Milky Sap of Greater Celandine (Chelidonium majus L.) and Anti-Viral Properties
Abstract
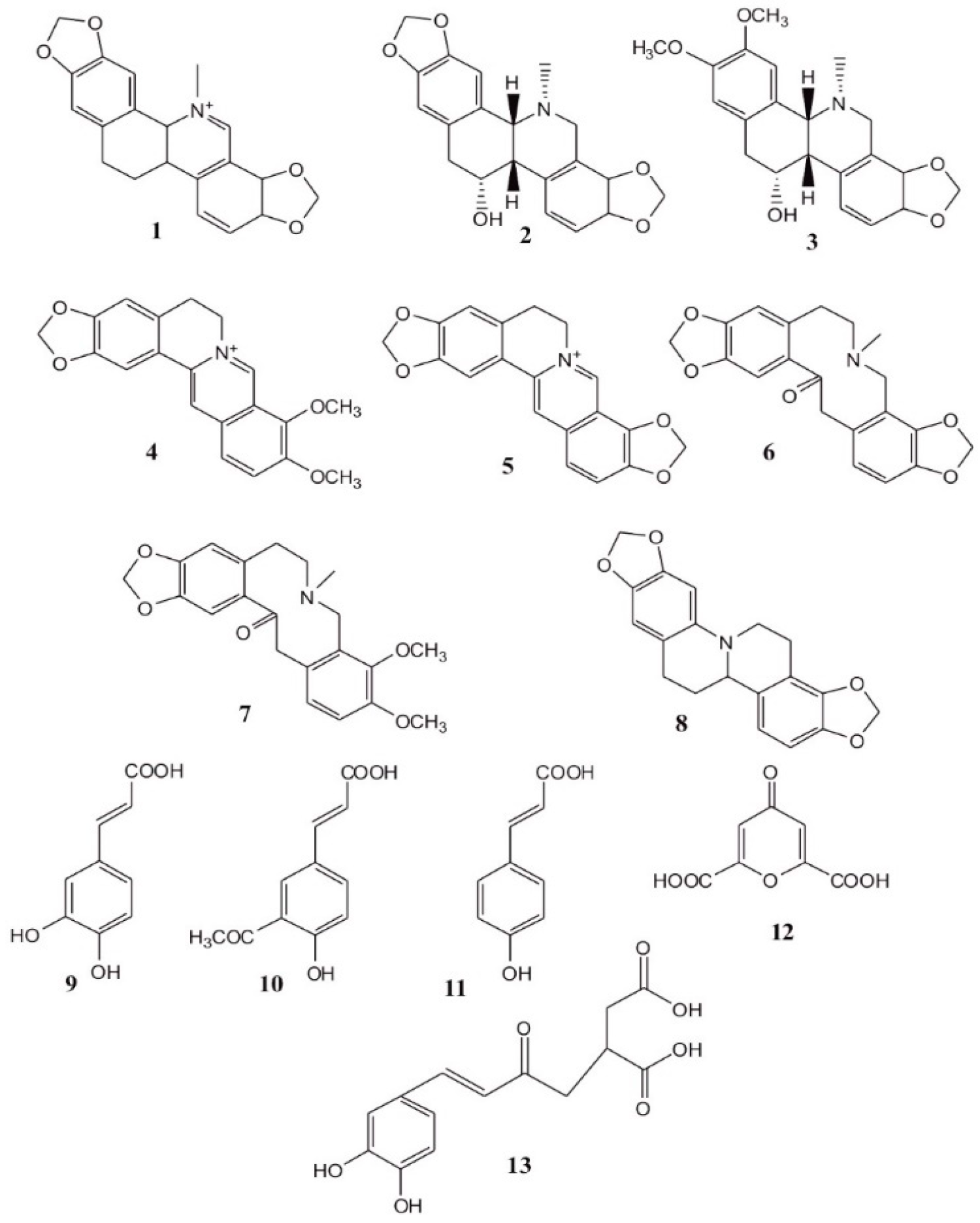
1. Introduction
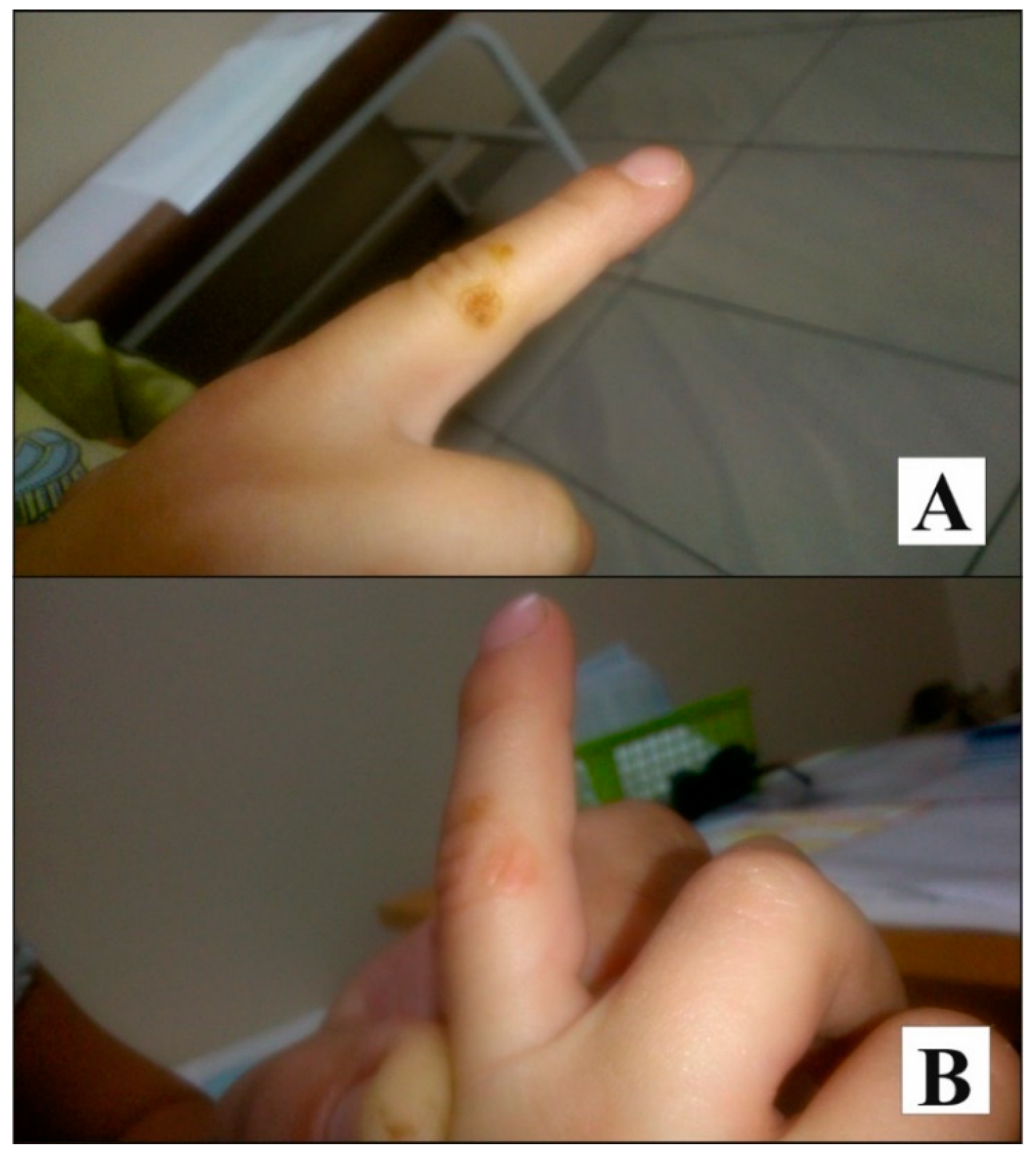
Case Presentation
2. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Biswas, S.J. Chelidoniummajus L.-A Review on Pharmacological activities and clinical effects. Glob. J. Res. Med. Plants Indigen. Med. 2013, 2, 238–245. [Google Scholar]
- Weiss, R.F. Lehrbuch der Phytotherapie; Hipppokrates Verlag GmbH: Stuttgart, Germany, 1980; p. 105. [Google Scholar]
- Dewick, P.M. Medicinal Natural Products a Biosynthetic Approach; John Wiley & Sons: Hoboken, NJ, USA, 1998; pp. 316–317. [Google Scholar]
- ESCOP Monographs. European Scientific Cooperative On Phytotherapy. In Chelidoniiherba, 2nd ed.; Thieme: New York, NY, USA, 2003; pp. 74–78. [Google Scholar]
- Glica, M.; Gaman, L.; Panait, E.; Stoian, I.; Atanasiu, V. Chelidoniummajus—An integrative review: Traditional knowledge versus modern findings. Forsch. Komplementmed. 2010, 17, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.F.; Fintelmann, V. Herbal Medicine, 2nd ed.; Thieme: Stuttgart, Germany; New York, NY, USA, 2000; p. 306. [Google Scholar]
- Colombo, M.L.; Bosisio, E. Pharmacological activities of Chelidoniummajus L. (Papaveraceae). Pharmacol. Res. 1996, 33, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Sokoloff, B.; Saelhof, C.C.; Takeuchi, Y.; Powella, R. The antitumor factors present in Chelidoniummajus L. Chelidonine an Protopine. Growth 1964, 28, 225–231. [Google Scholar] [PubMed]
- Kim, H.K.; Farnsworth, N.R.; Blomster, R.N.; Fong, H.H. Biological and phytochemical evaluation of plants. V. Isolation of two cytotoxic alkaloids from Chelidoniummajus. J. Pharm. Sci. 1969, 58, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Vavrecková, C.; Gawlik, I.; Müller, K. Benzophenanthridine alkaloids of Chelidoniummajus; II. Potent inhibitory action against the growth of human keratinocytes. Planta Med. 1996, 62, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Rogelj, B.; Popovic, T.; Ritonja, A.; Strukelj, B.; Brzin, J. Chelidocystatin, a novel phytocystatin from Chelidoniummajus. Phytochemistry 1998, 49, 1645–1649. [Google Scholar] [CrossRef]
- Philchenkov, A.; Kaminsky, V.; Zavelevich, M. Apoptogenic activity of two benzophenanthridine alkaloids from Chelidoniummajus L. does not correlate with their DNA damaging effects. Stoika R. Toxicol. In Vitro 2007, 22, 287–295. [Google Scholar] [CrossRef]
- Paul, A.; Bishayee, K.; Ghosh, S.; Mukherjee, A.; Sikdar, S.; Chakraborty, D.; Boujedaini, N.; Khuda-Bukhsh, A.R. Chelidonine isolated from ethanolic extract of Chelidoniummajus promotes apoptosis in HeLa cells through p38-p53 and PI3K/AKT signalling pathways. Zhong Xi Yi Jie He Xue Bao 2012, 10, 1025–1038. [Google Scholar] [CrossRef]
- Noureini, S.K.; Wink, M. Transcriptional down regulation of hTERT and senescence induction in HepG2 cells by chelidonine. World J. Gastroenterol. 2009, 15, 3603–3610. [Google Scholar] [CrossRef]
- Nawrot, R.; Wotuñ-Cholewa, M.; Gozdzicka-Józefiak, A. Nucleases isolated from Chelidoniummajus L. milky sap can induce apoptosis in human cervical carcinoma HeLa cells but not in Chinese Hamster Ovary CHO cells. Folia Histochem. Cytobiol. 2008, 46, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Culp, M.; Bragina, O. Capillary electrophoretic study of the synergistic biological effects of alkaloids from Chelidoniummajus L. in normal and cancer cells. Anal. Bioanal. Chem. 2013, 405, 3391–3397. [Google Scholar] [CrossRef] [PubMed]
- Riede, I. Chelidonium majus in der Therapie pramalinger Hautveranderungen. 2014. Available online: https://www.researchgate.net/publication/260197370_Chelidonium_majus_in_der_Therapie_pramaligner_Hautveranderungen (accessed on 26 February 2020).
- Song, J.Y.; Yang, H.O.; Pyo, S.N.; Jung, I.S.; Yi, S.Y.; Yun, Y.S. Immunomodulatory activity of protein-Bound polysaccharide extracted from Chelidoniummajus. Archives. Pharmacol. Res. 2002, 25, 158–164. [Google Scholar] [CrossRef]
- Chung, H.S.; An, H.J.; Jeong, H.J.; Won, J.H.; Hong, S.H.; Kim, H.M. Water extract isolated from Chelidoniummajus enhances nitric oxide and tumour necrosis factor-Alpha production via nuclear factorkappa B activation in mouse peritoneal macrophages. J. Pharm. Pharmacol. 2004, 56, 129–134. [Google Scholar] [CrossRef]
- Khmel’nitskaia, N.M.; Vorob’ev, K.V.; Kliachko, L.L.; Ankhimova, E.S.; Kosenko, V.A.; Tymova, E.V.; Mal’seva, G.S.; Medvedev, E.A. A comparative study of conservative treatment schemes in chronic tonsillitis in children. Vest. Otorinolaringol. 1998, 4, 39–42. [Google Scholar]
- Pavao, M.L.; Pinto, R.E. Sensitivity of Bacillus subtilis to water soluble alkaloid extracts from Azores Chelidonium majus L. (Papaveraceae) roots. Arquipdago. Life Mar. Sci. 1995, 13, 93–97. [Google Scholar]
- Zuo, G.Y.; Meng, F.Y.; Hao, X.Y.; Zhang, Y.L.; Wang, G.C.H.; Xu, G.L. Antibacterial alkaloids from Chelidoniummajus Linn (Papaveraceae) against clinical isolates of methicillin-Resistant Staphylococcus aureaus. J. Pharm. Pharmaceut. Sci. 2008, 11, 90–94. [Google Scholar] [CrossRef]
- Lendfeld, J.; Kroutil, M.; Marsalek, E.; Slavik, J.; Preininger, V.; Simanek, V. Isolation, chemistry and biology of alkaloids from plants of the papaveraceae: Antiinflammatory activity of quaternary benzophenanthridine alkaloids from Chelidoniummajus. Planta Med. 1981, 43, 161–165. [Google Scholar] [CrossRef]
- Gerencer, M.; Turecek, P.L.; Kistner, O.; Mitterer, A.; Savidis-Dacho, H.; Barrett, N.P. In vitro and in vivo anti-retroviral activity of the substance purified from the aqueous extract of Chelidoniummajus L. Antivir. Res. 2006, 72, 153–156. [Google Scholar] [CrossRef]
- Lozjuk, R.M.; Lisnyak, O.I.; Lozjuk, L.V. Theoretical grounds and experimental confirmation of the antiviral effect of the preparation Ukrain. Drugs Exp. Clin. Res. 1996, 22, 213–217. [Google Scholar]
- Kery, R.Y.; Horvath, J.; Nasz, I.; Verzar-Petri, G.; Kulcsar, G.; Dan, P. Antiviral alkaloid in Chelidoniummajus L. Acta Pharmaceu. Hungerica 1987, 57, 19–25. [Google Scholar]
- Horvath, J. Antiviral effect of Chelidonium extracts. In Proceedings of the 13th International Congress of Chemotherapy, Egermann, Vienna, 28 August–2 September 1983. [Google Scholar]
- Zielińska, S.; Jezierska-Domaradzka, A.; Wójciak-Kosior, M.; Sowa, I.; Junka, A.; Matkowski, A.M. Greater Celandine’s Ups and Downs-21 Centuries of Medicinal Uses of Chelidoniummajus From the Viewpoint of Today’s Pharmacology. Front Pharmacol. 2018, 9, 299. [Google Scholar] [CrossRef] [PubMed]
- Crijns, A.P.; de Smet, P.A.; van den Heuvel, M.; Schot, B.W.; Haagsma, E.B. Acute hepatitis after use of a herbal preparation with greater celandine (Chelidoniummajus). Ned. Tijdschr. Geneeskd. 2002, 146, 100–102. [Google Scholar]
- Haderman, E.; van Overbeke, L.; Ilegems, S.; Ferrante, M. Acute hepatitis induced by greater celandine (Chelidoniummajus). Acta Gastroenterol. Belg. 2008, 71, 281–282. [Google Scholar]
- Moro, P.A.; Cassetti, F.; Giugliano, G.; Falce, M.T.; Mazzanti, G.; Menniti-Ippolito, F.; Raschetti, R.; Santuccio, C. Hepatitis from greater celandine (Chelidoniummajus L.): Review of literature and report of a new case. J. Ethnopharmacol. 2009, 124, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe. Active Ingredients Used in Cosmetics: Safety Survey; Council of Europe Publishing: Strasbourg, France, 2008. [Google Scholar]
- Biswas, J.; Bhattacharjee, N.; Khuda-Bukhsh, A.R. Efficacy of a plant extract (Chelidoniummajus L.) in combating induced hepatocarcinogenesis in mice. Food Chem. Toxicol. 2008, 46, 1474–1487. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Sur, R.K.; Roy, A.; Mukherjee, A.S. Effect of Chelidoniummajus L. on experimental hepatic tissue injury. Phytother. Res. 1996, 10, 354–356. [Google Scholar] [CrossRef]
- Mitra, S.; Gole, M.; Samajdar, K.; Sur, R.K.; Chakraborty, B.N. Antihepatotoxic activity of Chelidoniummajus. Int. J. Pharmacogn. 1992, 30, 125–128. [Google Scholar] [CrossRef]
- Jakovljević, Z.D.; Stanković, S.M.; Topuzović, D.M. Seasonal variability of Chelidoniummajus L. secondary metabolites content and antioxidant activity. EXCLI J. 2013, 12, 260–268. [Google Scholar]
| Characteristics | Methods of Cutaneous Warts Therapy | ||||
|---|---|---|---|---|---|
| Cryotherapy (Freezing) | Peeling (Salicylic Acid) | Laser Treatment | Minor Surgery (Electrosurgery, Excision, Curettage) | Milky Sap from Ch. majus | |
| Complication (pain, blister formation, hemorrhage, infection, excessive granulation tissue formation, hyper-/hypo- pigmentation) | + | − | + | + | − |
| Availability | + | + | − | − | + |
| Low cost | + | + | − | − | + |
| Time (a long-term treatment, long recovery periods) | + | + | − | + | − |
| Lesions may become recalcitrant | − | + | − | − | − |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawrot, J.; Wilk-Jędrusik, M.; Nawrot, S.; Nawrot, K.; Wilk, B.; Dawid-Pać, R.; Urbańska, M.; Micek, I.; Nowak, G.; Gornowicz-Porowska, J. Milky Sap of Greater Celandine (Chelidonium majus L.) and Anti-Viral Properties. Int. J. Environ. Res. Public Health 2020, 17, 1540. https://doi.org/10.3390/ijerph17051540
Nawrot J, Wilk-Jędrusik M, Nawrot S, Nawrot K, Wilk B, Dawid-Pać R, Urbańska M, Micek I, Nowak G, Gornowicz-Porowska J. Milky Sap of Greater Celandine (Chelidonium majus L.) and Anti-Viral Properties. International Journal of Environmental Research and Public Health. 2020; 17(5):1540. https://doi.org/10.3390/ijerph17051540
Chicago/Turabian StyleNawrot, Joanna, Małgorzata Wilk-Jędrusik, Sylwia Nawrot, Krzysztof Nawrot, Barbara Wilk, Renata Dawid-Pać, Maria Urbańska, Iwona Micek, Gerard Nowak, and Justyna Gornowicz-Porowska. 2020. "Milky Sap of Greater Celandine (Chelidonium majus L.) and Anti-Viral Properties" International Journal of Environmental Research and Public Health 17, no. 5: 1540. https://doi.org/10.3390/ijerph17051540
APA StyleNawrot, J., Wilk-Jędrusik, M., Nawrot, S., Nawrot, K., Wilk, B., Dawid-Pać, R., Urbańska, M., Micek, I., Nowak, G., & Gornowicz-Porowska, J. (2020). Milky Sap of Greater Celandine (Chelidonium majus L.) and Anti-Viral Properties. International Journal of Environmental Research and Public Health, 17(5), 1540. https://doi.org/10.3390/ijerph17051540





