Biosorption Characteristics of Hg(II) from Aqueous Solution by the Biopolymer from Waste Activated Sludge
Abstract
1. Introduction
2. Materials and Methods
2.1. Biopolymer and Reagents
2.2. Adsorption Kinetics Analyses
2.3. Adsorption Isotherms and Thermodynamics Analyses
2.4. Physicochemical Analyses
3. Results and Discussion
3.1. Adsorption Kinetics of Hg(II) by the Biopolymer
3.2. Adsorption Isotherms and Thermodynamics of Hg(II) by the Biopolymer
3.3. Adsorption Mechanisms of Hg(II) by the Biopolymer
3.3.1. Functional Groups Analyses
3.3.2. Surface Morphology Analyses
3.3.3. Elemental Components Analyses
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Awual, M.R. Novel nanocomposite materials for efficient and selective mercury ions capturing from wastewater. Chem. Eng. J. 2017, 307, 456–465. [Google Scholar] [CrossRef]
- Gurgel, L.V.A.; Junior, O.K.; Gil, R.P.D.F.; Gil, L.F. Adsorption of Cu(II), Cd(II), and Pb(II) from aqueous single metal solutions by cellulose and mercerized cellulose chemically modified with succinic anhydride. Bioresour. Technol. 2008, 99, 3077–3083. [Google Scholar] [CrossRef]
- Turaga, R.M.R.; Howarth, R.B.; Borsuk, M.E. Perceptions of mercury risk and its management. Hum. Ecol. Risk Assess. 2014, 20, 1385–1405. [Google Scholar] [CrossRef]
- Hsu-Kim, H.; Kucharzyk, K.H.; Zhang, T.; Deshusses, M.A. Mechanisms regulating mercury bioavailability for methylating microorganisms in the aquatic environment: A critical review. Environ. Sci. Technol. 2013, 47, 2441–2456. [Google Scholar] [CrossRef]
- Mortazavi, S.M.J.; Mortazavi, G.; Paknahad, M. A review on the distribution of Hg in the environment and its human health impacts. J. Hazard. Mater. 2016, 310, 278–279. [Google Scholar] [CrossRef] [PubMed]
- Oehmen, A.; Vergel, D.; Fradinho, J.; Reis, M.A.M.; Crespo, J.G.; Velizarov, S. Mercury removal from water streams through the ion exchange membrane bioreactor concept. J. Hazard. Mater. 2014, 264, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Das, N. Recovery of precious metals through biosorption-a review. Hydrometallurgy 2010, 103, 180–189. [Google Scholar] [CrossRef]
- Lim, A.P.; Aris, A.Z. A review on economically adsorbents on heavy metals removal in water and wastewater. Rev. Environ. Sci. Biol. 2014, 13, 163–181. [Google Scholar] [CrossRef]
- Zhang, D.; Pan, X.; Mostofa, K.M.G.; Chen, X.; Mu, G.; Wu, F.; Liu, J.; Song, W.; Yang, J.; Liu, Y.; et al. Complexation between Hg(II) and biofilm extracellular polymeric substances: An application of fluorescence spectroscopy. J. Hazard. Mater. 2010, 175, 359–365. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Babalola, O.O. A New Strategy for Heavy Metal Polluted Environments: A Review of Microbial Biosorbents. Int. J. Environ. Res. Pub. Health 2017, 14, 94. [Google Scholar] [CrossRef]
- Toner, B.; Manceau, A.; Marcus, M.A.; Millet, D.B.; Sposito, G. Zinc sorption by a bacterial biofilm. Environ. Sci. Technol. 2005, 39, 8288–8294. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.B.; Wu, S.T.; Liu, G.; Liao, X.K.; Deng, X.; Sun, D.H.; Hu, Y.L.; Huang, Y.L. Simultaneous biosorption of cadmium (II) and lead (II) ions by pretreated biomass of Phanerochaete chrysosporium. Sep. Purif. Technol. 2004, 34, 135–142. [Google Scholar] [CrossRef]
- Kusvuran, E.; Yildirim, D.; Samil, A.; Gulnaz, O. A study: Removal of Cu(II), Cd(II), and Pb(II) ions from real industrial water and contaminated water using activated sludge biomass. Clean-Soil Air Water 2012, 40, 1273–1283. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, J.; Wen, Y.; Jiang, L.; Wu, Y. Biosorption of heavy metal ions (Cu2+, Mn2+, Zn2+, and Fe3+) from aqueous solutions using activated sludge: Comparison of aerobic activated sludge with anaerobic activated sludge. Appl. Biochem. Biotech. 2012, 168, 2079–2093. [Google Scholar] [CrossRef] [PubMed]
- Sulaymon, A.H.; Yousif, S.A.; Al-Faize, M.M. Single-multicomponent biosorption of lead mercury chromium and arsenic onto activated sludge in batch and fixed-bed adsorber. Desalin. Water Treat. 2015, 53, 3499–3512. [Google Scholar] [CrossRef]
- Sheng, G.; Xu, J.; Li, W.; Yu, H. Quantification of the interactions between Ca2+, Hg2+ and extracellular polymeric substances (EPS) of sludge. Chemosphere 2013, 93, 1436–1441. [Google Scholar] [CrossRef]
- Sheng, G.; Yu, H.; Li, X. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef]
- Zhou, Y.; Xia, S.; Nguyen, B.T.; Long, M.; Zhang, J.; Zhang, Z. Interactions between metal ions and the biopolymer in activated sludge: Quantification and effects of system pH value. Front. Environ. Sci. Eng. 2017, 11, 7. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Z.; Zhang, J.; Xia, S. Understanding key constituents and feature of the biopolymer in activated sludge responsible for binding heavy metals. Chem. Eng. J. 2016, 304, 527–532. [Google Scholar] [CrossRef]
- Zhou, Y.; Xia, S.; Zhang, J.; Nguyen, B.T.; Zhang, Z. Insight into the influences of pH value on Pb(II) removal by the biopolymer extracted from activated sludge. Chem. Eng. J. 2017, 308, 1098–1104. [Google Scholar] [CrossRef]
- Zhou, Y.; Xia, S.; Zhang, J.; Zhang, Z.; Hermanowicz, S.W. Adsorption characterizations of biosorbent extracted from waste activated sludge for Pb(II) and Zn(II). Desalin. Water Treat. 2016, 57, 9343–9353. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J. Characteristics of Cadmium(II) adsorbed by the extracellular polymeric substance extracted from waste-activated sludge after short-time aerobic digestion. Water Air Soil Poll. 2014, 225, 1850. [Google Scholar] [CrossRef]
- Bradford, M.M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Chaplin, M.F.; Kennedy, J.F. Carbohydrate Analysis: A Practical Approach, 2nd ed.; IRL Press: Oxford, UK, 1994. [Google Scholar]
- Sun, Y.D.; Clinkenbeard, K.D.; Clarke, C.; Cudd, L.; Highlander, S.K.; Dabo, S.M. Pasteurella haemolytica leukotoxin induced apoptosis of bovine lymphocytes involves DNA fragmentation. Vet. Microbiol. 1999, 65, 153–166. [Google Scholar] [CrossRef]
- Kushwaha, S.; Sudhakar, P.P. Adsorption of mercury(II), methyl mercury(II) and phenyl mercury(II) on chitosan cross-linked with a barbital derivative. Carbohyd. Polym. 2011, 86, 1055–1062. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Sari, A.; Tuzen, M. Removal of mercury(II) from aqueous solution using moss (Drepanocladus revolvens) biomass: Equilibrium, thermodynamic and kinetic studies. J. Hazard. Mater. 2009, 171, 500–507. [Google Scholar] [CrossRef]
- Rocha, C.G.; Morozin Zaia, D.A.; Da Silva Alfaya, R.V.; Da Silva Alfaya, A.A. Use of rice straw as biosorbent for removal of Cu(II), Zn(II), Cd(II) and Hg(II) ions in industrial effluents. J. Hazard. Mater. 2009, 166, 383–388. [Google Scholar] [CrossRef]
- Ho, Y.; Wang, C. Sorption equilibrium of mercury onto ground-up tree fern. J. Hazard. Mater. 2008, 156, 398–404. [Google Scholar] [CrossRef]
- Zhang, D.; Lee, D.; Pan, X. Desorption of Hg(II) and Sb(V) on extracellular polymeric substances: Effects of pH, EDTA, Ca(II) and temperature shocks. Bioresour. Technol. 2013, 128, 711–715. [Google Scholar] [CrossRef]
- Rozada, F.; Otero, M.; Moran, A.; Garcia, A.I. Adsorption of heavy metals onto sewage sludge-derived materials. Bioresour. Technol. 2008, 99, 6332–6338. [Google Scholar] [CrossRef] [PubMed]
- Vinod, V.T.P.; Sashidhar, R.B.; Sivaprasad, N.; Sarma, V.U.M.; Satyanarayana, N.; Kumaresan, R.; Rao, T.N.; Raviprasad, P. Bioremediation of mercury (II) from aqueous solution by gum karaya (Sterculia urens): A natural hydrocolloid. Desalination 2011, 272, 270–277. [Google Scholar] [CrossRef]
- Natarajan, R.; Manivasagan, R. Biosorptive removal of heavy metal onto raw activated sludge: Parametric, equilibrium, and kinetic studies. J. Environ. Eng. 2016, 142, C4015002. [Google Scholar] [CrossRef]
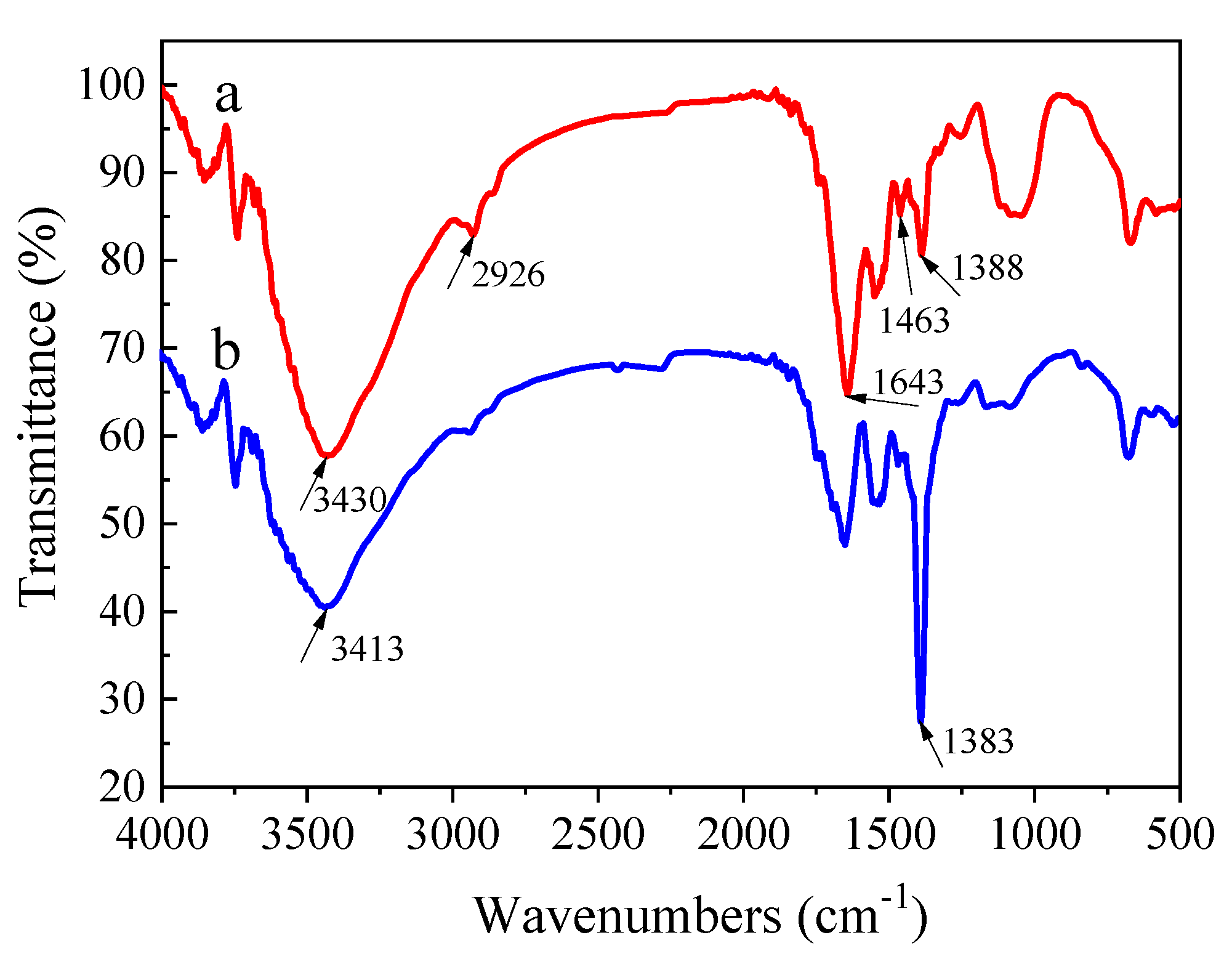
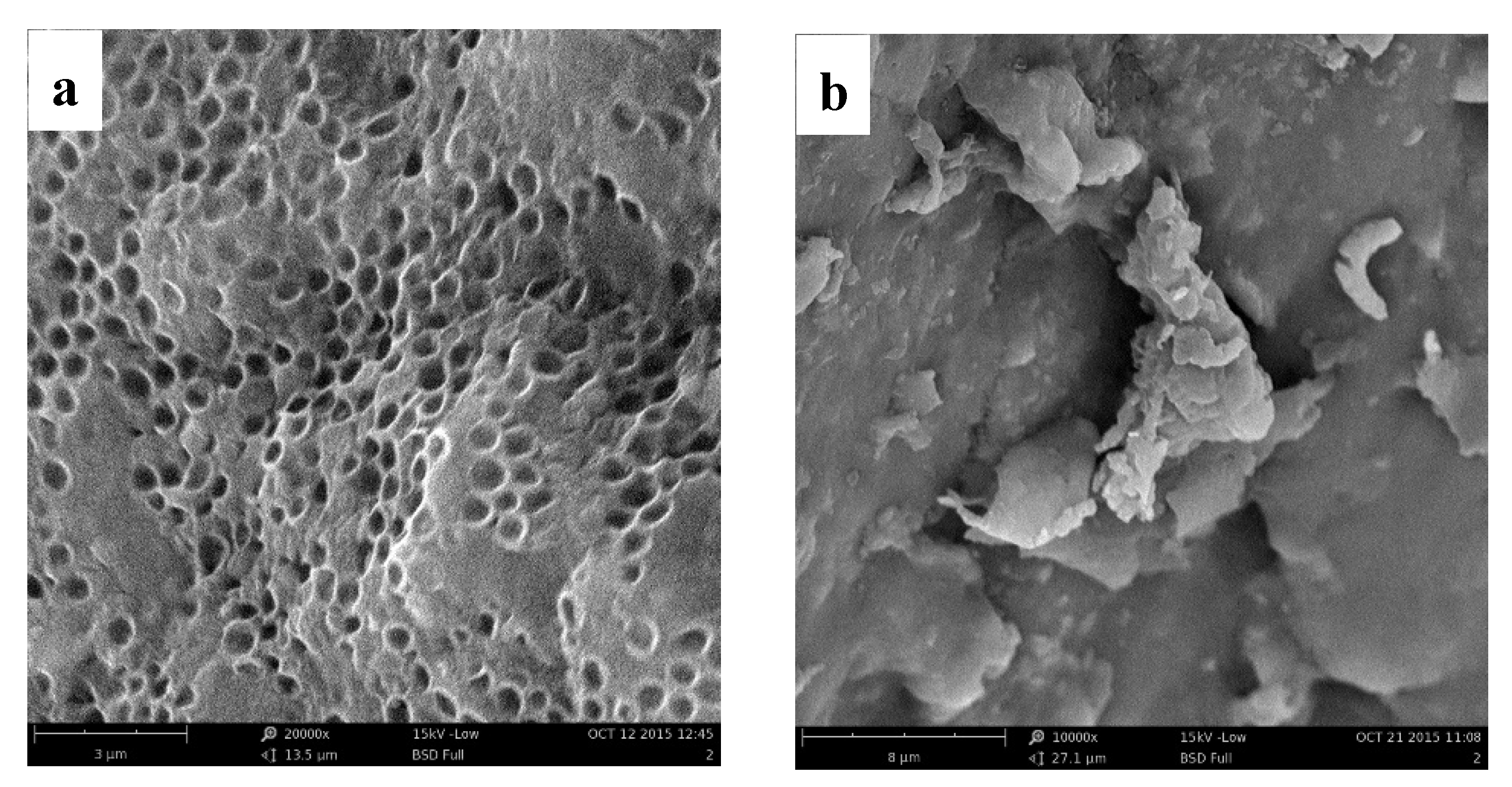
| C (mg/L) | qe, exp (mg/g) | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|
| qcal (mg/g) | K1 (min−1) | R2 | qcal (mg/g) | K2 (g/(mg·min)) | R2 | ||
| 10 | 441.4 | 225.7 | 0.06351 | 0.9042 | 471.0 | 0.0005250 | 0.9719 |
| 20 | 492.3 | 295.3 | 0.06433 | 0.9431 | 525.0 | 0.0004200 | 0.9605 |
| 40 | 497.4 | 254.7 | 0.05544 | 0.9059 | 519.6 | 0.0005180 | 0.9218 |
| Temperature (K) | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| qmax (mg/g) | KL (L/mg) | R2 | KF (mg/g)(L/mg)1/n | n | R2 | |
| 288 | 423.6 | 0.0953 | 0.9916 | 79.74 | 2.507 | 0.9401 |
| 298 | 452.9 | 0.1505 | 0.9748 | 129.8 | 3.358 | 0.9073 |
| 308 | 477.0 | 0.2217 | 0.9832 | 180.5 | 4.109 | 0.9689 |
| ΔG0 (kJ/mol) | ΔH0 (kJ/mol) | ΔS0 (J/mol·K) | R2 | ||
|---|---|---|---|---|---|
| 288 K | 298 K | 308 K | |||
| −23.61 | −25.54 | −27.39 | 30.93 | 189.4 | 0.9998 |
| Biosorbent | pH | Temperature (K) | qe | Reference |
|---|---|---|---|---|
| Rice straw | 5.0 | 298 | 22.1 | [29] |
| Dry activated sludge | 4.0 | 298 | 10.8 | [15] |
| Moss (Drepanocladus revolvens) biomass | 5.5 | 293 | 94.4 | [28] |
| EPS from activated sludge | 7.4 | 293 | 452.8 | [31] |
| Chemical activation of dried sewage sludge (AS) | 5.0 | 298 | 106.4 | [32] |
| Gum karaya | 6.0 | 298 | 62.6 | [33] |
| Raw activated sludge | 7.0 | 308 | 57.8 | [34] |
| Biopolymer | 5.0 | 308 | 477.0 | In this study |
| Elements | Atomic Percentage (%) before Adsorption of Hg(II) | Atomic Percentage (%) after Adsorption of Hg(II) |
|---|---|---|
| O | 38.9 | 7.4 |
| C | 30.0 | 41.3 |
| N | 24.1 | 4.0 |
| P | 3.1 | 0.1 |
| Na | 1.5 | 0.1 |
| Ca | 1.5 | 0 |
| K | 0.8 | 0 |
| Hg | 0 | 47.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, P.; Zhang, Z.; Xiang, P.; Xia, S. Biosorption Characteristics of Hg(II) from Aqueous Solution by the Biopolymer from Waste Activated Sludge. Int. J. Environ. Res. Public Health 2020, 17, 1488. https://doi.org/10.3390/ijerph17051488
Zhang J, Wang P, Zhang Z, Xiang P, Xia S. Biosorption Characteristics of Hg(II) from Aqueous Solution by the Biopolymer from Waste Activated Sludge. International Journal of Environmental Research and Public Health. 2020; 17(5):1488. https://doi.org/10.3390/ijerph17051488
Chicago/Turabian StyleZhang, Jiao, Pan Wang, Zhiqiang Zhang, Pengyu Xiang, and Siqing Xia. 2020. "Biosorption Characteristics of Hg(II) from Aqueous Solution by the Biopolymer from Waste Activated Sludge" International Journal of Environmental Research and Public Health 17, no. 5: 1488. https://doi.org/10.3390/ijerph17051488
APA StyleZhang, J., Wang, P., Zhang, Z., Xiang, P., & Xia, S. (2020). Biosorption Characteristics of Hg(II) from Aqueous Solution by the Biopolymer from Waste Activated Sludge. International Journal of Environmental Research and Public Health, 17(5), 1488. https://doi.org/10.3390/ijerph17051488





