Abstract
Ultraviolet B (UV-B, 280–320 nm) radiation causes complex molecular reactions in cells, including DNA damage, oxidative stress, and apoptosis. This study designed a mixture consisting of quercetin, luteolin and lycopene and used Caenorhabditis elegans as a model to study the resistance of these natural chemicals to UV-B. Specifically, we have confirmed that quercetin and its mixture can increase the resistance of Caenorhabditis elegans to UV-B through lifespan test, reactive oxygen species level assay, germ cell apoptosis test, embryonic lethal test and RT-qPCR experiments. The results show that quercetin and its mixture prolonged the lifespan of UV-B-irradiated Caenorhabditis elegans and reduced abnormal levels of reactive oxygen species, embryo death, and apoptosis induced by UV-B. The protective effect of quercetin and its mixture may be attributed to its down-regulation of HUS-1, CEP-1, EGL-1 and CED-13. Therefore, the results of this research could help the development of UV-B radiation protection agents.
1. Introduction
Radiation is divided into two forms: ionizing radiation and non-ionizing radiation. Ultraviolet (UV) radiation is the most common non-ionizing radiation in human daily life. Common UV rays are divided into UV-C (200–280 nm), UV-B (280–320 nm) and UV-A (315–340 nm) according to wavelength. UV radiation as an effective mutagen, which has been proved by epidemiology and molecular biology to causes human skin cancer [1]. UV irradiation can cause DNA double helixes to deform, producing thymine dimers, cyclobutane pyrimidine dimers (CPD) and other pyrimidine dimers. These deformed DNA fragments are usually recognized and removed by the nucleotide excision repair (NER) system [2]. In addition, UV radiation also increases the level of reactive oxygen species (ROS) including hydroxide (OH−), superoxide anion (O2−), singlet oxygen (O2) and hydrogen peroxide (H2O2) in mitochondria [3]. High levels of ROS activate the mitogen-activated protein kinase pathway, which causes damage to DNA, leading to cell cycle arrest and apoptosis [4]. Therefore, UV radiation can directly or indirectly cause damage to DNA. Among them, UV-A and UV-B are radiation that people often receive, and UV-B has stronger carcinogenic effect than UV-A [5].
As early as 1949, researchers first reported in vivo studies of the protective effect of antioxidants on ionizing radiation, demonstrating that cysteine protects rats from lethal doses of X-rays [6]. Later, in 1985, researchers first reported that 2% beta-carotene and 0.07% canthaxanthin can effectively prevent the development of skin tumors caused by UV-B [7]. Subsequently, a large number of chemicals like flavonoids with anti-oxidant activity have been reported to inhibit radiation damage [8,9]. The development of natural compounds with low side effects to inhibit UV-induced damage has become a popular subject.
Generally, UV radiation activates death receptors and mitochondrial apoptotic pathways in cells, which triggers apoptosis [10]. The CEP-1 in C. elegans is a homologous gene of p53 in mammals, and their functions are almost the same, mainly mediating apoptosis and meiotic chromosome separation [11]. The HUS-1 is a conserved checkpoint protein. The classical apoptotic pathway mediated by CEP-1 requires HUS-1 to activate, so it is necessary for DNA damage-induced cell cycle arrest and apoptosis [12]. After activation of the CEP-1-mediated apoptotic pathway, transcription of the pro-apoptotic genes EGL-1 and CED-13 is activated. The EGL-1 triggers apoptosis induction by antagonizing the functions of anti-apoptotic members of the BCL-2 family [12]. In addition, CED-13 is the only protein with a BH3 domain in C. elegans, which interacts with CED-9 to promote apoptosis when overexpressed [13]. These four genes are involved in the regulation of UV-induced apoptosis in C. elegans (Figure 1).
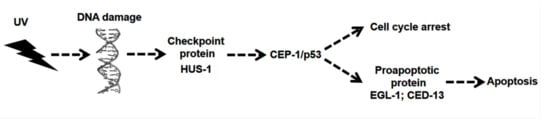
Figure 1.
UV-induced apoptosis pathway in C. elegans.
The natural chemicals used in this experiment are quercetin, luteolin and lycopene. The molecular formula of quercetin (3, 3′, 4′, 5, 7-pentahydroxyflavone) is C15H10O7. It has strong antioxidant activity [9], antitumor activity [14], and radiation resistance [15]. Similarly, luteolin (3′, 4′, 5, 7-tetrahydroxyflavone, C15H10O6) and lycopene (C40H56) also have great antioxidant and antitumor effects [16,17]. Therefore, this study designed a mixture using these three natural chemicals. The effect and mechanism of quercetin and its mixture on increasing stress resistance of C. elegans to UV-B were studied by lifespan test, ROS level assay, germ cell apoptosis test, embryonic lethal test and RT-qPCR.
2. Materials and Methods
2.1. Strain, Cultivation and Grouping of C. elegans
The N2 wild-type Bristol strain C. elegans used in this experiment were purchased from Caenorhabditis Genetics Center (University of Minnesota, MN, USA). They are usually cultured in nematode growth medium (NGM) plates (6 cm in diameter) at 20 °C and given E. coli OP50 as food [18]. Before each test, C. elegans were synchronized with the lysate (1 M NaOH solution: 5% NaClO solution = 1:1) to ensure that they could grow to L4 state at the same time. The eggs obtained by the synchronization operation could grow to the L4 stage after being cultured at 20 °C for 2.5 days in NGM plates [19].
All C. elegans are administered at the L4 stage. A temporary mixed solution (100 μL OP50 solution + 100 μL drug solution) was added dropwise to the surface of each NGM plate. The natural extracts used in this study were quercetin (Q4951, Sigma, St. Louis, MO, USA), luteolin (L107329, Aladdin, Los Angeles, CA, USA) and lycopene (FY1235, Feiyubio, Nantong, China). When these substances were dissolved, DMSO (D8370, Solarbio, Beijing, China) needed to be added so that the final concentration of DMSO was 0.5%. In the pre-experiment of the effect of monomers and mixtures on the inhibition of UVB-induced damage, the C. elegans were grouped as follows: (the dose is given in parentheses) CT (0.5% DMSO), UV (0.5% DMSO), Q (1 μM quercetin), Lu (1 μM luteolin), Ly (1 μM lycopene), QLu (0.5 μM quercetin + 0.5 μM luteolin), QLy (0.5 μM quercetin + 0.5 μM lycopene), Mixture (MIX, 0.33 μM quercetin + 0.33 μM luteolin + 0.33 μM lycopene). After that, we designed the mixture composition. In the experiment of quercetin and its mixture inhibiting UVB-induced damage, the grouping of elegans was as follows: CT (0.5% DMSO), UV (0.5% DMSO), Quercetin Low-dose (QL, 0.5 μM quercetin), Quercetin High-dose (QL, 1 μM quercetin), Mixture Low-dose (ML, 0.16 μM quercetin + 0.16 μM luteolin + 0.16 μM lycopene), Mixture High-dose (MH, 0.33 μM quercetin + 0.33 μM luteolin + 0.33 μM lycopene). We ensured that the total molar value of the drugs in each administration group was the same (1 μM). The NGM plates were changed daily with fresh drugs and OP50 were added during the administration period to prevent the drug from invaliding in the plate due to prolonged oxidation.
2.2. UV-B Irradiation
A UV Cross-linker (JRA03-II, Jieruian, WuXi, China) that can produce UV-B (312 nm) was used to irradiate the C. elegans at a dose of 100 J/M2, which is a more suitable dose that causes damage to C. elegans cells [1]. The irradiation operation was a multi-lamp irradiation mode (5 lamps) of the UV cross-linker. A UV integrator (UV-Int150, UV-Design, ZheJiang, China) was used to calibrate the exposure dose. When irradiating C. elegans, the covers of the NGM plates were removed, and it was confirmed that there was no liquid on the surface of the culture medium to exclude the interference of the liquid medium on the radiation dose.
2.3. Photographing of C. elegans
When photographing the results of each test, more than 10 C. elegans were transferred to a microscope slide with a 3% agarose pad. Ten microliters of a 30 μmol/mL NaN3 solution was added dropwise to anesthetize C. elegans [20]. Photographs of FITC (fluorescein isothiocyanate), and BF (bright field) were taken using a fluorescence electron microscope (Scope.A1, Carl Zeiss Jena Inc., Baden-Württemberg, Germany) as needed.
2.4. ROS Level Assay
There were two ROS level tests in this study, and two experiments were performed according to the group mentioned above. Thirty C. elegans grown to L4 state after synchronization in each group were transferred to a new NGM plate, and raised with OP50 solution mixed with different drugs for 48 h. After transferring them to new NGM plates, they were irradiated with UV-B (normal light was used in the CT group) at a dose of 100 J/M2, and then fed with OP50 without drugs for 6 h. The C. elegans was placed in 1.5 mL centrifuge tubes and washed twice with M9 buffer (3 g KH2PO4, 6 g Na2HPO4, 5 g NaCl, 1 mL of 1 M MgSO4, and H2O to 1 L), and then transferred to 100 μL M9 buffer containing 10 μmol 2′,7′-dichlorofluorescein (A601220, Sangon, Shanghai, China) for staining in the dark (20 °C, 30 min) [4]. Ten C. elegans were randomly selected from each group, and the dichlorofluorescein (DCF) was photographed using a fluorescence microscope (50X, FITC) as described above. Finally, Image J software (National Institutes of Health, Maryland) was used to analyze the fluorescence intensity of head muscle cells of C. elegans.
2.5. Lifespan Test
The synchronized L4 state C. elegans were transferred to NGM plates fed with different drugs according to groups. There was a total of 10 groups, and each group was repeated with 2 plates, with 25 worms on each plate [21]. Divided into UV treatment group (UV, QL-UV, QH-UV, ML-UV, MH-UV) and without UV treatment group (CT, QL, QH, ML, MH), a total of 500 worms were tested. The dosage was as described above. All groups were fed at 20 °C for 48 h, recorded as day 0, and they were no longer administered. On day 0, all C. elegans were transferred to new drug-free NGM plates and fed at 20 °C, and the UV-treated groups were irradiated with a dose of 100 J/M2 UV-B. From the first day, the number of alive and dead worms was recorded daily and the NGM medium was changed daily to prevent adult individuals from confounding with offspring individuals. The standard for the dead worms was no movement and swallowing, and no response after touching.
2.6. Gonadal Cell Apoptosis Test
The synchronized adult C. elegans were fed for 48 h using the same method of administration as described above, with 30 worms in each group. After transferring them to new NGM plates, they were irradiated with UV-B (normal light was used in the CT group) at a dose of 100 J/M2, and then fed with OP50 without drug for 6 h. They were washed twice with M9 buffer in 1.5 mL centrifuge tubes, and then transferred to 100 μL M9 buffer containing 1 mmol of Acridine orange (AO, A8120, Solarbio, Beijing, China) for staining in the dark (20 °C, 1 h). After that, they were transferred into new NGM plates for 1 h to let the worms excrete AO dye in the digestive tract. According to the above photographing method, the gonad arm cells of the worms were photographed under a fluorescence microscope 100× field of view (FITC) [22]. The apoptotic cells of each gonad arm were counted by the double-blind method.
2.7. Embryo Lethality Test
The synchronized L4 stage C. elegans were transferred to NGM plates with different drugs for 48 h. They were irradiated with UV-B at a dose of 100 J/M2 (normal light in the CT group). Six randomly selected individuals from each group were placed on blank NGM plates to lay eggs, with two worms per NGM plate, and three replicate plates per group. After allowing them to lay eggs at 25 °C for 4 h, they were transferred to new NGM plates and the number of eggs was counted. Subsequently, this operation was repeated twice. These C. elegans laid eggs for a total of 12 h at 25 °C. After 24 h at 25 ° C, non-hatched eggs (dead eggs) were counted on all NGM plates [23].
2.8. RT-qPCR Analysis of Transcription Levels of HUS-1, CEP-1, EGL-1, and CED-13
The synchronized L4 stage C. elegans were transferred to NGM plates with different drugs for 4 days, with 40 worms in each group. After transferring them to new NGM plates, they were irradiated with UV-B (normal light was used in the CT group) at a dose of 100 J/M2, and then fed with OP50 without drug for 6 h. After that, each group was transferred to a 1.5 mL centrifuge tube containing M9 buffer and washed 5 times to remove contaminants, respectively. Their RNA was extracted according to the instructions of the Ultrapure RNA Kit (CW0597, Cowin Bio, Beijing, China). According to the instructions, the obtained RNA was reverse transcribed into cDNA using PrimeScript ™ RT reagent Kit (RR047A, TaKaRa, Dalian, China), and quantitative PCR analysis was performed. The first strand of cDNA was synthesized in a multifunctional PCR instrument (ABI/ProFlex, Thermo Fisher Scientific, Waltham, MA, USA). Quantitative PCR was performed in the ABI 7300 PCR system (Thermo Fisher Scientific, Waltham, MA, USA) using TB Green Premix Ex Taq II with ROX (RR820A, TaKaRa, Dalian, China). Each PCR reaction system contained 20 uL, which consisted of 10 μL of TB Green Premix solution, 8 μM forward primer, 8 μM reverse primer, 0.4 μL Rox Dye and 2 μL cDNA. The reactions were performed in 200 μL PCR 8-Tube (F600552, BBI, Shanghai, China), and these tubes were loaded into 96 wells of the PCR system. The PCR cycle conditions were as follows: 1 cycle of 30 s at 95 °C for denaturation, and then 40 cycles of 5 s at 95 °C and 31 s at 60 °C for the PCR reaction. The melting curves were checked at the end of the cycle to confirm the specificity of the primers in each reaction. The PCR reaction was repeated three times. The results were calculated by the 2−ΔΔCt method. ACT-1 was used as a reference gene [24]. All primers used are shown in Table 1.

Table 1.
Primer sequence listing of PCR.
2.9. Statistical Analysis
All data in this paper were calculated and statistically analyzed (Student’s t-test and Log-rank test) using Microsoft Excel 2007 (Microsoft Corp., Washington, DC, USA) and GraphPad 7 (GraphPad Inc., San Diego, CA, USA) software packages.
3. Results
3.1. Initial Evaluation of Different Drugs on the Resistance to UV-B Stress by ROS Assay
In this experiment, the effects of different drugs on the resistance to UV-B stress were evaluated by the ROS level test in C. elegans, and the composition of quercetin mixture was designed based on the results. In the results of mean fluorescence intensity (MFI) of ROS (Figure 2), the MFI of UV group was significantly higher than that of other groups (** p < 0.01, Student’s t-test). The ROS level of MIX group (0.33 μM quercetin + 0.33 μM luteolin + 0.33 μM lycopene) decreased significantly and was lower than that of other drug treatment-groups (## p < 0.01, Student’s t-test). There were no significant differences between the mean fluorescence intensities of the other drug-treated groups. Therefore, in the subsequent experiments, we decided to use the composition of the MIX group as the mixture composition.
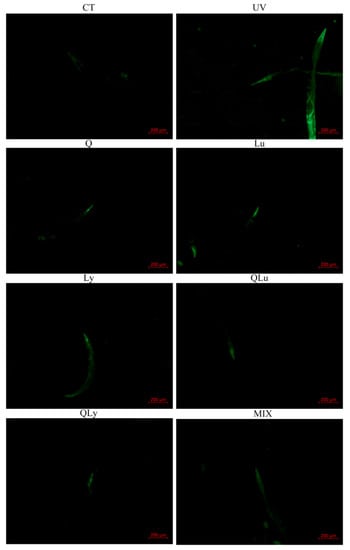
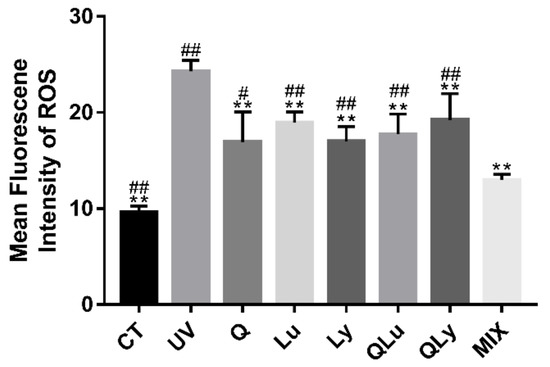
Figure 2.
The mean fluorescence intensity (MFI) results of C.elegans stained by 2′,7′-dichlorofluorescein show the reactive oxygen species (ROS) levels of every group. These pictures were taken in a 50× field of view (FITC). The higher the ROS level, the brighter the green fluorescence. All data are mean ± SEM and were analyzed by two-tailed Student’s t-test, n = 7, * p < 0.05, ** p < 0.01 compared to UV group. # p < 0.05, ## p < 0.01 compared to MIX group.
3.2. Quercetin and Its Mixture Helped C. elegans Resist UV-B Stress
Figure 3a shows the effect of quercetin and its mixture on the lifespan of C. elegans under UV-B stress. Among them, the CT group, UV group, and MH-UV group had a maximum survival duration of 24 days, 15 days, and 20 days, respectively. Table 2 shows the median survival duration of each group. The median survival duration of the CT group, UV group, and MH-UV group were 10.5 days, 4.5 days, and 7 days. The drug-treated group significantly prolonged worms after exposure to UV-B (Log-rank test), of which the MH-UV group performed the best (*** p < 0.001). Figure 3b shows the effect of quercetin and its mixture on the lifespan of normal C. elegans, which proves that the 48-h administration has no significant effect on the lifespan test (p > 0.05). These results indicate that quercetin and its mixture increase the resistance of C. elegans to UV-B stress.
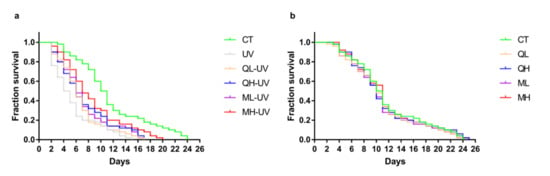
Figure 3.
Lifespan test results of C. elegans. (a) The survival curves of the drugs + UV (100 J/M2) treatment groups. (b) The survival curves of the drug treatment groups without UV irradiation (n = 50).

Table 2.
Analysis of survival curves for each UVB-treated group.
3.3. Quercetin and Its Mixture Inhibit UV-B-Induced Increase in ROS Levels
Figure 4 shows the inhibition effects of quercetin and its mixture at different dose on increased ROS levels caused by UV-B in C. elegans. UV-B dramatically increased ROS levels in worms. The levels of ROS in all drug treatment groups were significantly lower than those in the UV group (** p < 0.01, Student’s t-test), and the effect of the mixture high-dose (MH) was the most significant (## p < 0.01, Student’s t-test). In addition, Figure 3 shows bright-field photos of the same area of FITC.
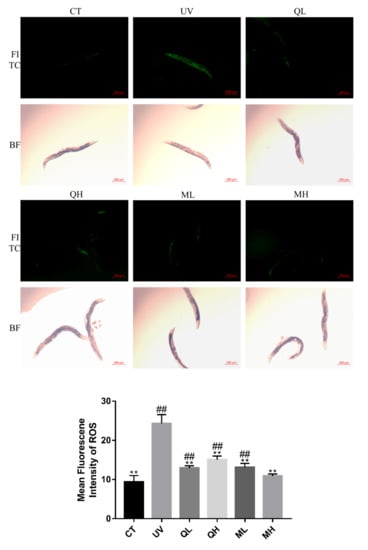
Figure 4.
The MFI results of C.elegans stained by 2′,7′-dichlorofluorescein show the ROS levels of every groups. These pictures were taken in a 50× field of view (FITC and BF). The higher the ROS level, the brighter the green fluorescence. All data are mean ± SEM and were analyzed by two-tailed Student’s t-test, n = 7, * p < 0.05, ** p < 0.01 compared to UV group. # p < 0.05, ## p < 0.01 compared to MH group.
3.4. Quercetin and Its Mixture Decreased DNA Damage of Germ Cells Caused by UV-B
Six hours after the C. elegans were exposed to UV-B radiation (100 J/M2), the apoptosis results (Figure 5) of the gonad arm show that UV-B caused DNA damage to germ cells, thereby increasing the level of apoptosis. The level of germ cell apoptosis in quercetin and its mixture group was significantly lower than that in the UV group (** p < 0.01). Similarly, in the results of dead embryo counts (Figure 6), the embryo lethality in the QH, ML, and MH groups was significantly lower than that in the UV group (** p < 0.01), and that of MH group was significantly lower than other drug-treatment groups (# p < 0.05). Therefore, quercetin and its mixture reduced DNA damage of gonad arm cells caused by UV-B in C. elegans.
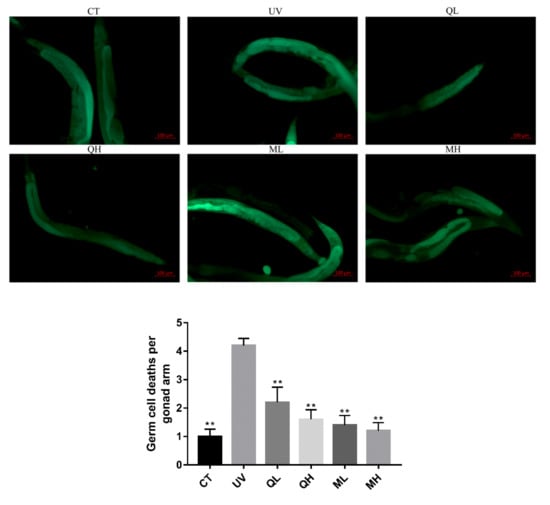
Figure 5.
Apoptosis results of gonad arm cells of every group 6 h after UV-B irradiation at a dose of 100 J/M2. These pictures were taken in a 100× field of view (FITC). The tiny yellow-green foci in the picture are the apoptotic cell stained by AO. All data are mean ± SEM and were analyzed by two-tailed Student’s t-test, n = 10, * p < 0.05, ** p < 0.01 compared to UV group.
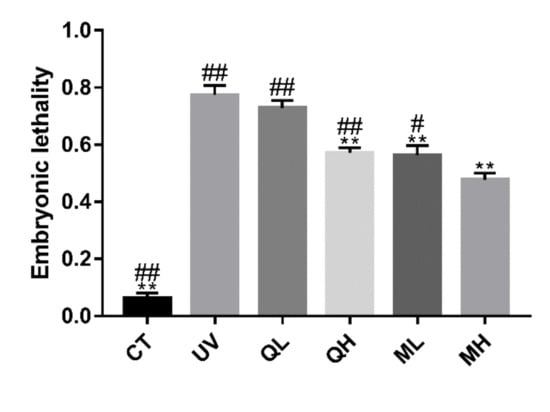
Figure 6.
Results of embryonic lethality of every group after UV-B irradiation (100 J/M2). All data are mean ± SEM and were analyzed by two-tailed Student’s t-test, n = 6, * p < 0.05, ** p < 0.01 compared to UV group. # p < 0.05, ## p < 0.01 compared to MH group.
3.5. Regulation of Quercetin and Its Mixture on Apoptosis Related Genes in C. elegans
Figure 7 shows that UV-B caused an increase in the expression level of apoptosis-related genes (HUS-1, CEP-1, EGL-1 and CED-13) in C. elegans. Except for the QL group, the drug treatment groups significantly reduced the expression levels of these genes (* p < 0.05, ** p < 0.01). The MH group had the most significant regulatory effect on HUS-1, CEP-1 and EGL-1 (# p < 0.05, ## p < 0.01). In the expression results of CED-13, the expression levels of MH and QH groups were both significantly lower than those of other treatment groups. This result indicates that quercetin and its mixture inhibited UV-B induced apoptosis in C. elegans.
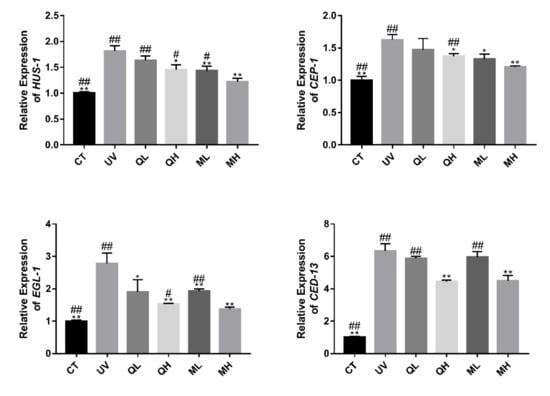
Figure 7.
Changes in the relative expression of apoptosis-related genes HUS-1, CEP-1, EGL-1 and CED-13. All data are mean ± SEM and were analyzed by two-tailed Student’s t-test, n = 3, * p < 0.05, ** p < 0.01 compared to UV group. # p < 0.05, ## p < 0.01 compared to MH group.
4. Discussion
In this study, we used ROS level assays to screen and design a mixture with UV-B resistance in C. elegans at first. After that, we verified that quercetin and its mixture can increase the resistance of C. elegans to UV-B stress by lifespan tests, ROS level tests, germ cell damage assessments, and RT-qPCR experiments of apoptosis-related genes. Among them, the high-dose mixture (0.33 μM quercetin + 0.33 μM luteolin + 0.33 μM lycopene) has the most significant resistance to cell damage caused by UV-B in C. elegans.
UV irradiation can cause a variety of cellular responses, including cell damage and apoptosis. The most critical mechanisms are the accumulation of reactive oxygen species and the mitochondrial pathway of apoptosis [25]. The large accumulation of ROS promotes oxidative stress and apoptosis, resulting in cellular DNA damage [26]. Therefore, in this study, the protective effect of quercetin, luteolin and lycopene on UV-B-induced damage was evaluated by using a convenient and rapid ROS level assay, and the composition of the quercetin mixture was designed based on the results. In subsequent results, quercetin and its mixture have been shown to significantly inhibit the increase in ROS levels caused by UV-B, similar to the results of previous studies by others [27]. In the lifespan experiment after UV-B irradiation, quercetin and its mixture significantly prolonged the maximum survival days and median survival days of C. elegans under UV-B stress. In addition, a 48-h drug treatment control experiment showed that this procedure did not significantly affect the lifespan of C. elegans without UV-B irradiation. These results indicate that quercetin and its mixture significantly improved the stress resistance of C. elegans, which is similar to the conclusions of others [28]. As the results show, the reduction in abnormal ROS levels and the increase in lifespan after UV irradiation means that quercetin and its mixture can reduce the level of oxidative stress. This may be one of the mechanisms that increase the resistance of C. elegans to UV-B stress.
Apoptosis is the process of programmed cell death controlled by intrinsic genes in order to maintain homeostasis under certain physiological conditions [29]. UV causes apoptosis through a complex process, including directly destroying DNA, increasing p53 levels, and directly or indirectly activating cell death receptors [30]. In the results of germ cell damage evaluation, quercetin and its mixture significantly reduced UV-B induced DNA damage, so the number of apoptotic cells in the gonad arms and dead embryos of the drug-treated group was significantly lower than that of the UV group. It shows that quercetin and its mixture reduce the DNA damage of C. elegans caused by UV-B by some mechanisms, thereby increasing the resistance stress of worms. Later, in RT-qPCR results of apoptosis-related genes, we discovered one of these mechanisms. After DNA is damaged by UV-B, the expression levels of pro-apoptotic genes EGL-1 and CED-13 are up-regulated [31]. During this classic apoptosis activation process, the mechanism of BH3 domain proteins in C. elegans is similar to that of mammals [12]. Therefore, the apoptosis response of EGL-1 and CED-13 corresponding DNA damage must be mediated by CEP-1 [32], and the activation of CEP-1 also requires the checkpoint protein HUS-1 [31]. The results of RT-qPCR show that quercetin and the mixture in the high-dose group could significantly inhibit the increase in expression levels of HUS-1, CEP-1, EGL-1 and CED-13 caused by UV-B, which explained their mechanism of inhibiting apoptosis [33]. However, the effects of quercetin on HUS-1, CEP-1 and CED-13 in the low-dose group were not significant.
Overall, this study found that a mixture of quercetin, luteolin, and lycopene can increase the resistance of C. elegans to UV-B stress. According to our findings in these experiments, these substances may increase the resistance of C. elegans by two mechanisms, including the inhibition of UV-B-induced abnormal oxidative stress and abnormal apoptosis. This is of great significance for the development of protective agents that increase UV resistance by using C. elegans as the animal model in the future. In addition, more detailed UV-resistant mechanisms of quercetin, luteolin and lycopene should be researched, and whether there is a biological synergy among them. Most importantly, future studies should be better performed using mice and monkeys to discover the protective mechanism of antioxidant drugs against UV-induced damage in mammals.
Author Contributions
Conceptualization, S.-m.L.; Data curation, S.-m.L.; Formal analysis, S.-m.L.; Funding acquisition, B.L.; Investigation, S.-m.L.; Methodology, B.L.; Project administration, B.L. and X.-h.C.; Resources, B.L. and X.-h.C.; Supervision, B.L. and X.-h.C.; Visualization, S.-m.L.; Writing—original draft, S.-m.L.; Writing—review & editing, S.-m.L., D.L. and Y.-l.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the 13th Five-year Plan of Marine Economic Innovation and Development Demonstration Project (No. HZHJ17), Fujian Agriculture and Forestry University International Cooperation Project (No. KXG15001A), and Fujian Agriculture and Forestry University Science and Technology Innovation Special Projects (No. CXZX2018055, CXZX2018056).
Acknowledgments
Authors wish to thank Ling-jun Zheng of University of Colorado Boulder for advice on experimental design.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| UV | ultraviolet |
| C. elegans | Caenorhabditis elegans |
| CPD | cyclobutane pyrimidine dimer |
| NER | nucleotide excision repair |
| ROS | reactive oxygen species |
| RT-qPCR | quantitative real time polymerase chain reaction |
| NGM | nematode growth medium |
| CT | control |
| Q | quercetin |
| Lu | luteolin |
| Ly | lycopene |
| MIX | mixture |
| QL | quercetin low dose |
| QH | quercetin high dose |
| ML | mixture low dose |
| MH | mixture high dose |
| DCF | dichlorofluorescein |
| AO | acridine orange |
| FITC | fluorescein isothiocyanate isomer |
| MFI | mean fluorescence intensity |
| BF | bright field. |
References
- Stergiou, L.; Doukoumetzidis, K.; Sendoel, A.; Hengartner, M.O. The nucleotide excision repair pathway is required for UV-C-induced apoptosis in Caenorhabditis elegans. Cell Death Differ. 2007, 14, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.P.; Hader, D.P. UV-induced DNA damage and repair: A review. Photochem. Photobiol. Sci. 2002, 1, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Artal-Sanz, M.; Tavernarakis, N. Prohibitin couples diapause signalling to mitochondrial metabolism during ageing in C. elegans. Nature 2009, 461, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Bian, P.; Liang, J.; Wang, Y.; Li, L.; Wang, J.; Yuan, H.; Chen, S.; Xu, A.; Wu, L. Synergistic Effects Induced by a Low Dose of Diesel Particulate Extract and Ultraviolet-A in Caenorhabditis elegans: DNA Damage-Triggered Germ Cell Apoptosis. Chem. Res. Toxicol. 2014, 27, 990–1001. [Google Scholar] [CrossRef]
- De Gruijl, F.R. Photocarcinogenesis: UVA vs. UVB Radiation. Skin Pharmacol. Physiol. 2002, 15, 316–320. [Google Scholar] [CrossRef]
- Patt, H.M.; Tyeee, E.B.; Straube, R.L.; Smith, D.E. Cysteine protection against X irradiation. Science 1949, 110, 213–214. [Google Scholar] [CrossRef]
- Mathews-Roth, M.M.; Krinsky, N.I. Carotenoid Dose Level And Protection Against Uv-B Induced Skin Tumors. Photochem. Photobiol. 1985, 42, 35–38. [Google Scholar] [CrossRef]
- Beggs, C.J.; Schneider-Ziebert, U.; Wellmann, E. UV-B Radiation and Adaptive Mechanisms in Plants; Springer: Berlin/Heidelberg, Germany, 1986; pp. 235–250. [Google Scholar]
- Weiss, J.F.; Landauer, M.R. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology 2003, 189, 1–20. [Google Scholar] [CrossRef]
- Sitailo, L.A.; Tibudan, S.S.; Denning, M.F. Activation of Caspase-9 Is Required for UV-induced Apoptosis of Human Keratinocytes. J. Biol. Chem. 2002, 277, 19346–19352. [Google Scholar] [CrossRef]
- Derry, W.B.; Putzke, A.P.; Rothman, J.H. Caenorhabditis elegans p53: Role in Apoptosis, Meiosis, and Stress Resistance. Science 2001, 294, 591–595. [Google Scholar] [CrossRef]
- Hofmann, E.R.; Milstein, S.; Boulton, S.J.; Ye, M.; Hofmann, J.J.; Stergiou, L.; Gartner, A.; Vidal, M.; Hengartner, M.O. Caenorhabditis elegans HUS-1 Is a DNA Damage Checkpoint Protein Required for Genome Stability and EGL-1-Mediated Apoptosis. Curr. Biol. 2002, 12, 1908–1918. [Google Scholar] [CrossRef]
- Fairlie, W.D.; Perugini, M.A.; Kvansakul, M.; Chen, L.; Huang, D.C.S.; Colman, P.M. CED-4 forms a 2:2 heterotetrameric complex with CED-9 until specifically displaced by EGL-1 or CED-13. Cell Death Differ. 2006, 13, 426–434. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, G.; Zhang, J.; Liu, L.; Sharma, S.; Dong, Q. Quercetin potentiates doxorubicin mediated antitumor effects against liver cancer through p53/Bcl-xl. PLoS ONE 2012, 7, e51764. [Google Scholar] [CrossRef] [PubMed]
- Erden Inal, M.; Kahraman, A. The protective effect of flavonol quercetin against ultraviolet an induced oxidative stress in rats1This research was supported by Osmangazi University Eskisehir-Turkey1. Toxicology 2000, 154, 21–29. [Google Scholar] [CrossRef]
- Kasala, E.R.; Bodduluru, L.N.; Barua, C.C.; Gogoi, R. Antioxidant and antitumor efficacy of Luteolin, a dietary flavone on benzo(a)pyrene-induced experimental lung carcinogenesis. Biomed. Pharmacother. 2016, 82, 568–577. [Google Scholar] [CrossRef]
- Kelkel, M.; Schumacher, M.; Dicato, M.; Diederich, M. Antioxidant and anti-proliferative properties of lycopene. Free Radical Res. 2011, 45, 925–940. [Google Scholar] [CrossRef]
- Brenner, S. The Genetics of Caenorhabditis Elegans. Genetics 1974, 77, 71–94. [Google Scholar] [PubMed]
- Au-Porta-de-la-Riva, M.; Au-Fontrodona, L.; Au-Villanueva, A.; Au-Cerón, J. Basic Caenorhabditis elegans Methods: Synchronization and Observation. JoVE 2012. [Google Scholar] [CrossRef]
- Gumienny, T.L.; Lambie, E.; Hartwieg, E.; Horvitz, H.R.; Hengartner, M.O. Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development 1999, 126, 1011. [Google Scholar] [PubMed]
- Murakami, S.; Johnson, T.E. A Genetic Pathway Conferring Life Extension and Resistance to UV Stress in Caenorhabditis elegans. Genetics 1996, 143, 1207–1218. [Google Scholar]
- Lettre, G.; Kritikou, E.A.; Jaeggi, M.; Calixto, A.; Fraser, A.G.; Kamath, R.S.; Ahringer, J.; Hengartner, M.O. Genome-wide RNAi identifies p53-dependent and -independent regulators of germ cell apoptosis in C. elegans. Cell Death Differ. 2004, 11, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhang, M.; Zheng, L.; Liang, Q.; Li, H.; Chen, J.-T.; Guo, H.; Oshina, S.Y.; Chen, Y.-Z.; Zhao, X.; et al. Cysteine protease cathepsin B mediates radiation-induced bystander effects. Nature 2017, 547, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Ly, K.; Reid, S.J.; Snell, R.G. Rapid RNA analysis of individual Caenorhabditis elegans. MethodsX 2015, 2, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, F.; Canonico, B.; Curci, R.; Battistelli, M.; Mannello, F.; Papa, S.; Tarzia, G.; Falcieri, E. Melatonin prevents apoptosis induced by UV-B treatment in U937 cell line. J. Pineal Res. 2006, 40, 158–167. [Google Scholar] [CrossRef]
- Simon, H.-U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-J.; Jeng, J.-Y.; Lin, C.-W.; Wu, C.-Y.; Chen, Y.-C. Quercetin inhibition of ROS-dependent and -independent apoptosis in rat glioma C6 cells. Toxicology 2006, 223, 113–126. [Google Scholar] [CrossRef]
- Kampkötter, A.; Nkwonkam, C.G.; Zurawski, R.F.; Timpel, C.; Chovolou, Y.; Wätjen, W.; Kahl, R. Investigations of protective effects of the flavonoids quercetin and rutin on stress resistance in the model organism Caenorhabditis elegans. Toxicology 2007, 234, 113–123. [Google Scholar] [CrossRef]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef]
- Kulms, D.; Schwarz, T. Molecular mechanisms of UV-induced apoptosis. Photodermatol. Photoimmunol. Photomed. 2000, 16, 195–201. [Google Scholar] [CrossRef]
- Nehme, R.; Conradt, B. egl-1: A key activator of apoptotic cell death in C. elegans. Oncogene 2008, 27, S30–S40. [Google Scholar] [CrossRef]
- Schumacher, B.; Schertel, C.; Wittenburg, N.; Tuck, S.; Mitani, S.; Gartner, A.; Conradt, B.; Shaham, S. C. elegans ced-13 can promote apoptosis and is induced in response to DNA damage. Cell Death Differ. 2005, 12, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, B.; Hanazawa, M.; Lee, M.-H.; Nayak, S.; Volkmann, K.; Hofmann, R.; Hengartner, M.; Schedl, T.; Gartner, A. Translational Repression of C. elegans p53 by GLD-1 Regulates DNA Damage-Induced Apoptosis. Cell 2005, 120, 357–368. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).