the Menstrual Cycle on Blood Markers of Muscle Damage and Inflammation Following Eccentric Exercise. International Journal of Environmental Research and Public Health. 2020; 17(5):1618. https://doi.org/10.3390/ijerph17051618
Chicago/Turabian StyleRomero-Parra, Nuria, Laura Barba-Moreno, Beatriz Rael, Víctor M. Alfaro-Magallanes, Rocío Cupeiro, Ángel E. Díaz, Francisco J. Calderón, and Ana B. Peinado. 2020. "Influence of the Menstrual Cycle on Blood Markers of Muscle Damage and Inflammation Following Eccentric Exercise" International Journal of Environmental Research and Public Health 17, no. 5: 1618. https://doi.org/10.3390/ijerph17051618
APA StyleRomero-Parra, N., Barba-Moreno, L., Rael, B., Alfaro-Magallanes, V. M., Cupeiro, R., Díaz, Á. E., Calderón, F. J., & Peinado, A. B. (2020). Influence of the Menstrual Cycle on Blood Markers of Muscle Damage and Inflammation Following Eccentric Exercise. International Journal of Environmental Research and Public Health, 17(5), 1618. https://doi.org/10.3390/ijerph17051618






