Effects of Cadmium on Bioaccumulation, Bioabsorption, and Photosynthesis in Sarcodia suiae
Abstract
1. Introduction
2. Materials and Methods
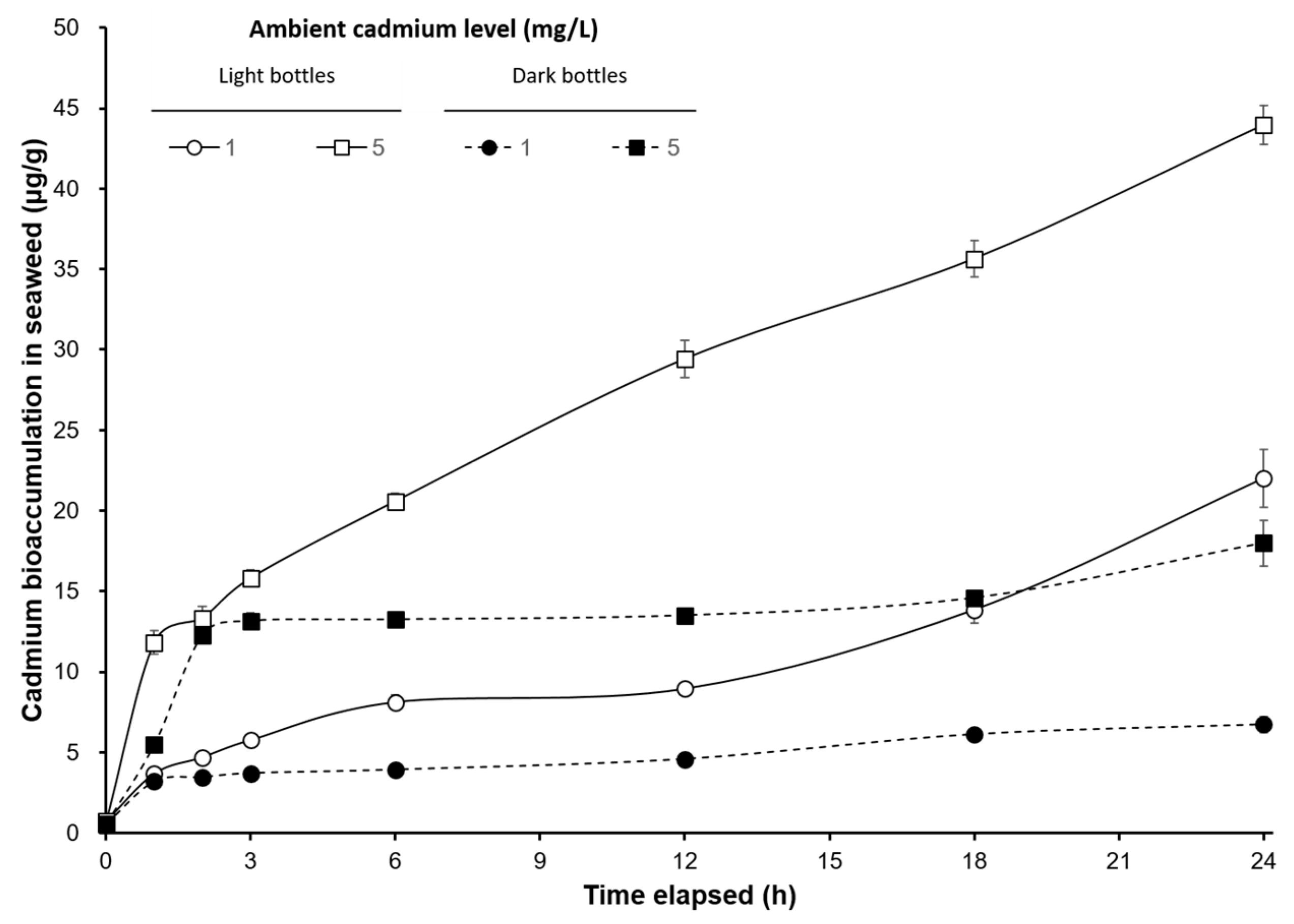
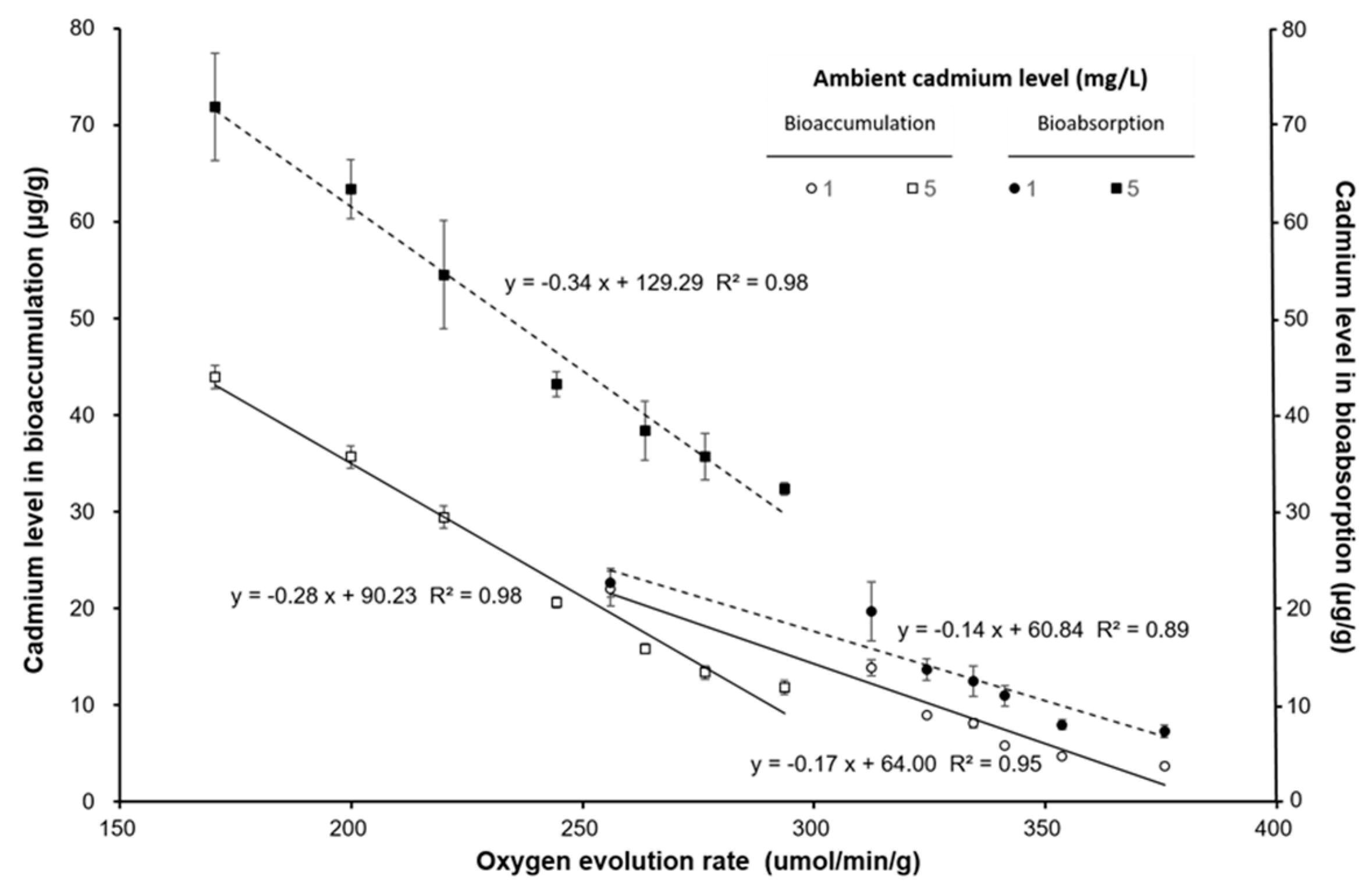
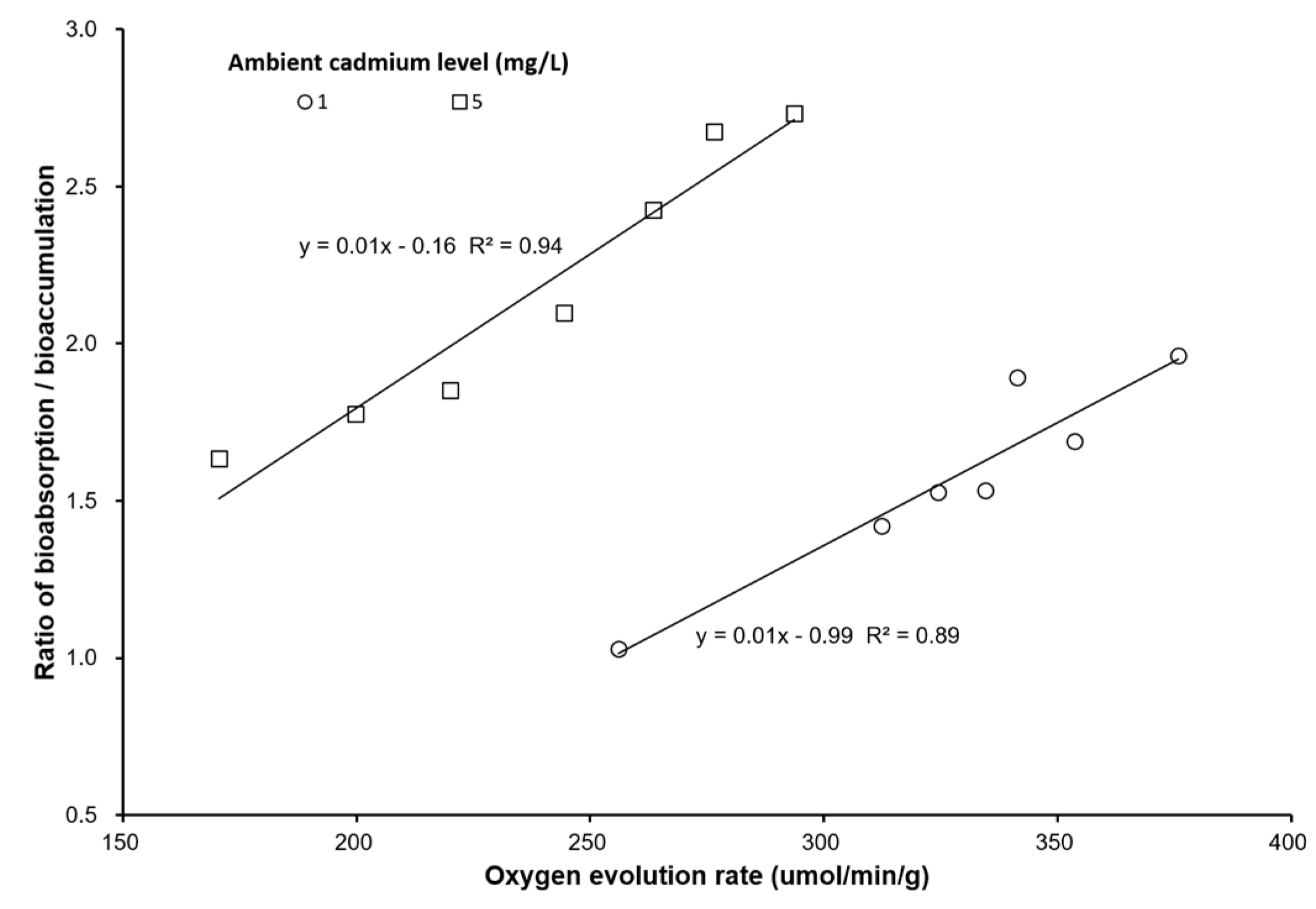
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yendo, K. Notes on algae new to Japan. VI. Bot. Mag. 1916, 30, 47–65. [Google Scholar] [CrossRef][Green Version]
- Rodríguez-Prieto, C.; Hommersand, M.H. Behaviour of the nuclei in pre- and post-fertilization stages in Kallymenia (Kallymeniaceae, Rhodophyta). Phycologia 2009, 48, 138–155. [Google Scholar] [CrossRef]
- Norris, R.E. Reproduction in Sarcodia dentata (Suhr) comb. nov. (Gigartinales, Rhodophyceae), with comments on the Sarcodiaceae. Br. Phycol. J. 1987, 22, 147–155. [Google Scholar] [CrossRef]
- Womersley, H.B.S. The Marine Benthic Flora of Southern Australia—Part IIIA; Australian Biological Resources Study: Canberra, Australia, 1994; p. 508. [Google Scholar]
- Renfro, W.C.; Fowler, S.W.; Heyraud, M.; La Rosa, J. Relative importance of food and water in long-term zinc-65 accumulation by marine biota. J. Fish. Res. Board Can. 1975, 32, 1339–1345. [Google Scholar] [CrossRef]
- Munshi, A.B.; Quan, S.Y.; Li, S.J. Acute toxicity of copper, cadmium and copper-cadmium mixture to the larvae of the shrimp Penaeus monodon. Pak. J. Sci. Ind. Res. 1996, 39, 68–71. [Google Scholar]
- Hsu, M.J.; Selvaraj, K.; Agoramoorthy, G. Taiwan’s industrial heavy metal pollution threatens terrestrial biota. Environ. Pollut. 2006, 143, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Rybak, A.; Messyasz, B.; Łeska, B. Freshwater Ulva (Chlorophyta) as a bioaccumulator of selected heavy metals (Cd, Ni and Pb) and alkaline earth metals (Ca and Mg). Chemosphere 2012, 89, 1066–1076. [Google Scholar] [CrossRef]
- Gresswell, T.; Simpson, S.L.; Smith, R.E.W.; Nugegoda, D.; Mazumder, D.; Twining, J. Bioaccumulation and retention kinetics of cadmium in the freshwater decapod Macrobrachium australiense. Aquat. Toxicol. 2014, 148, 174–183. [Google Scholar] [CrossRef]
- Monteiro, C.M.; Fonseca, S.C.; Castro, P.M.L.; Malcata, F.X. Toxicity of cadmium and zinc on two microalgae, Scenedesmus obliquus and Desmodesmus pleiomorphus, from Northern Portugal. J. Appl. Phycol. 2011, 23, 97–103. [Google Scholar] [CrossRef]
- Aguirre-von-Wobeser, E.; Figueroa, F.L. Photosynthesis and growth of red and green morphotypes of Kappaphycus alvarezii (Rhodophyta) from the Philippines. Mar. Biol. 2001, 138, 679–686. [Google Scholar] [CrossRef]
- Borlongan, I.A.; Gregory, N.N.; Shimada, S.; Terada, R. Photosynthetic performance of the red alga Solieria pacifica (Solieriaceae) from two different depths in the sublittoral waters of Kagoshima, Japan. J. Appl. Phycol. 2017, 29, 3077–3088. [Google Scholar] [CrossRef]
- Fukumoto, R.; Borlongan, I.A.; Nishihara, G.N.; Endo, H.; Terada, R. Effect of photosynthetically active radiation and temperature on the photosynthesis of two heteromorphic life history stages of a temperate edible brown alga, Cladosiphon umezakii (Chordariaceae, Ectocarpales), from Japan. J. Appl. Phycol. 2019, 31, 1259–1270. [Google Scholar] [CrossRef]
- Xia, J.R.; Li, Y.J.; Lu, J.; Chen, B. Effects of Copper and Cadmium on Growth, Photosynthesis, and Pigment Content in Gracilaria lemaneiformis. Bull. Environ. Contam. Toxicol. 2004, 73, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Dewez, D.; Geoffroy, L.; Vernet, G.; Popovic, R. Determination of photosynthetic and enzymatic biomarkers sensitivity used to evaluate toxic effects of copper and fludioxonil in alga Scenedesmus obliquus. Aquat. Toxicol. 2005, 74, 150–159. [Google Scholar] [CrossRef]
- Ji, Y.; Xie, X.J.; Wang, G.G. Effects of the heavy metal cadmium on photosynthetic activity and the xanthophyll cycle in Phaeodactylum tricornutum. J. Oceanol. Limnol. 2018, 36, 2194–2201. [Google Scholar] [CrossRef]
- Sunda, W.G.; Huntsman, S.A. Control of Cd concentrations in a coastal diatom by interactions among free ionic Cd, Zn, and Mn in seawater. Environ. Sci. Technol. 1998, 32, 2961–2968. [Google Scholar] [CrossRef]
- Reis, P.A.; Salgado, M.A.; Vasconcelos, V. Barnacles as biomonitors of metal contamination in coastal waters. Estuar. Coast. Shelf Sci. 2011, 93, 269–278. [Google Scholar] [CrossRef]
- Dos Santos, R.W.; Schmidt, E.C.; Martins, R.P.; Latini, A.; Maraschin, M.; Horta, P.A.; Bouzon, Z.L. Effects of Cadmium on Growth, Photosynthetic Pigments, Photosynthetic Performance, Biochemical Parameters and Structure of Chloroplasts in the Agarophyte Gracilaria domingensis (Rhodophyta, Gracilariales). Am. J. Plant. Sci. 2012, 3, 1077–1084. [Google Scholar] [CrossRef]
- Airs, R.L.; Temperton, B.; Sambles, C.; Farnham, G.; Skill, S.C.; Llewellyn, C.A. Chlorophyll f and chlorophyll d are produced in the cyanobacterium Chlorogloeopsis fritschii when cultured under natural light and near-infrared radiation. FEBS Lett. 2014, 588, 3770–3777. [Google Scholar] [CrossRef]
- Wolf, B.M.; Blankenship, R.E. Far-red light acclimation in diverse oxygenic photosynthetic organisms. Photosynth. Res. 2019, 142, 349–359. [Google Scholar] [CrossRef]
- Austin, P.A.; Ross, I.S.; Mills, J.D. Regulation of pigment content and enzyme activity in the cyanobacterium Nostoc sp. Mac grown in continuous light, a light-dark photoperiod, or darkness. Biochim. Biophys. Acta Bioenerg. 1996, 1277, 141–149. [Google Scholar] [CrossRef][Green Version]
- Jiang, T.; Zhang, J.P.; Chang, W.R.; Liang, D.C. Crystal structure of R-phycocyanin and possible energy transfer pathways in the phycobilisome. Biophys. J. 2001, 81, 1171–1179. [Google Scholar] [CrossRef][Green Version]
- Kwak, M.J.; Je, S.M.; Cheng, H.C.; Seo, S.M.; Park, J.H.; Baek, S.G.; Khaine, I.; Lee, T.Y.; Jang, J.; Li, Y.; et al. Night Light-Adaptation Strategies for Photosynthetic Apparatus in Yellow-Poplar (Liriodendron tulipifera L.) Exposed to Artificial Night Lighting. Forests 2018, 9, 74. [Google Scholar] [CrossRef]
- Küpper, H.; Küpper, F.; Spiller, M. In situ detection of heavy metal substituted chlorophylls in water plants. Photosynth. Res. 1998, 58, 123–133. [Google Scholar] [CrossRef]
- Ma, R.; Lu, F.; Bi, Y.; Hu, Z. Effects of light intensity and quality on phycobiliprotein accumulation in the cyanobacterium Nostoc sphaeroides Kützing. Biotechnol. Lett. 2015, 37, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, T.; Plusquin, M.; Hogervorst, J.; Roels, H.A.; Celis, H.; Thijs, L.; angronsveld, J.; Van Hecke, E.; Staessen, J.A. Environmental exposure to cadmium and risk of cancer: A prospective population-based study. Lancet Oncol. 2006, 7, 119–126. [Google Scholar] [CrossRef]
- Zhou, W.; Juneau, P.; Qiu, B. Growth and photosynthetic responses of the bloom-forming cyanobacterium Microcystis aeruginosa to elevated levels of cadmium. Chemosphere 2006, 65, 1738–1746. [Google Scholar] [CrossRef]
- Boyle, E.A.; Sclater, F.; Edmond, J.M. On the marine geochemistry of cadmium. Nature 1976, 263, 42–44. [Google Scholar]
- Hsu, S.C.; Lin, F.J.; Jeng, W.L.; Tang, T.Y. Spatial distribution of cadmium over a cyclonic eddy in the southern East China Sea. J. Mar. Syst. 2003, 39, 153–166. [Google Scholar] [CrossRef]
- Bruland, K.W.; Knauer, G.A.; Martin, J.H. Cadmium in northeast Pacific waters. Limnol. Oceanogr. 1978, 23, 618–625. [Google Scholar] [CrossRef]
- Licata, P.; Trombetta, D.; Cristani, M.; Martino, D.; Naccari, F. Organochlorine compounds and heavy metals in the soft tissue of the mussel Mytilus galloprovincialis collected from Lake Faro (Sicily, Italy). Environ. Int. 2004, 30, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Fargašová, A.; Bumbálová, A.; Havránek, E. Ecotoxicological effects and uptake of metals (Cu+, Cu2+, Mn2+, Mo6+, Ni2+, V5+) in freshwater alga Scenedesmus quadricauda. Chemosphere 1999, 38, 1165–1173. [Google Scholar] [CrossRef]
- Payne, C.D.; Price, N.M. Effects of cadmium toxicity on growth and elemental composition of marine phytoplankton. J. Phycol. 1999, 35, 293–302. [Google Scholar] [CrossRef]
- Kola, H.; Wilkinson, K.J. Cadmium uptake by a green alga can be predicted by equilibrium modelling. Environ. Sci. Technol. 2005, 39, 3040–3047. [Google Scholar] [CrossRef]
- Miao, X.; Wu, Q. Biodiesel production from heterotrophic microalgal oil. Bioresour. Technol. 2006, 97, 841–846. [Google Scholar] [CrossRef]
- Kapkov, V.I.; Belenikina, O.A.; Fedorov, V.D. Effect of heavy metals on marine phytoplankton. Mosc. Univ. Biol. Sci. Bull. 2011, 66, 32–36. [Google Scholar] [CrossRef]
- Echeveste, P.; Silva, J.C.; Lombardi, A.T. Cu and Cd affect distinctly the physiology of a cosmopolitan tropical freshwater phytoplankton. Ecotoxicol. Environ. Saf. 2017, 143, 228–235. [Google Scholar] [CrossRef]
- Zeraatkar, A.K.; Ahmadzadeh, H.; Talebi, A.F.; Moheimani, N.R.; McHenry, M.P. Potential use of algae for heavy metal bioremediation, a critical review. J. Environ. Manag. 2016, 181, 817–831. [Google Scholar] [CrossRef]
- Davis, T.A.; Volesky, B.; Vieira, R. Sargassum seaweed as biosorbent for heavy metals. Water Res. 2000, 34, 4270–4278. [Google Scholar] [CrossRef]
- Romera, E.; González, F.; Ballester, A.; Blázquez, M.; Munoz, J. Comparative study of biosorption of heavy metals using different types of algae. Bioresour. Technol. 2007, 98, 3344–3353. [Google Scholar] [CrossRef] [PubMed]
- Perales-Vela, H.V.; Pena-Casro, J.M.; Canizares-Villanueva, R.O. Heavy metal detoxification in eukaryotic microalgae. Chemosphere 2006, 64, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wilde, K.L.; Stauber, J.L.; Markich, S.J.; Franklin, N.M.; Brown, P.L. The effect of pH on the uptake and toxicity of copper and zinc in a tropical freshwater alga (Chlorella sp.). Arch. Environ. Contam. Toxicol. 2006, 51, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Macfie, S.M.; Tarmohamed, Y.; Welbourn, P.M. Effects of cadmium, cobalt, copper, and nickel on growth of the green alga Chlamydomonas reinhardtii: The influences of the cell wall and pH. Arch. Environ. Contam. Toxicol. 1994, 27, 454–458. [Google Scholar] [CrossRef]
- Bastosa, E.; Schneider, M.; de Quadros, D.P.C.; Welz, B.; Batista, M.B.; Horta, P.A.; Rörig, L.R.; Barufi, J.B. Phytoremediation potential of Ulva ohnoi (Chlorophyta): Influence of temperature and salinity on the uptake efficiency and toxicity of cadmium. Ecotoxicol. Environ. Saf. 2019, 174, 334–343. [Google Scholar] [CrossRef]
- Perreault, F.; Dionne, J.; Didur, O.; Juneau, P.; Popovic, R. Effect of cadmium on photosystem II activity in Chlamydomonas reinhardtii: Alteration of OJIP fluorescence transients indicating the change of apparent activation energies within photosystem II. Photosynth. Res. 2011, 107, 151–157. [Google Scholar] [CrossRef]
- Parmar, P.; Kumari, N.; Sharma, V. Structural and functional alterations in photosynthetic apparatus of plants under cadmium stress. Bot. Stud. 2013, 54, 45. [Google Scholar] [CrossRef]
- Sigfridsson, K.G.V.; Bernát, G.; Mamedov, F.; Styring, S. Molecular interference of Cd2+ with Photosystem II. Biochim. Biophys. Acta Bioenerg. 2004, 1659, 19–31. [Google Scholar] [CrossRef]
- Lu, F.; Dind, G.; Liu, W.; Zhan, D.M.; Wu, H.Y.; Guo, W. Comparative study of responses in the brown algae Sargassum thunbergii to zinc and cadmium stress. J. Oceanol. Limn. 2018, 36, 933–941. [Google Scholar] [CrossRef]
- Blot, N.; Wu, X.J.; Thomas, J.C.; Zhang, J.; Garczarek, L.; Bohm, S. Phycourobilin in trichromatic phycocyanin from oceanic cyanobacteria is formed post-translationally by a phycoerythrobilin lyase-isomerase. J. Biol. Chem. 2009, 284, 9290–9298. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; de Morais, R.M.; de Morais, A.M. Health applications of bio-active compounds from marine microalgae. Life Sci. 2013, 10, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Ikeuchi, M. Phycobilisome: Architecture of alight-harvesting supercomplex. Photosynth. Res. 2013, 116, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Mulders, K.J.M.; Lamers, P.P.; Martens, D.E.; Wijffels, R.H. Phototrophic pigment production with microalgae: Biological constraints and opportunities. J. Phycol. 2014, 50, 229–242. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, T.-W.; Tseng, C.-C.; Cai, M.; Chen, K.; Cheng, S.-Y.; Wang, J. Effects of Cadmium on Bioaccumulation, Bioabsorption, and Photosynthesis in Sarcodia suiae. Int. J. Environ. Res. Public Health 2020, 17, 1294. https://doi.org/10.3390/ijerph17041294
Han T-W, Tseng C-C, Cai M, Chen K, Cheng S-Y, Wang J. Effects of Cadmium on Bioaccumulation, Bioabsorption, and Photosynthesis in Sarcodia suiae. International Journal of Environmental Research and Public Health. 2020; 17(4):1294. https://doi.org/10.3390/ijerph17041294
Chicago/Turabian StyleHan, Tai-Wei, Chung-Chih Tseng, Minggang Cai, Kai Chen, Sha-Yen Cheng, and Jun Wang. 2020. "Effects of Cadmium on Bioaccumulation, Bioabsorption, and Photosynthesis in Sarcodia suiae" International Journal of Environmental Research and Public Health 17, no. 4: 1294. https://doi.org/10.3390/ijerph17041294
APA StyleHan, T.-W., Tseng, C.-C., Cai, M., Chen, K., Cheng, S.-Y., & Wang, J. (2020). Effects of Cadmium on Bioaccumulation, Bioabsorption, and Photosynthesis in Sarcodia suiae. International Journal of Environmental Research and Public Health, 17(4), 1294. https://doi.org/10.3390/ijerph17041294




