Parameters of Oxidative Stress in Reproductive and Postmenopausal Mexican Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Blood Samples
2.3. Biomarkers of Oxidative Stress
2.4. Sample Size
2.5. Statistics
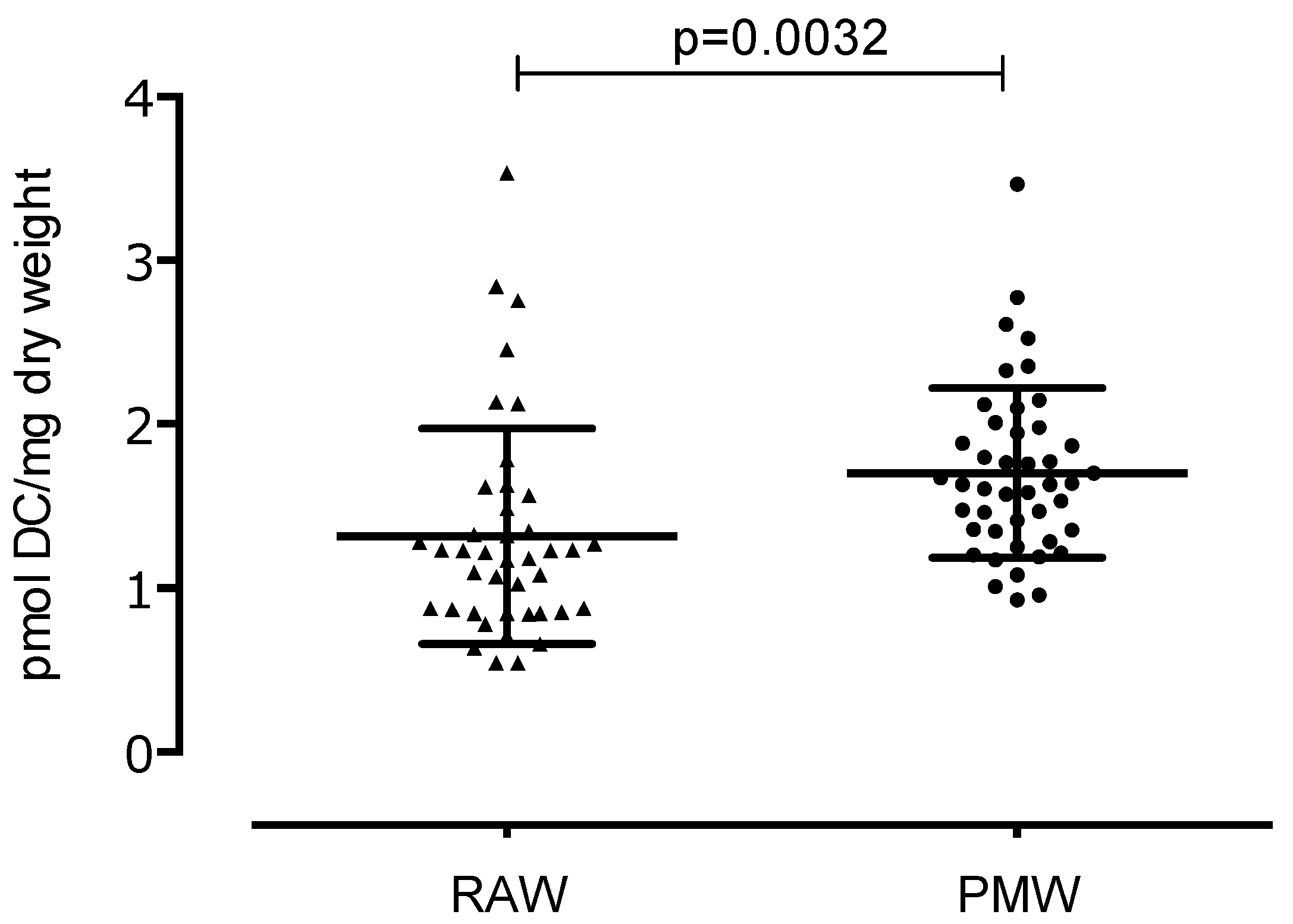
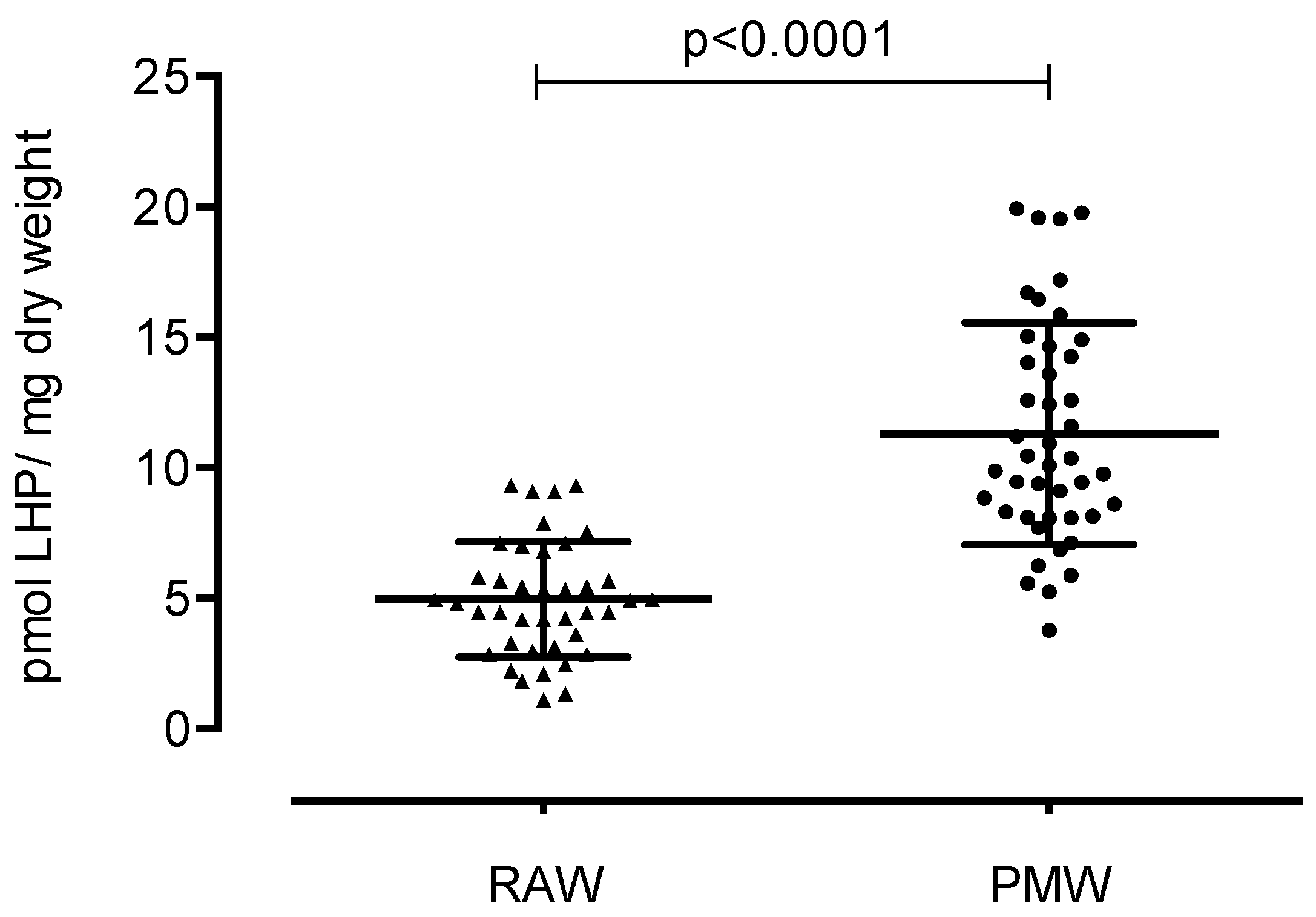
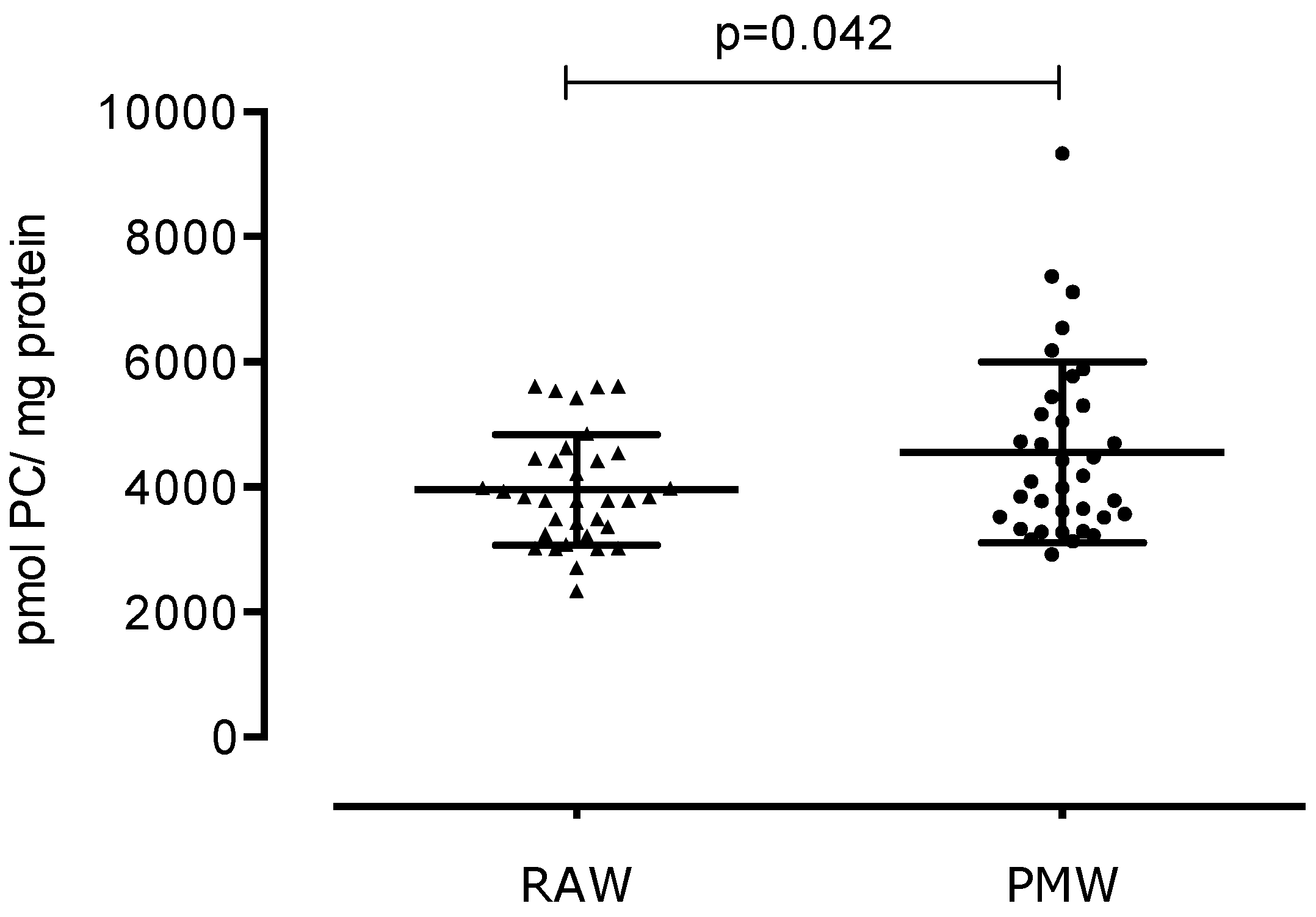
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hale, G.E.; Robertson, D.M.; Burger, H.G. The perimenopausal woman: Endocrinology and management. J. Steroid Biochem. Mol. Biol. 2014, 142, 121–131. [Google Scholar] [CrossRef]
- Unfer, T.C.; Figueiredo, C.G.; Zanchi, M.M.; Maurer, L.H.; Kemerich, D.M.; Duarte, M.M.F.; Konopka, C.K.; Emanuelli, T. Estrogen plus progestin increase superoxide dismutase and total antioxidant capacity in postmenopausal women. Climacteric 2015, 18, 379–388. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sekhon, L.; Shah, R. Redox considerations in female reproductive function and assisted reproduction: From molecular mechanisms to health implications. Antioxid Redox Signal. 2008, 10, 1375–1403. [Google Scholar] [CrossRef] [PubMed]
- Moldogazieva, N.; Mokhosoev, I.; Mel’Nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 3085756. [Google Scholar] [CrossRef]
- Kaur, G.; Kathariya, R.; Bansal, S.; Singh, A.; Shahakar, D. Dietary antioxidants and their indispensable role in periodontal health. J. Food Drug Anal. 2016, 24, 239–246. [Google Scholar] [CrossRef]
- Kolesnikova, L.; Semenova, N.; Madaeva, I.; Suturina, L.; Solodova, E.; Grebenkina, L.; Darenskaya, M.A. Antioxidant status in peri- and postmenopausal women. Maturitas 2015, 81, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk, M.; Nowicka, G. Obesity, DNA Damage, and Development of Obesity-Related Diseases. Int. J. Mol. Sci. 2019, 20, 1146. [Google Scholar] [CrossRef] [PubMed]
- Lizcano, F.; Guzmán, G. Estrogen Deficiency and the Origin of Obesity during Menopause. BioMed Res. Int. 2014, 2014, 757461. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive Species and Antioxidants. Redox Biology Is a Fundamental Theme of Aerobic Life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Keaney, J.; Larson, M.G.; Vasan, R.S.; Wilson, P.W.F.; Lipinska, I.; Corey, D.; Massaro, J.; Sutherland, P.; Vita, J.A.; Benjamin, E.J. Obesity and Systemic Oxidative Stress: Clinical correlates of oxidative stress in the Framingham Study. Arter. Thromb. Vasc. Boil. 2003, 23, 434–439. [Google Scholar] [CrossRef]
- Demirbag, R.; Yilmaz, R.; Erel, O. The association of total antioxidant capacity with sex hormones. Scand. Cardiovasc. J. 2005, 39, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Videla, L.A. The importance of the long-chain polyunsaturated fatty acid n-6/n-3 ratio in development of non-alcoholic fatty liver associated with obesity. Food Funct. 2011, 2, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Miquel, J.; Ramírez-Boscá, A.; Ramírez-Bosca, J.V.; Alperi, J.D. Menopause: A review on the role of oxygen stress and favorable effects of dietary antioxidants. Arch. Gerontol. Geriatr. 2006, 42, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Victorino, V.J.; Panis, C.; Campos, F.C.; Cayres, R.C.; Colado-Simão, A.N.; Oliveira, S.R.; Herrera, A.C.S.A.; Cecchini, A.; Cecchini, R. Decreased oxidant profile and increased antioxidant capacity in naturally postmenopausal women. Age 2013, 35, 1411–1421. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taleb-Belkadi, O.; Chaib, H.; Zemour, L.; Fatah, A.; Chafi, B.; Mekki, K. Lipid profile, inflammation, and oxidative status in peri- and postmenopausal women. Gynecol. Endocrinol. 2016, 32, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, N.; Vitaglione, P.; Granato, D.; Fogliano, V. Twenty-five years of total antioxidant capacity measurement of foods and biological fluids: Meritsand limitations. J. Sci. Food Agric. 2018. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhu, L.; Zhang, D.; Li, N.; Li, Q.; Dai, P.; Mao, Y.; Li, X.; Ma, J.; Huang, S. Oxidative Stress-Related Biomarkers in Postmenopausal Osteoporosis: A Systematic Review and Meta-Analyses. Dis. Markers 2016, 2016, 7067984. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Arguelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 1–31. [Google Scholar] [CrossRef]
- Recknagel, R.O.; Glende, E.A., Jr. Spectrophotometric detection of lipid conjugated dienes. Methods Enzymol. 1984, 105, 331–337. [Google Scholar]
- El-Saadani, M.; Esterbauer, H.; El-Sayed, M.; Goher, M.; Nassar, A.Y.; Jürgens, G. A spectrophotometric assay for lipid peroxides in serum lipoproteins using a commercially available reagent. J. Lipid Res. 1989, 30, 627–630. [Google Scholar]
- Gérard-Monnier, D.; Erdelmeier, I.; Régnard, K.; Moze-Henry, N.; Yadan, J.C.; Chaudière, J. Reactions of 1-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Analytical applications to a colorimetric assay of lipid peroxidation. Chem. Res. Toxicol. 1998, 11, 1176–1183. [Google Scholar]
- Dalle-Donne, I.; Aldini, G.; Carini, M.; Colombo, R.; Rossi, R.; Milzani, A. Protein carbonylation, cellular dysfunction, and disease progression. J. Cell. Mol. Med. 2006, 10, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S.E.; Altun, M. Total antioxidant capacity assay of human serum using copper(II)-neocuproine as chromogenic oxidant: The CUPRAC method. Free Radic. Res. 2005, 39, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Spickett, C.M. The lipid peroxidation product 4-hydroxy-2-nonenal: Advances in chemistry and analysis. Redox Biol. 2013, 1, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Lopes, H.F.; Morrow, J.D.; Stojiljkovic, M.P.; Goodfriend, T.L.; Egan, B.M. Acute hyperlipidemia increases oxidative stress more in African Americans than in white Americans. Am. J. Hypertens. 2003, 16, 331–336. [Google Scholar] [CrossRef]
- Morris, A.A.; Zhao, L.; Patel, R.S.; Jones, D.P.; Ahmed, Y.; Stoyanova, N.; Gibbons, G.H.; Vaccarino, V.; Din-Dzietham, R.; Quyyumi, A.A. Differences in systemic oxidative stress based on race and the metabolic syndrome: The Morehouse and Emory Team up to Eliminate Health Disparities (META-Health) study. Metab. Syndr. Relat. Disord. 2012, 10, 252–259. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, M.A.; Zacarías-Flores, M.; Arronte-Rosales, A.; Correa-Muñoz, E.; Mendoza-Núñez, V.M. Menopause as risk factor for oxidative stress. Menopause 2012, 19, 361–367. [Google Scholar] [CrossRef]
- Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin. Biochem. 2004, 37, 112–119. [Google Scholar] [CrossRef]
- Ganjifrockwala, F.; Joseph, J.; George, G. Decreased total anti- oxidant levels and increased oxidative stress in South African type 2 diabetes mellitus patients. J. Endocrinol. Metab. Diabetes S. Afr. 2017, 22, 21–25. [Google Scholar]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, M.A.; Zacarias-Flores, M.; Arronte-Rosales, A.; Mendoza-Nunez, V.M. Oxidative Stress Is Increased with the Severity of Hot Flashes in Postmenopausal Women. Free Radic. Biol. Med. 2015, 87, S132. [Google Scholar] [CrossRef]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex Differ. 2017, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.P.; Fouquerel, E.; Opresko, P.L. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech. Ageing Dev. 2019, 177, 37–45. [Google Scholar] [CrossRef]
- Zhang, Y.; Unnikrishnan, A.; Deepa, S.S.; Liu, Y.; Li, Y.; Ikeno, Y.; Sosnowska, D.; Van Remmen, H.; Richardson, A. A new role for oxidative stress in aging: The accelerated aging phenotype in Sod1-/- mice is correlated to increased cellular senescence. Redox Biol. 2017, 11, 30–37. [Google Scholar] [CrossRef]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019, 20, E4472. [Google Scholar] [CrossRef]
- Rangel-Zuñiga, O.A.; Cruz-Teno, C.; Haro, C.; Quintana-Navarro, G.M.; Camara-Martos, F.; Perez-Martinez, P.; Garcia-Rios, A.; Garaulet, M.; Tena-Sempere, M.; Lopez-Miranda, J.; et al. Differential menopause- versus aging-induced changes in oxidative stress and circadian rhythm gene markers. Mech. Ageing Dev. 2017, 164, 41–48. [Google Scholar] [CrossRef]
- Lacoste, M.G.; Ponce, I.T.; Golini, R.L.; Delgado, S.M.; Anzulovich, A.C. Aging modifies daily variation of antioxidant enzymes and oxidative status in the hippocampus. Exp. Gerontol. 2017, 88, 42–50. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef]
- Hernandez-Rodas, M.C.; Valenzuela, R.; Videla, L.A. Relevant Aspects of Nutritional and Dietary Interventions in Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2015, 16, 25168–25198. [Google Scholar] [CrossRef]


| RAW (n = 40) | PMW (n = 44) | p Values | |
|---|---|---|---|
| Age (years) | 25.4 ± 0.27 | 55.06 ± 3.69 | p < 0.0001 |
| Weight (kg) | 52.2 ± 2.14 | 68.9 ± 11.3 | p < 0.001 |
| BMI (kg/m2) | 22.3 ± 1.80 | 28.7 ± 4.60 | p < 0.001 |
| Beta | t | p Values | |
|---|---|---|---|
| DC | |||
| Age (years) | 0.298 | 2.036 | 0.045 |
| Weight (kg) | −0.105 | −0.328 | 0.744 |
| Body mass index (kg/m2) | 0.114 | 0.357 | 0.722 |
| LHP | |||
| Age (years) | 0.57 | 5.045 | <0.001 |
| Weight (kg) | −0.020 | −0.082 | 0.935 |
| Body mass index (kg/m2) | 0.169 | 0.683 | 0.496 |
| MDA | |||
| Age (years) | 0.165 | 1.103 | 0.274 |
| Weight (kg) | −0.013 | −0.038 | 0.97 |
| Body mass index (kg/m2) | 0.117 | 0.358 | 0.721 |
| PC | |||
| Age (years) | 0.113 | 0.711 | 0.48 |
| Weight (kg) | 0.816 | 2.358 | 0.021 |
| Body mass index (kg/m2) | −0.674 | −1.999 | 0.05 |
| TAC | |||
| Age (years) | 0.167 | 1.123 | 0.265 |
| Weight (kg) | 0.407 | 1.123 | 0.265 |
| Body mass index (kg/m2) | −0.348 | −1.068 | 0.289 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montoya-Estrada, A.; Velázquez-Yescas, K.G.; Veruete-Bedolla, D.B.; Ruiz-Herrera, J.D.; Villarreal-Barranca, A.; Romo-Yañez, J.; Ortiz-Luna, G.F.; Arellano-Eguiluz, A.; Solis-Paredes, M.; Flores-Pliego, A.; et al. Parameters of Oxidative Stress in Reproductive and Postmenopausal Mexican Women. Int. J. Environ. Res. Public Health 2020, 17, 1492. https://doi.org/10.3390/ijerph17051492
Montoya-Estrada A, Velázquez-Yescas KG, Veruete-Bedolla DB, Ruiz-Herrera JD, Villarreal-Barranca A, Romo-Yañez J, Ortiz-Luna GF, Arellano-Eguiluz A, Solis-Paredes M, Flores-Pliego A, et al. Parameters of Oxidative Stress in Reproductive and Postmenopausal Mexican Women. International Journal of Environmental Research and Public Health. 2020; 17(5):1492. https://doi.org/10.3390/ijerph17051492
Chicago/Turabian StyleMontoya-Estrada, Araceli, Karla Guadalupe Velázquez-Yescas, Daniela Belen Veruete-Bedolla, José David Ruiz-Herrera, Alma Villarreal-Barranca, José Romo-Yañez, Guillermo Federico Ortiz-Luna, Arturo Arellano-Eguiluz, Mario Solis-Paredes, Arturo Flores-Pliego, and et al. 2020. "Parameters of Oxidative Stress in Reproductive and Postmenopausal Mexican Women" International Journal of Environmental Research and Public Health 17, no. 5: 1492. https://doi.org/10.3390/ijerph17051492
APA StyleMontoya-Estrada, A., Velázquez-Yescas, K. G., Veruete-Bedolla, D. B., Ruiz-Herrera, J. D., Villarreal-Barranca, A., Romo-Yañez, J., Ortiz-Luna, G. F., Arellano-Eguiluz, A., Solis-Paredes, M., Flores-Pliego, A., Espejel-Nuñez, A., Estrada-Gutierrez, G., & Reyes-Muñoz, E. (2020). Parameters of Oxidative Stress in Reproductive and Postmenopausal Mexican Women. International Journal of Environmental Research and Public Health, 17(5), 1492. https://doi.org/10.3390/ijerph17051492








